Summary
Transmembrane AMPA receptor regulatory proteins (TARPs), including γ-2, γ-3, γ-4, and γ-8, are auxiliary subunits for AMPA receptors. Based on studies in single knockout mice, it has been suggested that nearly all native AMPA receptors are associated with TARPs. To study the interplay between TARP family members and AMPA receptors in vivo, we generated mice lacking multiple TARPs. Triple knockout mice lacking γ-3, γ-4, and γ-8 are viable and fertile, and synaptic AMPA receptor activity is reduced to a level comparable to that seen in γ-8 single knockout mice. In contrast, triple knockout mice lacking γ-2, γ-3, and either γ-4 or γ-8 cannot survive ex utero. In particular, γ-2, γ-3, γ-4 triple knockout mice are born apneic and paralyzed, despite normal AMPA receptor function in cortical and spinal neurons. We found that γ-8 is expressed at low levels in early post-natal mice and regulates AMPA receptor levels at this developmental time period. Thus, the early expression of γ-8 may be responsible for maintaining AMPA receptors function in neonatal neurons. Together, our data indicate that TARPs, in particular γ-2, are essential for early development, and that most neurons express multiple members of this functionally redundant protein family.
Keywords: AMPA receptor, stargazer, stargazin, TARP, knockout mice, glutamate, excitatory amino acid receptor, electrophysiology, neurotransmission, neurotransmitter, membrane trafficking, LTP, hippocampus, CA1, spinal cord, cortex, CACNG
Introduction
The discovery that the stargazer strain of ataxic, epileptic mice lacked AMPA receptor expression in cerebellar granule cells revealed the critical role for stargazin, the absent protein, as an auxiliary AMPA receptor subunit (Chen et al., 2000; Hashimoto et al., 1999). Stargazin, also known as γ-2, is a member of a family of transmembrane AMPA receptor regulatory proteins (TARPs) (Nicoll et al., 2006; Ziff, 2007) that includes γ-3, γ-4, and γ-8 (Tomita et al., 2003), as well as γ-7, which exhibits some but not all TARP properties (Kato et al., 2007; Milstein et al., 2007). Like classical auxiliary subunits of voltage-gated channels, TARPs regulate many functional aspects of AMPA receptors; they augment AMPA receptor surface trafficking, enhance synaptic clustering, increase glutamate affinity, increase kainate efficacy, determine antagonist pharmacology, and slow channel deactivation and desensitization (Cho et al., 2007; Korber et al., 2007; Menuz et al., 2007; Milstein et al., 2007; Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005; Yamazaki et al., 2004; Zhang et al., 2006). TARP regulation is emerging as a universal trait of native AMPA receptors, including those localized synaptically and extrasynaptically in both excitatory and inhibitory neurons (Chen et al., 1999; Chen et al., 2000; Hashimoto et al., 1999; Menuz et. al., in submission; Rouach et al., 2005). Although much is known about the distinct properties of individual TARP family members when expressed in heterologous systems, their individual roles in vivo remain elusive.
γ-2 is the only TARP whose deletion results in a behavioral phenotype (Noebels et al., 1990), as mice lacking γ-3, γ-4 or γ-8 appear behaviorally indistinguishable from littermates (Kato et al., 2007; Letts et al., 2005; Menuz et al., in submission; Rouach et al., 2005). Perhaps this reflects the widespread distribution of γ-2; whereas γ-2 is found in virtually all brain regions, expression of γ-3 is highest in the cortex, γ-4 in the olfactory bulb, striatum, and glia, and γ-8 in the hippocampus (Lein et al., 2007; Fukaya et al., 2005; Klugbauer et al., 2000; Tomita et al., 2003). However, significant overlap exists, with lower levels of γ-3, γ-4, and γ-8 also detectable in most other brain regions in adult mice (Lein et al., 2007; Fukaya et al., 2005). In the neonate, γ-4 was reported to be the sole TARP expressed (Tomita et al., 2003). However, its deletion does not preclude normal development (Kato et al., 2007; Letts et al., 2005), implying that TARPs may not be required at all developmental time periods, or that compensatory upregulation by other family members may occur.
We generated mice lacking multiple TARPs to determine the relationship between TARP expression and AMPA receptor function. We report for the first time the generation of γ-3−/−;γ-4−/− (γ-3,4 KO) mice, as well as triple γ-3,4,8 KO and γ-2,3,4 KO strains. We found preserved AMPA receptor function in the hippocampus, cortex, and spinal cord, suggesting that a single remaining TARP is sufficient and thus highlighting TARP functional redundancy. Our data further suggest that γ-2 plays a special role in survival.
