Abstract
Background
D-serine is an allosteric modulator of the brain N-methyl-D-aspartate (NMDA) receptor and a potential novel treatment of schizophrenia. Double-blind studies have been performed at 30 mg/kg/day (~2 g/day) with encouraging results, but no formal dose escalation studies have been performed. We describe the first evaluation of the efficacy and safety of D-serine at doses >30 mg/kg/day; a 4-week, open-label trial of adjunctive D-serine (30, 60 or 120 mg/kg/day).
Methods
42 antipsychotic-stabilized patients with schizophrenia or schizoaffective disorder participated. PANSS was obtained bi-weekly and neuropsychological (MATRICS) was obtained pre- and post medication phase. The pharmacokinetics/pharmacodynamics (PK/PD), and safety of doses ≥30 mg/kg was also evaluated.
Results
Significant improvement in symptoms and neuropsychological measures was noted across doses. On the PANSS, improvement was observed for positive (p=0.006;d=0.46), negative (p<0.001;d=0.68), general (p=0.001;d=0.53), and total (p<0.0001;d=0.74) symptoms. On MATRICS, while only non-significant improvement was noted at 30 mg/kg, highly significant, large effect size improvement was noted on the composite score (p<0.01;d=1.0) for doses ≥60 mg/kg, leading to a significant dose-by-time interaction (p<0.01).
In PK analyses, significant dose-dependent increases in plasma D-serine levels were seen during the study, predictive of significantly increased brain levels. Furthermore, increases in plasma levels correlated with improved symptomatic and neuropsychological function.
Discussion
These findings support double-blind investigation of D-serine at doses ≥60 mg/kg/d, and suggest effectiveness in treatment of both persistent symptoms and neurocognitive dysfunction.
Keywords: schizophrenia, NMDA, D-serine, negative symptoms, cognition
1. Introduction
Phencyclidine (PCP) and related compounds induce a psychotic state that closely resembles schizophrenia by blocking N-methyl-D-aspartate (NMDA)-type glutamate receptors (Javitt and Zukin, 1991). Agonists of the NMDA positive allosteric modulatory site, such as glycine and D-serine, reverse behavioral effects of PCP preclinically, suggesting potential therapeutic utility. Disturbances in D-serine metabolism are also reported (Kantrowitz and Javitt, 2009). To date, several studies of D-serine in schizophrenia have been conducted using a dose of ~30 mg/kg with positive results across studies (Javitt, 2008). Nevertheless, ideal doses of D-serine in clinical studies remains to be determined. In this study, we evaluated clinical effects of D-serine at doses >30 mg/kg/d with emphasis on both safety and efficacy.
2. Methods
2.1 Subjects
Inclusion criteria: 1) Aged 18–60; 2) SCID diagnosis of schizophrenia/schizoaffective disorder (First, et al., 1997); 3) Positive and Negative Symptom Scale (PANSS) (Kay, et al., 1987) negative symptom score >20 and total score between 60–110; 4) Stable Clinical Global Impression Scale (CGI) (Guy, 1976) for two consecutive weeks; 5) Simpson Angus Scale (SAS) (Simpson and Angus, 1970) score ≤12; 6) Calgary Depression Inventory for Schizophrenia (CDDS) (Addington, et al., 1994) score ≤10 and suicide less than moderate (<2); 7) No medication changes within 4 weeks prior to study; 8) Chlorpromazine (CPZ) equivalent ≤1500 mg (Woods, 2003).
Exclusion criteria: 1) Treatment with ≥2 antipsychotics/clozapine; 2) Renal impairment; 3) Alcohol/substance abuse and use within past month or dependence and use within past six months; 4) Known neurological disorder.
2.2 Study Design
The study was performed in 3 dose-level phases. Following enrollment, patients underwent a 24-hour pharmacokinetics/pharmacodynamics (PK/PD) session on Day 1 of treatment. Subjects then received four weeks of open-label D-serine. A 4-hour, PK/PD session was completed at study end in the 60 and 120 mg/kg phases. Safety measures (urinalysis, chemistry, CBC, LFT’s) were obtained weekly, with a follow-up two weeks after the last D-serine dose. Inter-rater reliability was maintained by biweekly teleconferences.
