Abstract
Objectives
Yoga and exercise have beneficial effects on mood and anxiety. γ-Aminobutyric acid (GABA)-ergic activity is reduced in mood and anxiety disorders. The practice of yoga postures is associated with increased brain GABA levels. This study addresses the question of whether changes in mood, anxiety, and GABA levels are specific to yoga or related to physical activity.
Methods
Healthy subjects with no significant medical/psychiatric disorders were randomized to yoga or a metabolically matched walking intervention for 60 minutes 3 times a week for 12 weeks. Mood and anxiety scales were taken at weeks 0, 4, 8, 12, and before each magnetic resonance spectroscopy scan. Scan 1 was at baseline. Scan 2, obtained after the 12-week intervention, was followed by a 60-minute yoga or walking intervention, which was immediately followed by Scan 3.
Results
The yoga subjects (n = 19) reported greater improvement in mood and greater decreases in anxiety than the walking group (n = 15). There were positive correlations between improved mood and decreased anxiety and thalamic GABA levels. The yoga group had positive correlations between changes in mood scales and changes in GABA levels.
Conclusions
The 12-week yoga intervention was associated with greater improvements in mood and anxiety than a metabolically matched walking exercise. This is the first study to demonstrate that increased thalamic GABA levels are associated with improved mood and decreased anxiety. It is also the first time that a behavioral intervention (i.e., yoga postures) has been associated with a positive correlation between acute increases in thalamic GABA levels and improvements in mood and anxiety scales. Given that pharmacologic agents that increase the activity of the GABA system are prescribed to improve mood and decrease anxiety, the reported correlations are in the expected direction. The possible role of GABA in mediating the beneficial effects of yoga on mood and anxiety warrants further study.
Introduction
Yoga has been used to reduce symptoms of depression, anxiety, and epilepsy.1–3 Reduced activity in γ-aminobutyric acid (GABA) systems has been found in mood disorders, anxiety disorders, and epilepsy.4–6 All three of these conditions respond to pharmacologic agents known to increase GABA system activity, raising the possibility that some of the therapeutic effect may be via increased GABA activity.7 In a previous study using magnetic resonance spectroscopy (MRS) to obtain brain GABA levels, our group demonstrated that experienced yoga practitioners had a significant (27%) increase in whole-slab GABA levels after a 60-minute session of yoga postures compared to no change in GABA levels in controls after a 60-minute reading session.8 These findings raise the question of whether the associated increase in GABA levels was specific to yoga or related to physical activity in general.
There is a large body of research on the beneficial effects of exercise on depression and anxiety.2,9,10 The results of exercise as a treatment for mild to moderate depression compare favorably to psychotherapy and pharmacologic treatment, supporting the contention that a behavioral intervention can have an effect similar to a pharmacologic intervention on mood.11
This study extends the findings of self-reported mood changes by exploring a possible mechanism: changes in thalamic GABA levels measured on MRS. This study was designed to correlate changes in mood, anxiety, and brain GABA levels, and to determine whether such changes are specific to a practice of yoga postures or whether they occur in a metabolically matched walking intervention. We hypothesize that improvement in mood scores correlate positively with GABA levels, while anxiety scores will correlate negatively with GABA levels.
Materials and Methods
Subjects were recruited from the community by newspaper ads, flyers, and the Internet. Screening interviews and written informed consent were obtained at Boston University School of Medicine General Clinical Research Unit. Eligible subjects were randomized in permuted blocks (n = 4) to a 12-week intervention of either Iyengar yoga or walking for three 60-minute sessions per week, with a maximum of 36 sessions. All subjects had three MRS scans: Scan 1 at baseline; Scan 2 after their 12-week intervention; immediately after Scan 2, all subjects completed a 60-minute yoga or walking intervention, depending on group assignment, which was immediately followed by Scan 3.