Methods
Knockout mice
All experiments followed animal welfare guidelines established by the University of California, San Francisco I.A.C.U.C. Stargazer mice (γ-2 KO mice), γ-3 KO, γ-4 KO, and γ-8 KO mouse strains have been described previously (Letts et al., 1998; Menuz et. al., in submission; Milstein et al., 2007; Rouach et al., 2005). PCR genotyping of mouse tail DNA was performed with the following primers: γ-2: F-WT: CATTTGTTATACATGCTCTAG, R-WT: ACTGTCACTCTATCTGGAATC, F-KO: GAGCAAGCAGGTTTCAGGC, R-KO: ACTGTCACTCTATCTGGAATC; γ-3: F-WT: AACTAGGTTCCCAGATAGCC, R-WT: GCTTCTAATGGGTTGCGCCC, F-KO: GGCTGCTCTTTGGTTAATCGG, R-KO: TACCCGGTAGAATTGACCTGC; γ-4: F-WT: GGACTCCTGGGAGAGATGCC, R-WT: CGGCTGTAGATCCTCCCAGC, F-KO: GGTGATGGCGTTCAGTGCACGG R-KO: TACCCGGTAGAATTGACCTGC; γ-8: F-WT: TCGCGCTTTCCTCTCGTCCC, R-WT: GCTGCCACGAACAGGATCCC, F-KO: CGTTTAGGATCTACCCAGATC, R-KO: TACCCGGTAGAATTGACCTGC.
As illustrated in Table 1, selective breeding generated a stable line of mice lacking both γ-3 and γ-4. Further crossbreeding generated γ-3−/−;γ-4−/−;γ-8+/− mice, who produced the γ-3,4 KO and γ-3,4,8 KO littermates used for experiments. The nonviable γ-2,3,4 KO mice that we studied resulted from breedings between viable γ-2+/−; γ-3−/−; γ-4−/− parents.
Table 1.
Survival of mice lacking multiple TARPs
| Colony | Breeding scheme | Age(1) | +/+ | +/− | −/− | Litters | Chi square |
|---|---|---|---|---|---|---|---|
| γ-2,3 | γ-2+/−;γ-3−/− | P0 | 59 | 111 | 41 | 37 | P = 0.16 |
| X γ-2+/−;γ-3−/− | (28%) | (53%) | (19%) | ||||
| P14 | 62 | 106 | 7 | 46 | P < 0.0001 | ||
| (35%) | (61%) | (4%) | |||||
| Died | 5 | 23 | 27 | 30 | P < 0.0001 | ||
| <P14 | (9%) | (42%) | (49%) | ||||
|
| |||||||
| γ-2,4 | γ-2+/−;γ-4−/− | (2) | 13 | 32 | 11 | 23 | P = 0.52 |
| X γ-2+/−;γ-4−/− | (23%) | (57%) | (20%) | ||||
|
| |||||||
| γ-2,8 | γ-2+/−;γ-8−/− | (3) | |||||
| X γ-2+/−;γ-8+/− | |||||||
|
| |||||||
| γ-2,3,4 | γ-2+−;γ -3−/−;γ -4−/− | E18.5 | 15 | 36 | 5 | 10 | P < 0.05 |
| X γ-2+/−;γ -3−/−;γ -4−/− | (27%) | (64%) | (9%) | ||||
|
| |||||||
| γ-2,3,8 | γ-2+/−;γ-3−/−;γ-8−/− | (4) | |||||
| X γ-2+/−;γ-3−/−;γ-8+/− | |||||||
|
| |||||||
| γ-3,4 | γ-3−/−;γ-4−/− | (2) | — | — | (100%) | (5) | |
| X γ-3−/−;γ-4−/− | |||||||
|
| |||||||
| γ-3,4,8 | γ-3−/−;γ-4−/−;γ-8+/− | (2) | 38 | 74 | 25 | 17 | P = 0.27 |
| X γ-3−/−;γ-4−/−;γ-8+/− | (28%) | (54%) | (18%) | ||||
Genotypes of offspring are tabulated by postnatal (P) or embryonic (E) age in days.
Age did not influence the proportions of offspring in these crossings, in which no early deaths were observed.
In our limited experience with this breeding, we did successfully produce γ-2,8 KO offspring, some of whom died soon after birth and some who lived long enough for acute slice experiments (Rouach et al., 2005).
In our limited attempts to breed these mice, no living γ-2,3,8 KO pups resulted (12.5% predicted); of the dead pups genotyped, approximately half were γ-2,3,8 KOs.