2.3 Procedures
2.3.1 Clinical Assessments
The predesignated primary clinical outcomes were the PANSS total and the CGI (http://clinicaltrials.gov/ct2/show/NCT00322023). Individual PANSS subscales, the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1982), SAS; the Abnormal Involuntary Movement Scale (AIMS) for tardive dyskinesia (TD) (Guy, 1976); Barnes Akathisia Scale (BAS) (Barnes, 1989); and CDDS were secondary outcomes.
2.3.2 Neurocognitive
The predesignated neurocognitive rating outcome was the composite T-score of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery (Nuechterlein, et al., 2008) which was obtained at baseline and end-of-treatment.
2.4 Statistical Analysis
Baseline data was compared by a Chi-square test and a one-way ANOVA for categorical and continuous data, respectively. Despite baseline differences in symptoms and cognition between sites, reflecting greater impairments in inpatients, there were no significant site-by-time effects. Within-dose baseline-final comparisons were made with a paired t-test, and across dose/site–by-time comparisons with a repeated measure ANOVA (rmANOVA). Within-group effect sizes (Cohen’s d) were calculated for the baseline-final change scores (Cohen, 1988). Exploratory pharmacodynamic relationships were determined by Spearman’s correlation coefficients (rs). Reported values are Mean±SD.
2.5 Plasma D-serine level determination
D- and L-serine levels were determined using liquid chromatography with fluorescence detection (Hashimoto, et al., 1992). Intra-assay variability did not exceed 6.4% for the 7 calibration concentrations (n=8 for each concentration). Inter-assay variation, measured using two sets of quality controls at 50 and 500 nM/mL of D-serine prepared in drug-free plasma, did not exceed 9.1% and 6.0% for the respective concentrations (n=13 days).
2.6 Subject Disposition
42 individuals were enrolled. 34 individuals completed at least one phase, with eight completing at least two [three participating in the 60 and 120 mg/kg) and five others in all three], yielding 47 evaluable phases (Table 1). For repeaters, there were a mean 7.0 months and a mean 10.8 months between the 30 and 60 and the 60 and 120 mg/kg trials, respectively.
Table 1.
Study Demographics for Study Completers
| Nominal relative D-serine dose (mg/kg/d) | 30mg/kg (n=12) | 60 mg/kg (n=19) | 120 mg/kg (n=16) |
|---|---|---|---|
| Age | 41.7±11.4 | 43.5±9.4 | 43.2±9.6 |
| Male (%) | 75% | 95% | 88% |
| Schizophrenia (n) | 9 | 16 | 13 |
| Schizoaffective (n) | 3 | 3 | 3 |
| Inpatient (n) | 5 | 8 | 6 |
| Highest Education | 12.1±2.3 | 11.7±1.9 | 11.9±2.8 |
| Age of 1st Treatment | 21.9±5.4 | 21.1±6.4 | 20.8±8.9 |
| Age of 1st Hospitalization1 | 22.3±4.5 | 22.6±5.4 | 24.0±8.6 |
| Antipsychotic dose (CPZ Equivalents/d) | 468.8±252 | 602.6±295 | 493.7±319 |
| Atypical (%) | 67% | 68% | 75% |
| Dose Repeaters | N/A | 5 | 8 |
| Body weight (kg) | 88.9±17.2 | 99.8±17.5 | 99.5±21.0 |
| Achieved absolute D-serine dose (g/d) | 2.58±0.31 | 5.58±0.45 | 11.1±1.1 |
Except for number of dose repeaters, there were no significant baseline differences on a one-way ANOVA (all p>0.29).
Data unavailable for one patient in 30 mg/kg dose and one in 120 mg/kg dose.
Baseline scores did not differ between completers and non-completers (p>0.05). Of the non-completers, two withdrew after the PK/PD session, two for reported side effects, three for minor laboratory abnormalities and two for reasons unrelated to study medication.