Participants were 18–45 years old with no current Axis I diagnosis. Nonpsychoactive medications were allowed if the subject had been on a stable dose for at least 1 month with no anticipated changes during the study. The following items were exclusionary: any yoga practice in the previous 3 months, or a lifetime history of one yoga session/week for ≥4 weeks; current participation in psychotherapy, prayer groups, or any mind–body disciplines; a neurological disorder or medical condition that would compromise subject safety or scan data; treatment within the previous 3 months with medications that might affect the GABA system; use of tobacco products (known to affect GABA levels)12; alcohol consumption >4 drinks/day; and contraindication to magnetic resonance evaluation.
The following instruments were used for screening: the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV to identify Axis I Disorders13 and the Time Line Follow Back to assess alcohol consumption.14,15 Two (2) reliable and valid psychologic scales were selected to monitor the effects of the interventions on mood and anxiety over time. Mood was assessed with the Exercise-Induced Feeling Inventory (EIFI), which has four subscales: Positive Engagement, Revitalization, Tranquility, and Physical Exhaustion.16 Anxiety was assessed with the State scale of the Spielberger State–Trait Anxiety Inventory (STAI).17 The EIFI and STAI-State were given before each scan, prior to the first intervention session (week 0), and after completion of sessions at weeks 4, 8, and 12.
Metabolic equivalents (METs) are used to rate and compare the physical demands of various activities.18 The American College of Sports Medicine list of metabolic equivalents was consulted to match the 60-minute Iyengar yoga intervention with a 60-minute walking intervention at 2.5 miles per hour (mph) on a flat surface rated at 3.0 METs. During the intervention, the Physical Activity Recall (PAR), a valid and widely used instrument, was used to convert each subject's weekly physical activity outside of the intervention into a METs score.19,20 For each group, the mean weekly PAR METs scores was computed.
Certified Iyengar yoga instructors taught the yoga interventions, which were monitored by the Principal Investigator to ensure consistency in presentation of weekly posture sequences. Written lists of the weekly sequences and pictures of the postures were given to the subjects.21 After 4 weeks of instruction, subjects were encouraged to practice at home. The intrascan yoga sequence was taught in class and monitored by research staff during Imaging Session II. The structure of the walking intervention was designed to be similar to that of the yoga intervention, with weekly group sessions in which subjects walked around the gym perimeter at 2.5 mph for 60 minutes. The intrascan walking session was done on a treadmill set to 2.5 mph with 0 incline. This design controlled for group effects and interaction time with research staff.
Imaging
Subjects were scanned on a 4-Tesla full-body MR scanner (Varian/UnityInova, Varian Inc., Palo Alto, CA) at Mclean Hospital in Belmont, MA. Scout images confirmed optimal positioning. After global shimming on unsuppressed water, T1-weighted anatomical images were taken in sagittal and axial planes [echo time (TE)/repetition time (TR) = 6.2 seconds/11.4 milliseconds, field-of-view = 22 × 22 × 8 cm (sagittal) and 22 × 22 × 16 cm (axial), readout duration = 4 ms, receive bandwidth = ±32 kHz, in-plane matrix size = 128 × 256 × 16 (sagittal) and 256 × 256 × 64 (axial), in-plane resolution = 0.94 × 1.9 mm (sagittal) and 0.94 × 0.94 mm (axial), readout points = 512, slice-thickness = 2.5 mm, flip-angle = 11°].
In our previous study, a post-hoc regional analysis that used multivoxel spectroscopic imaging showed that the greatest increase in GABA levels after the yoga intervention was in the thalamus.8 The selection of the left thalamus was based on evidence that the left side has greater parasympathetic innervations and that GABA levels are lower in the left thalamus in post-traumatic stress disorder subjects.22,23 For this study, an algorithm was developed to position a 2 × 2 × 3-cm voxel over the left thalamus. Proton spectroscopy implemented a MEGAPRESS [MEscher-GArwood Point-Resolved Echo Spectroscopy Sequence] difference-editing sequence specifically tuned for GABA.24 Manual voxel shimming yielded global water-line widths ranging from 8 to 15 Hz. The MEGAPRESS sequence collected 68-millisecond echo-time spectra in an interleaved fashion where the GABA editing pulse was applied on every second transient. Additional MEGAPRESS acquisition parameters were: TR = 2 seconds, spectral-bandwidth = 2 kHz, readout-duration = 512 milliseconds, Number of Excitations (NEX) = 384, and total scan duration = 13 minutes.