These homozygous breeding pairs produced normal litters of healthy offspring. During creation of this line, γ-3,4 KO mice and their heterozygous siblings appeared in normal Mendelian ratios (P = 0.34)
Electrophysiology in juvenile mice
Acute transverse hippocampal slices (300 μm) were prepared from postnatal day 15–22 animals using a cutting solution at 4–6 °C containing (in mM): 50 NaCl, 150 sucrose, 25 NaHCO3, 1 NaH2PO4, 10 glucose, 7 MgCl2, and 0.5 CaCl2 bubbled continuously with 95% O2 / 5% CO2. Slices were submerged in artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 26.3 NaHCO3, 1 NaH2PO4, 11 glucose, 1.3 MgSO4, and 2.5 CaCl2, adjusted to osmolarity 290–300 mOsm and bubbled continuously as above. After incubation at 30–34 °C for 30 min, slices remained at room temperature for experiments that took place between 1 and 8 hours after dissection. Slices were transferred to a recording chamber on the stage of an upright IR-DIC microscope (Olympus, Inc.) and perfused with ACSF containing picrotoxin (100 μM) (Sigma-Aldrich). Whole-cell recordings were obtained from visualized hippocampal CA1 pyramidal cells with borosilicate glass pipettes (2–6 MΩ) filled with a solution containing (in mM): 130 CsCH3SO3, 10 CsCl, 10 HEPES, 2 NaCl, 1 QX314-Cl, 5 TEA-Cl, 8 Na-phosphocreatine, 4 Na-ATP, 0.5 Na-GTP, 4 MgCl2, 0.1 spermine, and 0.2 EGTA, adjusted to pH 7.3 and osmolarity 300 mOsm. Pipette capacitance was compensated, and series resistance, ranging 10–25 MΩ was monitored, uncompensated, throughout experiments. Cells or epochs were discarded when series resistance changed by more than 10–15%. Monosynaptic EPSCs were evoked by a monopolar stimulating electrode placed in the stratum radiatum. Data were amplified and filtered at 4 kHz with a MultiClamp 700B amplifier (Molecular Devices, Inc.), digitized at 10 kHz (National Instruments, Inc.), and analyzed with customized software for IgorPro (Wavemetrics, Inc.).
Rectification index (RI) was calculated by obtaining AMPA-only EPSC amplitudes at −60, 0, and +40 mV:
X0 is the voltage at which current is zero, extrapolated from EPSC amplitudes at −60 and 0 mV (typically 0–5 mV). An RI value of 0 corresponds to complete inward rectification, whereas 1 corresponds to a purely linear response.
Preparation of acute slices from perinatal mice
Transverse hippocampal slices (300 μm) from P0–P1 mice were cut in cold (4–6 °C) cutting solution containing (in mM): 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 25 glucose, 4 MgCl2, and 1 CaCl2 saturated with 95% O2 / 5% CO2. Hippocampal slices were incubated at 30–34°C for 30 minutes, and then maintained at room temperature. For cortical and spinal cord slice preparation, timed-pregnant γ-2+/−;γ -3−/−;γ -4−/− mother mice were sacrificed by cervical dislocation, and E16.5–E18.5 embryos were removed. As γ-2,3,4 KO fetuses are motionless, one motionless pup and one normal-appearing littermate were selected for an experiment, and the genotypes were confirmed later. For recording cortical neurons, coronal brain slices (300 μm) were prepared in the same manner as hippocampal slices. For spinal cord slices, the meninges surround the spinal cord were removed and the spinal cord was set into an agarose block for cutting slices (300–400 μm). The cutting solution was the same as described above, but at a temperature of 0–4 °C. Spinal cord slices were incubated at 37 °C and then left at room temperature prior to recordings.
Preparation of dissociated neuron cultures
Dissociated neuronal cultures were prepared from the hippocampus of E16–E19 wild type and γ-8 KO mice, spinal cord of E13–E16 wild type and γ-2,3,4 KO mice, and cerebral cortex of E14–E18 γ-2,3,4 KO mice and their littermates. The tissues were dissociated by papain digestion and brief mechanical trituration. Cells were plated on poly-D-lysine–coated glass coverslips (10–12 mm). Hippocampal and cortical cultures were plated in Neurobasal media (Gibco) with B-27 and 5% FBS (Invitrogen) (Craven et al., 1999). After three days, the media was replaced by serum free media. Spinal cultures were plated and maintained in L15 media with FBS. After 3 days in culture, cytosine arabinoside was added. Recordings were performed after 10–19 days in vitro (DIV).
Electrophysiology in perinatal slices or cultured neurons
Slices or coverslips were transferred to a chamber on the stage of an upright IR-DIC microscope (Olympus, Inc.) and perfused with ACSF containing tetrodotoxin (500 nM) (Tocris, Inc.) and picrotoxin (100 μM) (Sigma-Aldrich); strychnine (3 μM) (Sigma-Aldrich) was also added during spinal neuron recordings. Whole-cell patch-clamp recordings were obtained from visually identified neurons using 3–6 MΩ glass electrodes filled with an internal solution containing (in mM): 115 CsCH3SO3, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 Na2-ATP, 0.4 Na-GTP, 10 Na-phosphocreatine, 0.6 EGTA, 0.1 spermine, and 5 QX-314, pH 7.2–3, adjusted to 295–305 mOsm. CA1 pyramidal neurons and cortical plate neurons were chosen from acute hippocampus and cortex slices respectively. Neurons from intermediate layers of the acute spinal cord slices were chosen for recordings as few superficial dorsal horn neurons or motor neurons survived the dissection and slice preparation. Neurons were voltage-clamped at −60 or −70 mV, and miniature EPSCs (mEPSCs) or agonist-evoked currents were acquired and analyzed with customized software for IgorPro (Wavemetrics, Inc.).