3. Results
3.1 PANSS
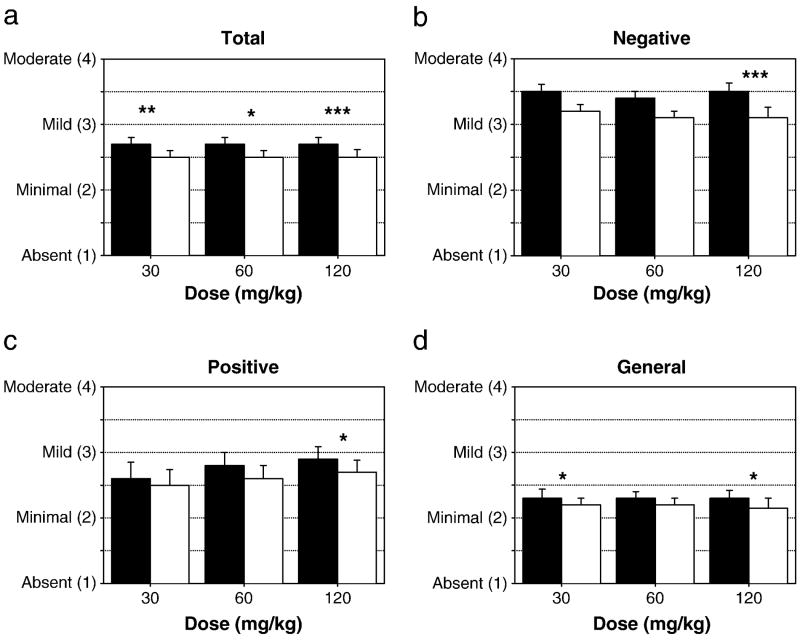
Across doses, significant, moderate effect-size improvements were observed on PANSS total and subscales (Figure 1a–d and Supplement 1). Significant improvement was seen on the total PANSS within all three phases (p<0.05), and across all three subscales for the 120 mg/kg dose.
Figure 1. Total and PANSS outcomes.
Baseline (filled bars) and final (open bars) mean item scores for (a) total, (b) negative, (c) positive and (d) general symptoms on the PANSS. For the study as a whole, PANSS, improvement was observed for positive (p=0.006; d=0.46), negative (p<0.001; d=0.68), general (p=0.001; d=0.53), and total (p<0.0001; d=0.74) symptoms. *p<0.05, **p<0.01, ***p<0.001 paired t-test, baseline vs. final
3.2 MATRICS
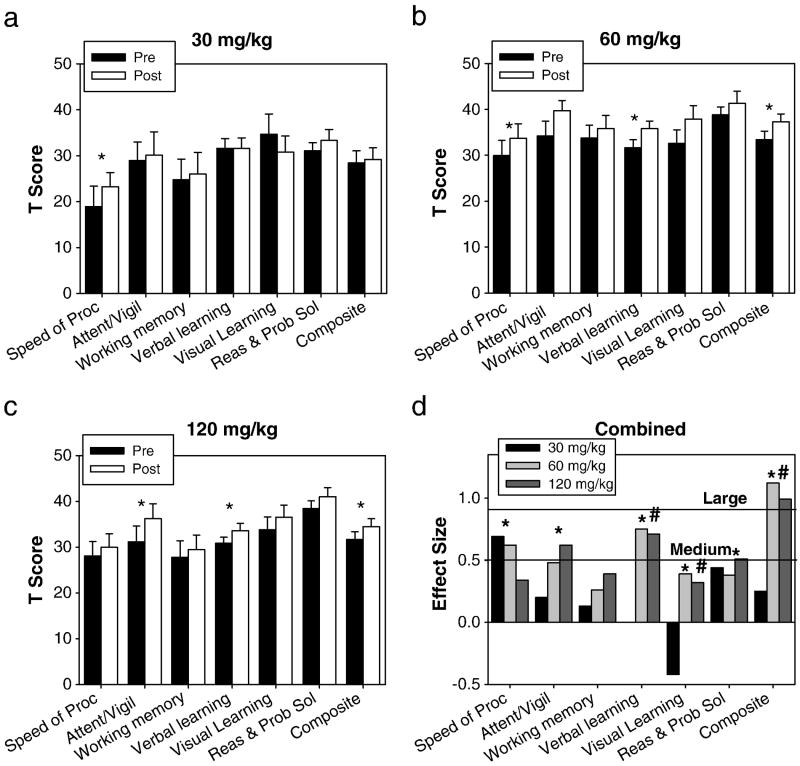
Baseline scores were ~2 sd below normative levels on the MATRICS, suggesting clinically significant baseline cognitive deficits (Figure 2). There were no significant differences in baseline mean composite (F2,44=1.5;p=0.23) or individual MATRICS domain scores by dose, except for a significantly lower baseline Reasoning & Problem Solving Domain for 30 mg/kg (F2,44=5.6;p<0.01).
Figure 2. MATRICS Outcomes.
