Abstract
Transforming growth factor–β (TGFβ) signaling drives aneurysm progression in multiple disorders, including Marfan syndrome (MFS), and therapies that inhibit this signaling cascade are in clinical trials. TGFβ can stimulate multiple intracellular signaling pathways, but it is unclear which of these pathways drives aortic disease and, when inhibited, which result in disease amelioration. Here we show that extracellular signal–regulated kinase (ERK) 1 and 2 and Smad2 are activated in a mouse model of MFS, and both are inhibited by therapies directed against TGFβ. Whereas selective inhibition of ERK1/2 activation ameliorated aortic growth, Smad4 deficiency exacerbated aortic disease and caused premature death in MFS mice. Smad4-deficient MFS mice uniquely showed activation of Jun N-terminal kinase–1 (JNK1), and a JNK antagonist ameliorated aortic growth in MFS mice that lacked or retained full Smad4 expression. Thus, noncanonical (Smad-independent) TGFβ signaling is a prominent driver of aortic disease in MFS mice, and inhibition of the ERK1/2 or JNK1 pathways is a potential therapeutic strategy for the disease.
The transforming growth factor–β (TGFβ) ligands belong to a family of cytokines that regulates diverse cellular functions, including proliferation, differentiation, and synthetic repertoire. TGFβ is secreted from cells as part of a large latent complex that binds to extracellular matrix (ECM) proteins including fibrillin-1 (1), the deficient gene product in Marfan syndrome (MFS). Current models posit that ECM sequestration of TGFβ inhibits its activation, thereby limiting its ability to stimulate cell surface receptors, TβRI and TβRII (2, 3). In canonical signaling, the TβRI/II complex phosphorylates receptor-activated Smad2 and/or Smad3 (to pSmad2 and pSmad3, respectively), which leads to recruitment of Smad4, translocation to the nucleus, and the transcription of Smad-dependent genes (4). Recent work has shown that TGFβ also induces other (noncanonical) pathways, including the RhoA and mitogen-activated protein kinase (MAPK) cascades, the latter of which includes extracellular signal–regulated kinase (ERK), Jun N-terminal kinase (JNK), and p38 (5–7). TGFβ activates these by phosphorylation to pERK, pJNK, and pp38, respectively. In light of these findings, the exclusive focus on Smad signaling in TGFβ-related pathogenetic models needs to be reconsidered.
Increased Smad2/3 activation and increased expression of Smad-responsive genes (e.g., connective tissue growth factor and plasminogen-activator inhibitor–1, PAI-1) have been observed in the lung, skeletal muscle, mitral valve, and aortic wall in humans and a mouse model of MFS (8–11). Treatment of MFS mice with TGFβ-neutralizing antibody (TGFβNAb) ameliorates the phenotype in all of these tissues, in association with attenuated pSmad2/3 signaling (8–11). A similar rescue is achieved by using the angiotensin II type 1 receptor–blocker losartan (8–11), which is known to reduce the expression of TGFβ ligands, receptors, and activators (12–14). It has also been shown that mutations in TβRI or II, which lead to a paradoxical increase in pSmad2 signaling in the aortic wall, cause Loeys-Dietz syndrome, a condition that has considerable phenotypic overlap with MFS, including aortic aneurysm (15, 16). Together, these earlier observations suggested that canonical TGFβ signaling drives disease pathogenesis in MFS. We have now explored the relative contributions of canonical and noncanonical TGFβ signaling cascades in MFS mice, by either genetically or pharmacologically inhibiting each cascade and analyzing the resultant phenotypic consequences.