Fast agonist application was achieved using a gravity-fed perfusion system. A Valve-Link 8 controller and pinch-valves connected to a 360 μm flowpipe (AutoMate Scientific) were used for rapid solution exchange. The control line contained (in mM): 140 NaCl, 2.4 KCl, 10 HEPES, 10 glucose, 2.5 CaCl2, 4 MgCl2, and cyclothiazide (100–250 μM) (Tocris). The agonist solution, made by adding AMPA (500 μM) (Tocris) to an aliquot of the control solution, was applied for 2–5 seconds to cortical or spinal neurons in whole-cell mode or to outside-out hippocampal patches.
Immunoblotting
The rabbit polyclonal antibody to γ-8 has been characterized previously (Tomita et al., 2003). Hippocampi and spinal cords were extracted from P2 mice, homogenized in 4.5 volumes of 320 mM sucrose buffer, and then sonicated in 2% final SDS. Equal amounts (20 μg) of protein were separated on 8% polyacrylamide gels followed by transfer to PVDF membranes. Proteins were detected by immunoblotting using the HRP-ECL kit from Amersham. Similar methods were used for immunoblotting protein from spinal cord cultures on 10 mm glass coverslips. Several coverslips from each genotype were pooled to attain 20 μg of protein.
Statistics
Statistical significance was determined by either a Student’s t-test for comparisons between two groups or a one-way ANOVA for comparisons between multiple groups. All data shown is the mean ± SEM.
Results
AMPA receptors in hippocampal CA1 neurons from multiple TARP knockout mice
Prior work identified a crucial role for γ-8 in AMPA receptor synthesis and surface expression in hippocampal neurons, compared to smaller deficits in synaptic AMPA receptor localization as well as in the capacity for further synaptic receptor recruitment, as measured by a long term potentiation (LTP) protocol (Rouach et al., 2005). We hypothesize that other TARPs mediate this residual AMPA receptor function in γ-8 KO animals. Additional removal of γ-2 (γ-2,8 KO) resulted in a further reduction, but not elimination, of synaptic AMPA receptors (Rouach et al., 2005). Therefore, we examined the roles of γ-3 and γ-4 in hippocampal AMPA receptor function. We obtained viable and fertile γ-3,4 KO mice (Table 1) that exhibited no behavioral abnormalities. With further selective breeding, we generated γ-3−/−;γ-4−/−;γ-8+/− mice, whose γ-3,4 KO and γ-3,4,8 KO offspring we examined for alterations in AMPA receptor function.
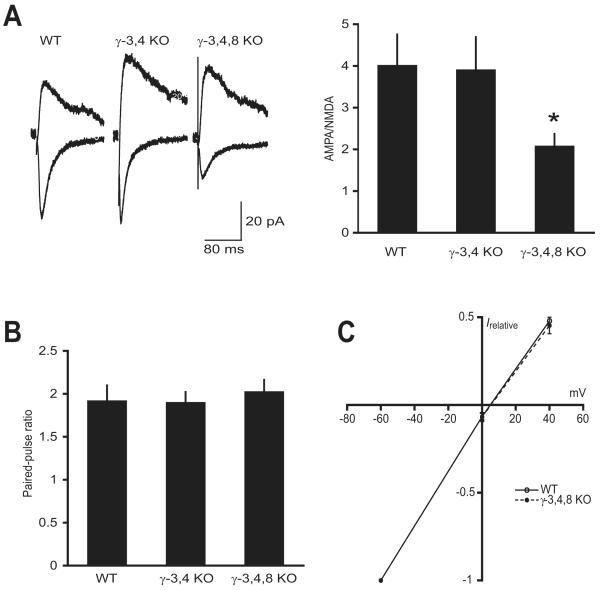
The most effective way to assay for defects in AMPA receptor transmission is to measure the AMPA/NMDA ratio. A selective defect in AMPA-mediated transmission causes a reduction in this ratio independent of stimulus intensity or the number of synapses activated. Previous studies have found the AMPA/NMDA ratio to be normal in the hippocampi of stargazer and γ-2,3 KO mice (Hashimoto et al., 1999; Menuz et al., in submission). In γ-3,4 KO animals, this ratio also appeared normal compared to age-matched wild type (WT) C57BL/6 mice (WT 4.02 ± 0.73, γ-3,4 KO 3.91 ± 0.78; n = 15 and 17, respectively; P = 0.92) (Figure 1A). Littermate γ-3,4,8 KO mice exhibited a reduced AMPA/NMDA ratio (2.09 ± 0.29; n = 14; P < 0.05 compared to WT or γ-3,4 KO) (Figure 1A) to a degree comparable with the effect of γ-8 removal alone (P = 0.31) (Rouach et al., 2005). We hypothesize that any single TARP is sufficient for AMPA receptor expression in hippocampal CA1 pyramidal cells, with a bottleneck observed only in the case of γ-8 deletion, commensurate with its higher level of native expression in the hippocampus relative to other TARPs. Unfortunately, we have so far been unable to generate the γ-2,3,4,8 quadruple knockout mice necessary to address this hypothesis more directly.
Figure 1. AMPA receptors in hippocampal CA1 pyramidal neurons from WT, γ-3,4 KO and γ-3,4,8 KO mice.