Baseline (filled bars) and final (open bars) normalized MATRICS domains and overall mean composite (mean T-score of six tested domains) for (a) 30, (b) 60, and (c) 120 mg/kg. d. Within group effect sizes for each dose. *p<0.05 in a paired t-test between baseline and final (a–c) or on a paired t-test for doses >60mg/kg (d). #Significant (p<0.05) dose by time interaction for 30 vs. ≥60 mg/kg.
For the 30 mg/kg dose, there was no significant main effect of time on the MATRICS composite score (t11=−0.89;p=0.39;d=0.25), although a moderate improvement in the Speed of Processing Domain (t11=2.4;p=0.04;d=0.69) was seen. In contrast, significant, statistically large treatment effects were observed at both the 60 (t18=−4.9;p<0.001;d=1.1) and 120 (t15=−3.9;p=0.01;d=0.99) mg/kg doses, leading to a significant dose-by-time interaction (F2,44=3.6;p=0.035) for the three phases. Moreover, in an exploratory analysis (Figure 2d), a significant, large effect-size improvement was seen for the composite score (t34=−6.3;p<0.001;d=1.1), with greater improvement at ≥60 mg/kg vs. 30 mg/kg dose noted for the overall composite (F1,44=6.2;p=0.017), and the Visual (F1,44=4.8;p=0.034) and Verbal (F1,44=4.7;p=0.035) Memory Domains. In addition, for ≥60 mg/kg doses, significant change was observed across all domains except Working Memory (t33=1.6;p=0.12).
3.3 CGI and secondary outcomes
On the CGI-Improvement scale, 36.2% of subjects were rated as at least mildly improved (t46=−3.5;p<0.001), with no significant difference across doses (F2,44=0.11;p=0.895). While no significant changes were seen in lower doses (all p>0.19), in the 120 mg/kg phase, both total SANS and Global Affective Flattening significantly improved (all p<0.02). There were no significant changes in CDSS. The AIMS showed a modest, significant improvement (Table 2).
Table 2.
Baseline and Final extrapyramidal scales by dose.
| Baseline | Final | Change | ||||
|---|---|---|---|---|---|---|
| Dose | AIMS | SAS | AIMS | SAS | AIMS | SAS |
| 30 | 1.0±1.3 | 3.4±3.4 | 0.7±1.3 | 3.5±3.6 | −0.3±0.7 | 0.1±2.4 |
| 60 | 1.3±2.7 | 3.0±2.3 | 1.2±2.6 | 2.4±2.0 | −0.2±0.8 | −0.6±2.2 |
| 120 | 1.8±3.1 | 2.3±2.0 | 1.4±3.0 | 1.6±1.8 | −0.3±0.9 | −0.8±2.2 |
| All | 1.4±2.5 | 2.9±2.5 | 1.1±2.5 | 2.4±2.5 | −0.3±0.8* | −0.5±2.2 |
p<0.05
3.4 Pharmacokinetics
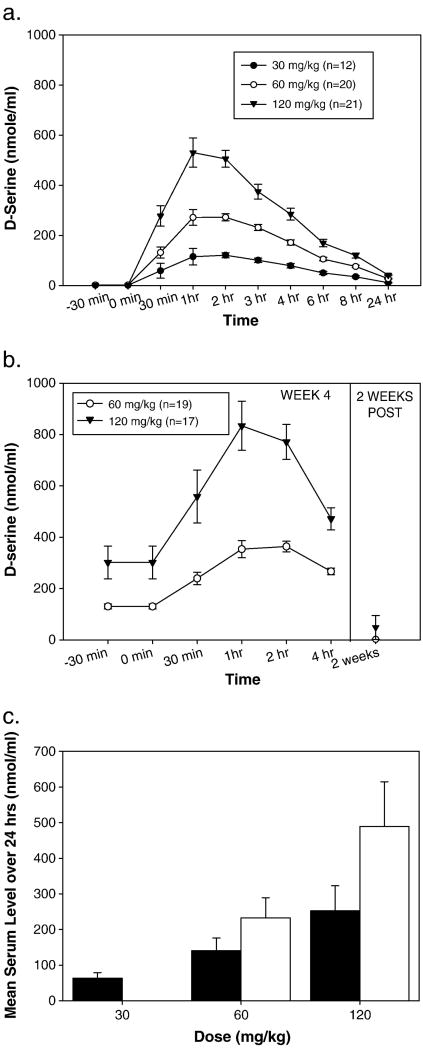
For all doses, D-serine exhibited linear kinetics, with a Tmax and t½ of ~1–2 hr and ~3.3 hr, respectively (Figure 3a and Table 3). Furthermore, mean levels observed during PK assessments at week 4 were significantly greater than those at week 1 (t46=4.7;p<0.001), suggesting accumulation over time (Figure 3b and 3c).