We performed Western blot analysis on the proximal ascending aorta of 12-month-old mice heterozygous for a missense mutation in Fbn1 (Fbn1C1039G/+), a validated animal model of MFS (17). Compared with wild-type (WT) littermates, Fbn1C1039G/+ mice showed a significant increase in activation of Smad2, ERK1/2, and MAPK kinase 1 (MEK1), the upstream activator of ERK1/2 (P < 0.05, P < 0.001, and P < 0.05, respectively) (Fig. 1A). In contrast, there was no difference in the activation of Smad3; JNK1; p38; ERK5; Rho-associated coiled-coil containing protein kinase–1 (ROCK1); or LIMK1, a downstream target of ROCK1 (Fig. 1A and fig. S1) (18). Furthermore, an in vivo trial of fasudil, a well-established inhibitor of the RhoA/ROCK pathway [details discussed in (18)] failed to attenuate aortic root growth in Fbn1C1039G/+ mice (fig. S2).
Fig. 1.
Canonical and noncanonical TGFβ signaling in the proximal ascending aorta. (A) Western blot analysis of 4 WT and Fbn1C1039G/+ mice. Note that only pSmad2, pERK1/2, and pMEK1 signaling are increased in Fbn1C1039G/+ mice. The graphs show normalization to β-actin, but the same outcomes were observed with normalization to the respective total proteins. (B) Western blot analysis of three each of WT and of Fbn1C1039G/+ mice treated with placebo, TGFβNAb, or losartan. Note the significant reduction in pERK1/2 signaling after treatment with TGFβNAb (NAb) or losartan (Los). (C) Aortic root growth in placebo-treated WT (n = 5), placebo-treated Fbn1C1039G/+ (n = 6), RDEA119-treated WT (n = 3), and RDEA119-treated Fbn1C1039G/+ (n = 7) mice. Note that RDEA119 therapy selectively reduced growth in Fbn1C1039G/+ mice. Final absolute aortic root diameter (mm): WT (1.62 ± 0.08), placebo-treated Fbn1C1039G/+ (2.15 ± 0.17), RDEA119-treated WT (1.64 ± 0.09), RDEA119-treated Fbn1C1039G/+ (1.94 ± 0.07). (D) Western blot analysis of three placebo- and three RDEA119-treated Fbn1C1039G/+ mice, showing a selective reduction in pERK1/2 signaling in RDEA119-treated mice. Plac, placebo. Values are means ± 2SEM. *P < 0.05; **P < 0.01; †P < 0.001; NS, not significant.
Because TGFβNAb and losartan attenuate aortic root growth in Fbn1C1039G/+ mice (fig. S3) (10), if Smad2 or ERK1/2 are important mediators of aortic disease in MFS, one would expect their activation to be reduced by these agents. Prior work demonstrated that Smad2 activation is decreased by both therapies (10). We now find that compared with placebo-treated littermates, Fbn1C1039G/+ mice treated with either TGFβNAb or losartan also show a significant reduction in ERK1/2 activation (P < 0.01 for both) (Fig. 1B).
To confirm that ERK1/2 is a driver, rather than simply a marker, of aortic aneurysm progression, 2-month-old Fbn1C1039G/+ mice were treated for 2 months with the selective MEK1/2 inhibitor RDEA119 (19). Aortic root size was measured by echocardiography at 2 months (baseline before treatment) and 4 months of age (Fig. 1C). Aortic root growth was significantly greater in placebo-treated Fbn1C1039G/+ mice, compared with WT littermates (P < 0.05). Aortic root growth in RDEA119-treated Fbn1C1039G/+ mice was significantly less than that of placebo-treated Fbn1C1039G/+ littermates (P < 0.01) and indistinguishable from that observed in WT mice (P = 0.15). RDEA119 therapy had no significant effect in WT mice (P = 0.24), which illustrated that inhibition of ERK1/2 activation specifically targets MFS-associated pathological aortic root growth, while still allowing for normal physiological growth.
The specificity of RDEA119 was confirmed by Western blot analysis of the proximal ascending aorta. Compared with placebo-treated Fbn1C1039G/+ littermates, RDEA119-treated Fbn1C1039G/+ mice showed a significant reduction in ERK1/2 activation (P < 0.01), whereas Smad2, JNK1, p38 and ERK5 activation was unchanged (Fig. 1D). This result also shows that Smad2 activation in Fbn1C1039G/+ mice is not ERK-dependent. Together, these data suggest that TGFβ-driven ERK1/2 activation contributes to aortic aneurysm progression in MFS mice and that antagonism of this pathway may be therapeutically useful.