A, AMPA/NMDA ratios were calculated by dividing the peak amplitude of EPSCs evoked at −60 mV by the amplitude of dual-component EPSCs evoked at +40 mV at 150 ms after the stimulus artifact, by which time the AMPA receptor-mediated component had entirely decayed. Traces at left are from representative experiments, and the graph at right shows summarized data. *P < 0.05 compared to WT and γ-3,4 KO.
B, Summary of paired-pulse ratios, calculated by dividing the amplitude of the second EPSC by the amplitude of the first (interval 50 ms).
C, Summarized current-voltage relationship of AMPA-mediated EPSCs recorded in AP-5 (50 μM), normalized to the peak amplitude at −60 mV.
Consistent with a purely postsynaptic effect of TARP elimination, we observed no change in the probability of transmitter release in either the γ-3,4 KO or γ-3,4,8 KO animals, as measured by the paired-pulse ratio (WT 1.92 ± 0.18, γ-3,4 KO 1.90 ± 0.12, γ-3,4,8 KO 2.03 ± 0.13; n = 13, 17, and 14, respectively; ANOVA P = 0.80) (Figure 1B). Although one could imagine a scenario whereby individual TARP family members interact selectively with individual AMPA receptor subunits, the current-voltage (IV) relationship remained perfectly linear in the absence of γ-3, γ-4, and γ-8 (rectification index for WT 1.10 ± 0.18, γ-3,4,8 KO 1.06 ± 0.13; n = 5 and 6, respectively), indicating at least that GluR2 remains a steadfast component of synaptic AMPA receptors regardless of TARP composition (Figure 1C).
AMPA receptors in cortical neurons from multiple TARP knockout mice
In contrast to the γ-3,4,8 KO mice, we found that multiple TARP knockout mice lacking γ-2 were often impaired. We have previously reported that γ-2,3 KO mice typically die within the first weeks after birth (see Table 1 and Menuz et al., in submission)) and have a near complete elimination of synaptic AMPA receptors in cerebellar Golgi cells. We did obtain viable γ-2,8 KO (Rouach et al., 2005) and γ-2,4 KO mice (but see (Letts et al., 2005)). However, further TARP elimination was lethal: Preliminary attempts at generating γ-2,3,8 KO mice produced no viable triple knockout offspring (see Table 1 for breeding scheme). Likewise, γ-2,3,4 KO mice were unable to survive past birth, although their γ-2+/−; γ-3−/−; γ-4−/− and γ-2+/+; γ-3−/−; γ-4−/− littermates were viable. Furthermore, genotyping complete litters just before birth (E17–18.5) revealed significantly fewer γ-2,3,4 KO mice than predicted by Mendelian inheritance (Table 1), indicating that this knockout combination is often embryonic lethal. These triple knockout mice were indistinguishable from their littermates in terms of size and color at the moment of birth; however, they did not breathe or move. Furthermore, they did not respond to a forelimb pinch and remained motionless upon decapitation, indicating paralysis or a loss of spinal cord reflexes. These observations suggest that unlike their γ-3,4,8 KO counterparts, γ-2,3,4 KO mice cannot compensate for the missing TARPs, which must be critical for early development.
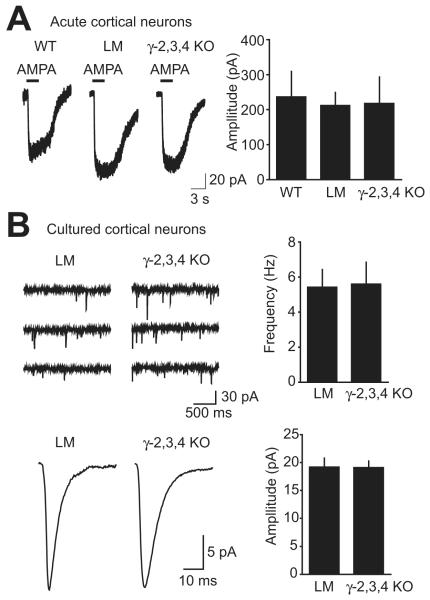
Given the dramatic phenotype of the γ-2,3,4 KO pups, we sought to determine which populations of neurons might have impaired AMPA receptor function, potentially contributing to early lethality. We turned first to the neocortex, an area that expresses high levels of γ-2, γ-3, γ-4, and γ-8 in the adult (Fukaya et al., 2005; Lein et al., 2007; Tomita et al., 2003). We recorded whole-cell responses to brief applications of AMPA with cyclothiazide in cortical plate neurons in slices prepared from E18.5 γ-2,3,4 KO mice, their littermates, and WT mice. No difference was found in the average peak amplitude (WT 238 ± 70 pA, γ-2,3,4 littermates 214 ± 35 pA, γ-2,3,4 KO 219 ± 74 pA; n = 14, 20, and 9, respectively; ANOVA P = 0.94) (Figure 2A).
Figure 2. AMPA receptors in cortical neurons from γ-2,3,4 KO mice.