Figure 3. D-serine Pharmacokinetics.
a. 24-hour pharmacokinetics of an acute dose of D-serine on day 1 of treatment. b. 4-hour pharmacokinetics after 4 weeks of chronic dosing. c. Mean plasma level over 24 hours of acute and chronic D-serine during week 1 (filled bar) and week 4 (open bar) pharmacokinetics. The Week 4 pharmacokinetics session was not done in the 30 mg/kg phase.
Table 3.
Cmax levels by dose.
| Dose | Cmax (nmol/ml) |
|---|---|
| 30 mg/kg | 120.6±34.6 |
| 60 mg/kg | 272.3±62.0 |
| 120 mg/kg | 530.3±266.8 |
3.5 Correlational analyses
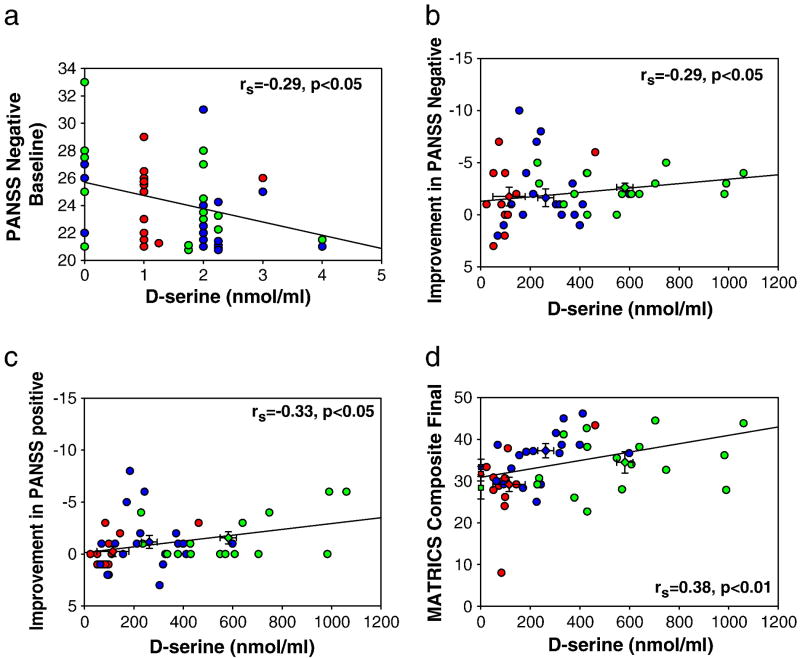
Despite low baseline D-serine levels (1.51±1.0 nmol/ml) a significant range (0–5 nmol/ml) was observed across subjects. Pretreatment levels were significantly correlated with the pretreatment PANSS negative symptoms for the sample as a whole (rs=−0.29, p<0.05) (Figure 4a). Peak D-serine at study initiation correlated with the magnitude of improvement in negative (Figure 4b) and positive (Figure 4c) symptoms independently. Composite final MATRICS score also correlated with peak levels (Figure 4d). Similar relationships were observed with peak levels obtained during week 4 PK/PD studies, suggesting a continued relationship to sustained elevation in D-serine level.
Figure 4. D-serine Pharmacodynamics.
Regression scatter plots of D-serine plasma level vs. behavioral outcome. Circles
 indicate an individual patient, diamonds
indicate an individual patient, diamonds
 and
and
 indicate final and baseline mean±SEM, respectively. a. Pretreatment D-serine level vs. pretreatment PANSS negative. As PANSS scores are reported as whole numbers, circles were vertically offset to avoid superimposing subjects. b and c. Peak D-serine level on Day 1 of treatment vs. improvement in (b) PANSS negative, (c) PANSS positive. d. Peak D-serine level on Day 1 of treatment vs. post-treatment MATRICS composite.
indicate final and baseline mean±SEM, respectively. a. Pretreatment D-serine level vs. pretreatment PANSS negative. As PANSS scores are reported as whole numbers, circles were vertically offset to avoid superimposing subjects. b and c. Peak D-serine level on Day 1 of treatment vs. improvement in (b) PANSS negative, (c) PANSS positive. d. Peak D-serine level on Day 1 of treatment vs. post-treatment MATRICS composite.