To determine whether canonical signaling contributes to aortic disease progression in MFS, we introduced haploinsufficiency for Smad4, a critical mediator of canonical TGFβ signaling, into our MFS mouse model. We bred Fbn1C1039G/+ mice to mice harboring a deletion of exon 8 of the Smad4 gene. Homozygosity for this Smad4 allele (S4−/−) results in the death of embryos (20). In contrast, haploinsufficient mice (S4+/−) are fertile, have normal life spans, and show clinically relevant attenuation of Smad-dependent signaling in several tissues, including the stomach, breast, and intestine (20).
The Fbn1C1039G/+ MFS mouse model shows progressive aortic root dilatation, but does not typically progress to aortic dissection or premature death. Whereas almost all WT, S4+/− and Fbn1C1039G/+ mice survived to 8 months of age, S4+/−:Fbn1C1039G/+ mice died prematurely. This was first evident by 1 month of age; by 3 months 40% had died, and by 8 months 70% had died (Fig. 2A). Necropsy of these animals revealed hemothorax and hemopericardium in all cases, indicative of proximal aortic rupture; there was no evidence of aortic rupture in any WT, S4+/−, or Fbn1C1039G/+ mice.
Fig. 2.
Effect of Smad4 haploinsufficiency (S4+/−) on aortic phenotype. (A) Survival curve of WT (n = 112), S4+/− (n = 56), Fbn1C1039G/+ (n = 107) and S4+/−:Fbn1C1039G/+ (n = 85) mice. Note the high rate of premature death due to aortic dissection in S4+/−:Fbn1C1039G/+ mice. (B) Aortic root and ascending aortic diameter, measured by echocardiography, at 3 months of age in WT (n = 9), S4+/− (n = 11), Fbn1C1039G/+ (n = 24), and S4+/−:Fbn1C1039G/+ (n = 26) mice. Although Fbn1C1039G/+ mice showed a selective increase in aortic root diameter compared with WT littermates, S4+/−:Fbn1C1039G/+ mice demonstrated an increase in both aortic root and ascending aortic diameter, compared with all other genotypes. (C) VVG staining of representative sections of the proximal ascending aorta. Compared with WT littermates, Fbn1C1039G/+ mice demonstrated medial thickening and elastic fiber fragmentation, both of which are exacerbated in S4+/−:Fbn1C1039G/+ mice. Values are means ± 2 SEM. †P < 0.001; ††P < 0.0001; NS, not significant.
Echocardiography at 3 months of age revealed significant enlargement of both the aortic root and the ascending aorta in S4+/−:Fbn1C1039G/+ mice, compared with Fbn1C1039G/+ littermates (P < 0.0001 and P < 0.001, respectively) (Fig. 2B). These data provide a conservative estimate of the effect of Smad4 haploinsufficiency, because premature deaths in the S4+/−:Fbn1C1039G/+ cohort effectively eliminated more severe cases from the analysis. No difference in aortic root or ascending aortic size was observed between WT and S4+/− mice (P = 0.20 and P = 0.20, respectively) (Fig. 2B), which indicated that the deleterious effect of Smad4 haploinsufficiency was limited to MFS mice.
After death of the mice, we performed Verhoeff–Van Gieson (VVG) staining of the proximal ascending aorta to assess whether there were any abnormalities in aortic architecture (Fig. 2C). Compared with WT littermates, Fbn1C1039G/+ mice showed increased aortic medial thickening, elastic fiber fragmentation, and elastic fiber disarray, collectively quantified as an aortic architecture score (P < 0.0001) (fig. S4). S4+/−:Fbn1C1039G/+ mice showed an exaggeration of these pathologic changes (P < 0.05). By contrast, there was no significant difference between WT and S4+/−mice (P = 0.94). The histological changes in the aorta therefore paralleled the echocardiography findings and support the conclusion that Smad4 haploinsufficiency exacerbates aortic disease in MFS mice.