A, Acute coronal slices were prepared from E18.5 WT, γ-2,3,4 KO, and γ-2,3,4 littermates. Whole-cell recordings were obtained from cortical neurons, and AMPA was briefly applied in the presence of cyclothiazide. The average holding current change was similar in the three genotypes.
B, On the upper left are three consecutive traces of mEPSCs from γ-2,3,4 littermates and γ-2,3,4 KOs. Below are averaged mEPSCs from representative cells of each genotype. Only cells with at least 100 mEPSCs were used for analysis. At right are summarized data showing no change in mEPSC amplitude or frequency in the γ-2,3,4 KO mice compared to their littermates.
To determine whether synaptic AMPA receptor function is affected in γ-2,3,4 KO mice, we recorded mEPSCs in cultured cortical neurons. mEPSC amplitude and frequency were similar in γ-2,3,4 KO mice and their littermates (amplitude: γ-2,3,4 littermates 19.3 ± 1.5 pA, γ-2,3,4 KO 19.2 ± 1.1 pA; frequency: γ-2,3,4 littermates 5.46 ± 0.97 Hz, γ-2,3,4 KO 5.63 ± 1.21 Hz; n= 10 and 9 respectively; P > 0.9 for each measurement) (Figure 2B). Furthermore, mEPSC decay kinetics were not significantly different in the γ-2,3,4 KO mice (γ-2,3,4 littermates 3.88 ± 0.5 ms, γ-2,3,4 KO 4.69 ± 0.82 ms; n= 8 and 8 respectively; P = 0.41). Thus, loss of γ-2, -3, and -4 does not affect expression of extrasynaptic or synaptic AMPA receptors in cortical neurons.
AMPA receptors in spinal cord neurons from γ-2,3,4 KO mice
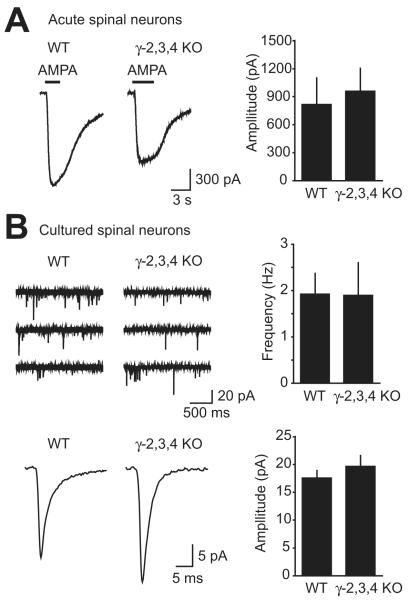
Because γ-2,3,4 KO mice appeared paralyzed and areflexic, we hypothesized that spinal cord neurons may have dysfunctional AMPA receptor activity. To test this possibility, we voltage-clamped neurons from E18.5 spinal cord slices (see Methods) and measured responses to brief whole-cell application of 500 μM AMPA in the presence of cyclothiazide. We did not find any significant difference between γ-2,3,4 KO mice and WT mice (WT 822 ± 279 pA, γ-2,3,4 KO 965 ± 240 pA; n = 5 and 8 respectively; P = 0.76) (Figure 3A).
Figure 3. AMPA receptors in spinal neurons from γ-2,3,4 KO mice.
A, Short pulses of AMPA were applied in the presence of cyclothiazide to neurons from E18.5 spinal cord slices. No significant difference in average holding current change was observed between WT, γ-2,3,4 KO, and γ-2,3,4 littermates.
B, Neurons were cultured from WT or γ-2,3,4 KO spinal cords. Typical mEPSC activity is shown by three consecutive 2s sweeps. The average mEPSC for a neuron from each genotype is illustrated. Neurons were included for analysis only if at least 50 mEPSCs were detected. The average amplitude and frequency of mEPSCs was similar in WT and γ-2,3,4 KO mice.
To investigate synaptic AMPA receptor currents in γ-2,3,4 KO mice, we recorded mEPSCs in cultured spinal neurons. No difference was found in mEPSC amplitude, frequency, or decay kinetics in γ-2,3,4 KO mice compared to WT (amplitude: WT 17.7 ± 1.2 pA, γ-2,3,4 KO 19.8 ± 1.8 pA; frequency: WT 1.94 ± 0.43 Hz, γ-2,3,4 KO 1.91 ± 0.69 Hz; decay: WT 3.00 ± 0.31 ms, γ-2,3,4 KO 2.59 ± 0.29 ms, n = 12 and 9, respectively, P > 0.3 for each measurement) (Figure 3B). Therefore, both extrasynaptic and synaptic AMPA receptors appear normal in γ-2,3,4 KO mice in the subset of spinal neurons that we were able to test (see Methods).