3.6 Safety Measures
There were no safety issues at doses <120 mg/kg. At the 120 mg/kg dose, 1 subject showed 2+ proteinuria without glycosuria during the final week of treatment with no significant change in creatinine that resolved following D-serine discontinuation. Two patients in the 120 mg/kg phase were discontinued from this study for asymptomatic transaminitis, which resolved completely. Two additional subjects withdrew consent for reported side effects (insomnia after one dose and GI distress) in the 120 mg/kg phase.
4. Discussion
Similar to prior studies (Javitt, 2008), significant improvement was observed on PANSS positive, negative and total scores. Significant baseline correlations were observed between reduced D-serine levels and negative symptoms and between peak D-serine on day 1 and subsequent improvement in negative symptoms, consistent with prior clinical findings (Neeman, et al., 2005, Sumiyoshi, et al., 2004). As plasma D-serine levels were unknown at the time when ratings were performed, these are unlikely to reflect placebo response and therefore support the hypothesis of a true therapeutic response to D-serine.
Despite repeated studies of symptoms, neurocognitive data with NMDA agonists to date have been sparse. In the present study, highly significant, dose-dependent, within-subject change was observed on composite score, with large effect-size improvement (d>1.0) being seen at both 60 and 120 mg/kg doses. Furthermore, changes were significant not only within dose, but also relative to the 30 mg/kg dose that has been the subject of prior clinical studies. Peak D-serine levels on day 1 correlated significantly with post-treatment neurocognition scores, supporting a dose-response relationship.
As in prior studies with NMDA glycine-site agonists (Heresco-Levy and Javitt, 2004), significant improvement was noted in TD. Improvement has been noted as well in an animal model of TD (Shoham, et al., 2004). Despite the introduction of atypical antipsychotics, TD remains an issue in clinical practice (Correll and Schenk, 2008).
No nephrotoxicity was observed at dose <120 mg/kg/d, supporting safety. One patient did show a nephrotoxic-like pattern at 120 mg/kg, which resolved upon D-serine discontinuation.
Overall, present results are encouraging of further study of higher-dose D-serine. As our present efficacy results are from a relatively brief open-label study, they must be interpreted with caution, and replicated in larger, longer-duration, randomized, double-blind clinical investigations.
Supplementary Material
Acknowledgments
5.1 Role of funding source
This project was funded by NIH grant (U01 MH074356). Preclinical/clinical toxicology in support of the project was provided by 5R44AT000810 and a grant from the Stanley Foundation. The NIH and Stanley Foundation had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the paper for publication.
Funded by NIH grant (U01 MH074356) to DCJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington D, Addington J, Maticka-Tyndale E. Specificity of the Calgary Depression Scale for Schizophrenics. Schizophr Res. 1994;11(3):239–244. doi: 10.1016/0920-9964(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Assoc; Hillsdale, NJ: 1988. [Google Scholar]
- Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21(2):151–156. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) 1997. [Google Scholar]
- Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology Revised. National Institute of Mental Health; Bethesda, MD: 1976. [Google Scholar]
- Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert.-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J Chromatogr Biomed Appl. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: a retrospective analysis. Schizophr Res. 2004;66(2–3):89–96. doi: 10.1016/S0920-9964(03)00129-4. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry. 2008;63(1):6–8. doi: 10.1016/j.biopsych.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. Handbook of Neurochemistry and Molecular Neurobiology. 3 2009. Glutamatergic Approaches to the Conceptualization and Treatment of Schizophrenia. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Neeman G, Blanaru M, Bloch B, Kremer I, Ermilov M, Javitt DC, Heresco-Levy U. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am J Psychiatry. 2005;162(9):1738–40. doi: 10.1176/appi.ajp.162.9.1738. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Shoham S, Mazeh H, Javitt DC, Heresco-Levy U. Glycine and D-cycloserine attenuate vacuous chewing movements in a rat model of tardive dyskinesia. Brain Res. 2004;1004(1–2):142–7. doi: 10.1016/j.brainres.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psych Scand. 1970;212(suppl):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Anil AE, Jin D, Jayathilake K, Lee M, Meltzer HY. Plasma glycine and serine levels in schizophrenia compared to normal controls and major depression: relation to negative symptoms. Int J Neuropsychopharmacol. 2004;7(1):1–8. doi: 10.1017/S1461145703003900. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.