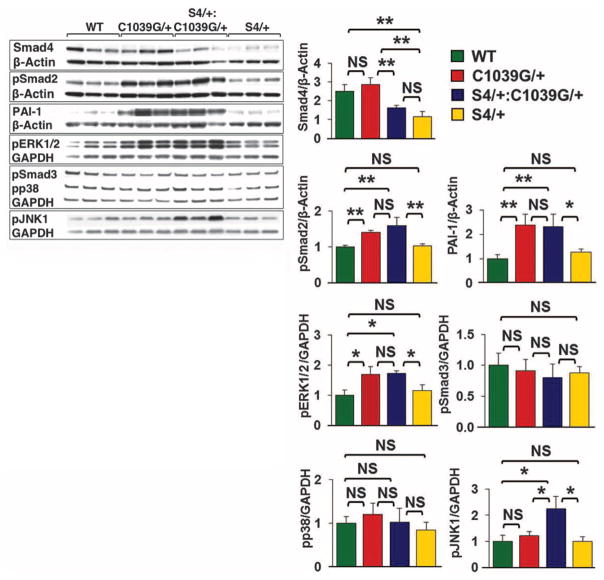
Using Western blot analysis, we evaluated the effect of Smad4 haploinsufficiency on canonical and noncanonical TGFβ signaling in the proximal ascending aorta (Fig. 3). As anticipated, S4+/−and S4+/−:Fbn1C1039G/+ mice showed a roughly 50% reduction in expression of Smad4 protein, compared with WT and Fbn1C1039G/+ mice. Compared with WT animals, Fbn1C1039G/+ mice showed significantly greater activation of Smad2 and ERK1/2 (P < 0.01 and P < 0.05, respectively) and a significant increase in expression of the Smad2-responsive gene product PAI-1 (P < 0.01). However, there was no further increase in Smad2 activation, ERK1/2 activation, or PAI-1 expression in S4+/−:Fbn1C1039G/+ mice (P = 0.35, P = 0.90, and P = 0.85, respectively). This indicates that Smad4 haploinsufficiency did not attenuate Smad-dependent signaling and that increased Smad2 or ERK1/2 activation could not be invoked as the cause of the aortic disease exacerbation seen in S4+/−:Fbn1C1039G/+ mice.
Fig. 3.
Effect of Smad4 haploinsufficiency (S4+/−) on aortic signaling. Western blot analysis of the proximal ascending aorta in three mice each: WT, S4+/−, Fbn1C1039G/+, and S4+/−:Fbn1C1039G/+. Note the unique activation of JNK1 in S4+/−:Fbn1C1039G/+ mice compared with all other genotypes. Values are means ± 2 SEM. *P < 0.05; **P < 0.01; NS, not significant.