Expression of γ-8 in young mice
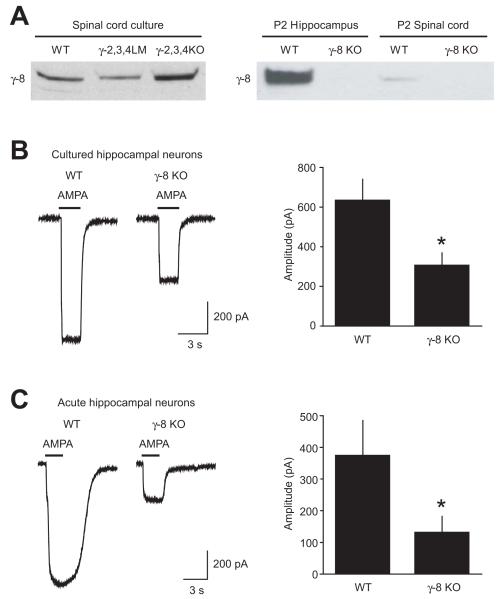
AMPA receptor function persisted despite elimination of γ-2, -3, and -4, possibly reflecting TARP-less receptors or compensation by another TARP family member. Although γ-8 expression does not become prominent until after the first postnatal week (Fukaya et al., 2005; Tomita et al., 2003), there may be a lower level of expression earlier. We detected γ-8 in Western blots of acute hippocampal extracts from P2 WT mice (Figure 4A). We were not able to detect a band in γ-8 KO mice, demonstrating that the antibody is specific. We also found γ-8 immunoreactivity in extracts from cultured spinal cord neurons and acute spinal cords from P2 WT mice (Figure 4A). Finally, we verified that γ-8 could be detected in cultured spinal neurons prepared from γ-2,3,4 KO mice and their littermates.
Figure 4. γ-8 is present and functional in young animals.
A, Protein extracts from either spinal cord cultures (left) or P2 hippocampi or spinal cords (right) were blotted with an anti-γ-8 antibody, revealing γ-8 immunoreactivity in all conditions, except in γ-8 KO mice.
B, Outside-out patches were pulled from cultured dissociated hippocampal neurons prepared from WT and γ-8 KO mice. The response to a brief application of AMPA in the presence of cyclothiazide was significantly reduced in γ-8 KO mice.
C, Acute hippocampal slices were prepared from P0–1 WT and γ-8 KO mice, and outside-out patches were pulled from CA1 pyramidal neurons. Response to AMPA application in the presence of cyclothiazide was significantly reduced in γ-8 KO mice.
To test whether the amount of γ-8 in very young mice is sufficient to regulate AMPA receptor expression, we investigated neonatal and cultured neurons from γ-8 KO mice. In outside-out patches from cultured dissociated hippocampal neurons, AMPA receptor currents were 50% smaller in γ-8 KO mice compared to WT (WT 638 ± 103 pA, γ-8 KO 310 ± 60 pA, n = 20 and 14 respectively, P < 0.05) (Figure 4C). In outside-out patches pulled from CA1 pyramidal neurons in P0–P1 acute hippocampal slices, we found a 65% reduction of AMPA receptor currents in γ-8 KO mice compared to WT controls (WT 377 ± 108 pA, γ-8 KO 134 ± 48 pA, n = 6 and 8 respectively, P < 0.05) (Figure 4B). By comparison, we had reported an approximately 90% reduction in AMPA currents in acute slices from older γ-8 KO animals (Rouach et al., 2005), suggesting that the dependence upon γ-8 increases with age, along with the expression level of γ-8. In any case, γ-8 is expressed in neonatal mice at sufficient levels to influence AMPA receptor function significantly, potentially allowing it to compensate for the loss of other TARPs in γ-2,3,4 KO mice.
Discussion
This study examined the effect of multiple TARP deletions on AMPA receptor function and mouse survival. Despite distinct effects on AMPA receptor pharmacology and kinetics in heterologous expression systems, individual TARP subtypes appear to be functionally interchangeable in vivo, at least when an organism is faced with loss of one or more family members. Most neurons express two, three, or even four TARPs, likely explaining the persistent AMPA receptor function that we found in our multiple knockout animals. Cerebellar granule cells stand out as the only cell type known that relies on one TARP, with no capacity for compensation.
Accordingly, we report here that γ-3,4 KO mice exhibit normal AMPA receptor function in the hippocampus. Like γ-8 KO mice, γ-3,4,8 KOs exhibit a similar reduction in hippocampal synaptic AMPA receptor currents. Taken together with previous reports showing normal responses in γ-2 KO and γ-2,3 KO mice (Hashimoto et al., 1999; Menuz et al., in submission), these data suggest that hippocampal CA1 neurons can use any TARP to traffic AMPA receptors, relying more on γ-8 than the other family members. Until we can study AMPA receptors in the absence of γ-2, -3, -4, and -8 (perhaps also γ-7), we cannot exclude the formal possibility that some degree of TARP-less AMPA receptor expression may occur in some neurons.
Perhaps because its expression was found in every neuron type examined (Fukaya et al., 2005), γ-2 deletion is more difficult to overcome than other TARPs. Knockout of γ-2 alone produces the well-known stargazer phenotype. γ-2,3 KO mice are unable to survive past the first few weeks after birth and are profoundly ataxic (Menuz et al., in submission). Although our γ-2,4 KO mice are viable, a different γ-2,4 KO strain reported by Letts and colleagues (Letts et al., 2005) is lethal, suggesting that these mice are impaired in at least some genetic backgrounds. Most strikingly, γ-2,3,4 KO and γ-2,3,8 KO mice are unable to survive past birth, in contrast to their γ-3,4,8 KO counterparts, who develop normally. Thus, although TARPs are functionally redundant in vivo, γ-2 plays a singular role that is critical for survival.