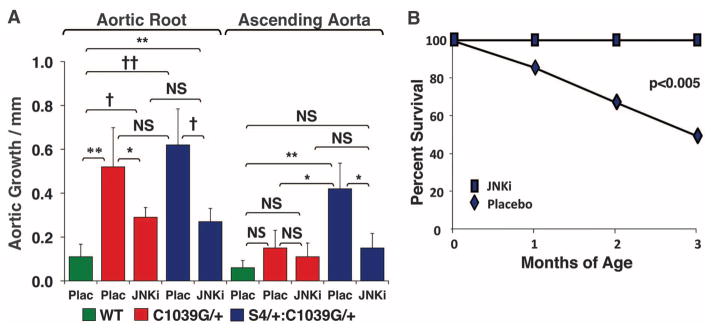
We next assessed whether other TGFβ-dependent canonical or noncanonical pathways could account for these changes (Fig. 3). There was no significant difference in Smad3 or p38 activation in WT, Fbn1C1039G/+, or S4+/−:Fbn1C1039G/+ mice. Although there was no significant difference in JNK1 activation between Fbn1C1039G/+ and WT mice, S4+/−:Fbn1C1039G/+ mice demonstrated unique activation of JNK1 (P < 0.05). We therefore treated a cohort of S4+/−:Fbn1C1039G/+ mice with SP600125, a selective JNK inhibitor (21). SP600125 treatment led to a significant reduction in both aortic root and ascending aortic growth in S4+/−:Fbn1C1039G/+ mice, compared with placebo-treated littermates (P < 0.001 and P < 0.05, respectively) (Fig. 4A). Furthermore, SP600125 treatment prevented the premature death due to aortic dissection seen in these animals. At 3 months of age, 50% of placebo-treated S4+/−:Fbn1C1039G/+ mice had died from aortic dissection, whereas all of the SP600125-treated S4+/−:Fbn1C1039G/+ mice were still alive (Fig. 4B).
Fig. 4.
Effect of JNK antagonism in the presence of SP600125. (A) Aortic root and ascending aortic growth, as measured by echocardiography, in WT mice (n = 6) and Fbn1C1039G/+ placebo- (n = 5) or SP600125-treated (n = 5) mice, as well as placebo- (n = 8) or SP600125-treated (n = 11) S4+/−:Fbn1C1039G/+ littermates. Note that JNK inhibition decreased aortic root growth in S4+/−:Fbn1C1039G/+ and Fbn1C1039G/+ mice and reduced ascending aortic growth in S4+/−:Fbn1C1039G/+ mice. Final absolute aortic root and ascending aortic diameter (mm): WT (1.66 ± 0.06; 1.33 ± 0.06), placebo- (2.31 ± 0.02; 1.43 ± 0.10) or SP600125-treated (1.97 ± 0.16; 1.38 ± 0.06) Fbn1C1039G/+ mice, placebo- (2.33 ± 0.38; 1.85 ± 0.37) or SP600125-treated (2.09 ± 0.16; 1.47 ± 0.14) S4+/−:Fbn1C1039G/+ mice. (B) Survival curve for S4+/−:Fbn1C1039G/+ mice treated with either placebo (n = 8) or SP600125 (n = 11), showing prevention of premature death in SP600125-treated animals. JNKi, JNK inhibitor SP600125; Plac, placebo. Values are means ± 2 SEM. *P < 0.05; **P < 0.01; †P < 0.001; ††P < 0.0001; NS, not significant.
Although JNK1 activation is not increased in the aortas of Fbn1C1039G/+mice, SP600125 treatment ameliorated their aortic root growth, compared with placebo-treated Fbn1C1039G/+ littermates (P < 0.05) (Fig. 4A). This correlated with a reduction of JNK1 activation to levels below baseline, whereas ERK1/2 activation remained unaffected, in SP600125-treated Fbn1C1039G/+ animals (fig. S5). This observation is consistent with prior work showing that SP600125 can ameliorate abdominal aortic aneurysm induced by the periaortic application of calcium chloride, in association with reduced JNK1 activation (22). These data suggest that both ERK1/2 and JNK1 can contribute to aortic disease in fibrillin-1–deficient mice; whether or not this relies upon downstream cross-talk between these signaling cascades remains to be determined. Taken together, these data further support the conclusion that noncanonical TGFβ signaling is a prominent determinant of aortic aneurysm progression in MFS mice.
ERK activation was recently shown to occur in the aorta of a fibulin-4–deficient mouse model of cutis laxa with aneurysm, although a mechanistic link remains to be elucidated (23). ERK activation also appears to be central to the pathogenesis of cardiovascular disease in Noonan syndrome (24, 25). Although aortic aneurysm has been described in this condition (26, 27), it is not highly penetrant, which suggests as yet undefined context specificity. It is also notable that the combination of aortic root and ascending aortic aneurysm seen in S4+/−:Fbn1C1039G/+ mice is similar to that observed in individuals with either Loeys-Dietz syndrome or bicuspid aortic valve and aneurysm. Both conditions are associated with increased TGFβ signaling in the aortic wall (15, 28), but the contribution of noncanonical TGFβ signaling cascades has not been revealed.
In summary, this work defines a critical role for noncanonical TGFβ-dependent signaling in aneurysm pathogenesis in MFS mice. It also defines inhibition of ERK1/2 or JNK1 activation as possible therapeutic strategies for MFS and suggests that such therapies may find broader application. Finally, it focuses attention on noncanonical TGFβ signaling cascades in MFS-related conditions, where etiology or pathogenesis remains poorly understood.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (H.C.D. and D.P.J), Howard Hughes Medical Institute (H.C.D.), National Marfan Foundation (H.C.D., J.P.H., and J.J.D.), Cellular and Molecular Medicine Training Program (J.J.D.), National Human Genome Research Institute Intramural Research Program and the NIH Therapeutics for Rare and Neglected Diseases Program (C.J.T., S.P., and J.J.M.), and the Smilow Center for Marfan Syndrome Research (H.C.D.). We thank Dr. C. Deng for the Smad4-targeted mice. Johns Hopkins University and the authors (H.C.D., J.P.H., D.P.J., and R.C.) have filed a patent relating to the use of TGFβ antagonists, including angiotensin II type 1 receptor blockers, for the treatment of Marfan syndrome.
Footnotes
References and Notes
- 1.Isogai Z, et al. J Biol Chem. 2003;278:2750. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 2.Dietz HC. J Clin Invest. 2010;120:403. doi: 10.1172/JCI42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO. Science. 2009;326:1216. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JS, Liu C, Derynck R. Trends Cell Biol. 2009;19:385. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Derynck R, Zhang YE. Nature. 2003;425:577. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 6.Lee MK, et al. EMBO J. 2007;26:3957. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita M, et al. Mol Cell. 2008;31:918. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neptune ER, et al. Nat Genet. 2003;33:407. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 9.Ng CM, et al. J Clin Invest. 2004;114:1586. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habashi JP, et al. Science. 2006;312:117. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn RD, et al. Nat Med. 2007;13:204. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf G, Ziyadeh FN, Stahl RA. J Mol Med. 1999;77:556. doi: 10.1007/s001099900028. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda N, et al. Am J Hypertens. 2000;13:191. doi: 10.1016/s0895-7061(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 14.Naito T, et al. Am J Physiol Renal Physiol. 2004;286:F278. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- 15.Loeys BL, et al. Nat Genet. 2005;37:275. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 16.Loeys BL, et al. N Engl J Med. 2006;355:788. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 17.Judge DP, et al. J Clin Invest. 2004;114:172. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Materials and methods are available as supporting material on Science Online.
- 19.Iverson C, et al. Cancer Res. 2009;69:6839. doi: 10.1158/0008-5472.CAN-09-0679. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, et al. Oncogene. 2000;19:1868. doi: 10.1038/sj.onc.1203504. [DOI] [PubMed] [Google Scholar]
- 21.Bennett BL, et al. Proc Natl Acad Sci USA. 2001;98:13681. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura K, et al. Nat Med. 2005;11:1330. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, et al. Circ Res. 2010;106:583. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki T, et al. Nat Med. 2004;10:849. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, et al. J Clin Invest. 2007;117:2123. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan JM, Coupe MO, Honey M, Miller GA. Eur Heart J. 1989;10:190. doi: 10.1093/oxfordjournals.eurheartj.a059462. [DOI] [PubMed] [Google Scholar]
- 27.Purnell R, Williams I, Von Oppell U, Wood A. Eur J Cardiothorac Surg. 2005;28:346. doi: 10.1016/j.ejcts.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Gomez D, et al. J Pathol. 2009;218:131. doi: 10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.