During fetal development, γ-4 is widely expressed, with γ-2, -3, and -8 appearing after birth (Fukaya et al., 2005; Tomita et al., 2003). However, given that our γ-4 KO and γ-2,4 KO mice develop normally and that γ-2,3,4 KO mice die at or before birth, γ-3 must play some developmental role. Even perinatal γ-2,3,4 KO mice exhibited normal AMPA receptor responses in cortical and spinal neurons, which could be a result of compensation by the γ-8. Indeed, we found evidence for γ-8 expression in Western blots of neonatal hippocampal and spinal cord tissue; γ-8 is functional at this early age, as AMPA receptor responses are reduced in hippocampal cells from neonatal γ-8 KO mice. We suspect that γ-8 facilitates remaining AMPA receptor expression in γ-2,3,4 KO mice, although we acknowledge the possibility of TARP-less receptors, as noted above.
Recently, γ-7 was identified as a new member of the TARP family (Kato et al., 2007), and it may also be capable of maintaining AMPA receptor function in the γ-2,3,4 KO mice. However, γ-7 only partially rescued surface AMPA receptors expression and failed to rescue synaptic responses in stargazer cerebellar granule cells (Milstein et al., 2007). It remains to be shown whether γ-7 functions as a TARP in vivo.
One of the most intriguing findings of this study is that although we could find no defect in AMPA receptor function in the spinal cord of γ-2,3,4 KO mice, spinal cord reflexes appeared absent. It is plausible that spinal motor neurons in these mice lack functional AMPA receptors; our data set may not have included these neurons, since they almost uniformly died during acute dissections. Inexcitable motor neurons could account for the observed paralysis, apnea, and lack of reflexes in γ-2,3,4 KO mice. On the other hand, if AMPA responses persist in these cells as in other spinal neurons, then there must be another explanation for the observed phenotype. Perhaps AMPA responses are missing from some other critical neuron subpopulation that we did not sample. Alternatively, TARPs may exert other functions aside from AMPA receptor trafficking. The only other role proposed for TARPs is as calcium channel auxiliary subunits (Arikkath and Campbell, 2003; Black, 2003). If TARPs also function to regulate calcium channels or have additional unknown partners, the γ-2,3,4 KO mice may have a defect at the neuromuscular junction or a more general effect on neuronal survival or nervous system development, despite normal gross structure. Further investigation into the lethal abnormality in γ-2,3,4 KO mice may reveal novel roles for the TARP family of proteins.
Acknowledgements
We thank Kirsten Bjorgan for help with genotyping. This work was supported by N.I.H. grants to R.A.N. L’Oreal UNESCO and Epilepsy Foundation pre-doctoral fellowships provided support for K.M., and the Larry L. Hillblom Foundation supported G.A.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Black JL., 3rd The voltage-gated calcium channel gamma subunits: a review of the literature. J Bioenerg Biomembr. 2003;35:649–660. doi: 10.1023/b:jobb.0000008029.22650.c5. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Qiao X, Thompson RF. Impaired cerebellar synapse maturation in waggler, a mutant mouse with a disrupted neuronal calcium channel gamma subunit. Proc Natl Acad Sci U S A. 1999;96:12132–12137. doi: 10.1073/pnas.96.21.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Dai S, Specht V, Lacinova L, Marais E, Bohn G, Hofmann F. A family of gamma-like calcium channel subunits. FEBS Lett. 2000;470:189–197. doi: 10.1016/s0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- Korber C, Werner M, Kott S, Ma ZL, Hollmann M. The transmembrane AMPA receptor regulatory protein gamma4 is a more effective modulator of AMPA receptor function than stargazin (gamma2) J Neurosci. 2007;27:8442–8447. doi: 10.1523/JNEUROSCI.0424-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Letts VA, Mahaffey CL, Beyer B, Frankel WN. A targeted mutation in Cacng4 exacerbates spike-wave seizures in stargazer (Cacng2) mice. Proc Natl Acad Sci U S A. 2005;102:2123–2128. doi: 10.1073/pnas.0409527102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K, O’Brien JL, Karmizadegan S, D.S. B, Nicoll RA. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. doi: 10.1523/JNEUROSCI.1319-08.2008. (in submission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K, Stroud RM, Nicoll RA, Hayes F. TARPs switch AMPA receptor antagonists into agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990;7:129–135. doi: 10.1016/0920-1211(90)90098-g. [DOI] [PubMed] [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Robert A, Vogensen SB, Howe JR. The relationship between agonist potency and AMPA receptor kinetics. Biophys J. 2006;91:1336–1346. doi: 10.1529/biophysj.106.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff EB. TARPs and the AMPA Receptor Trafficking Paradox. Neuron. 2007;53:627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]