In order to quantify GABA, the difference-edited spectra were processed and then fitted with LCModel using basis sets acquired at 4 T. A separate LCModel template was used to fit the unedited 68-milisecond subspectrum to obtain creatine (Cr). All fitted metabolite areas were normalized to the fitted Cr resonance from the 68-millisecond subspectrum. One (1) spectrum from the MEGAPRESS acquisition in the thalamus was excluded from analysis due to low signal-to-noise. GABA/Cr ratios are referred to as GABA levels. In order to ascertain the gray and white matter contribution to each voxel, the axial T1-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid compartments using the commercial software package FSL 4.1 (FMRIB Software Library; Analysis Group, FMRIB; Oxford, UK).
Statistical analysis
The primary outcome variables were mood scores, anxiety scores, and thalamic GABA levels. Continuous measures were summarized by means ± standard deviations; within-group comparisons were performed using paired t-tests, while between-group comparisons were performed using two-sample t-tests. Discrete measures were summarized by raw counts for numerators and denominators, as well as the associated percentages, and were compared by Fisher's exact test due to the limited sample size. Linear regression analysis was used to quantify the association between the primary outcome variables and potential predictor variables. In order to take into account within-subject correlations arising from repeated longitudinal measurements, generalized estimated equations (GEEs) were used to analyze within-group trends in mood and anxiety scores, as well as to perform between-group analyses.25,26 All hypothesis tests were two-tailed and conducted at the α = 0.05 significance level. Confidence intervals were two-sided and were constructed with 95% confidence. Stata 10.0 (College Station, TX) was used for analysis.
Results
Demographics and study participation
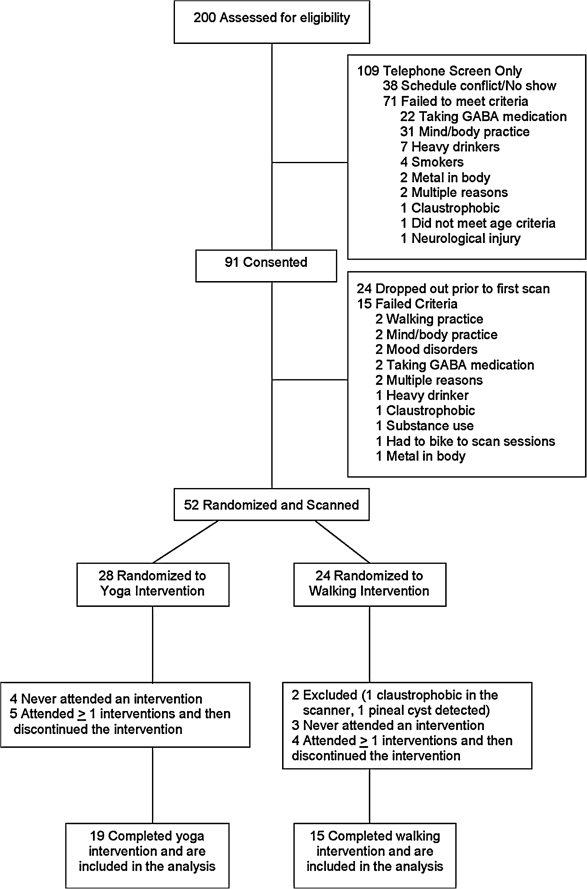
Thirty-four (34) subjects completed the study: 19 in the yoga group and 15 in the walking group (Fig. 1). There was no significant difference between groups for demographic or descriptive variables except for height, which although statistically significant due to a relatively small standard deviation was clinically not significant. There was no difference in demographics between study completers and dropouts, with dropouts equally divided between interventions (Table 1). The means for the weekly PAR METs during the 12-week intervention showed the walking group to have a significantly greater level of activity outside the intervention than the yoga group (p = 0.02); however, there was no difference between groups in activity levels on the week before Imaging Session II. Out of 36 sessions, each group attended about two thirds, with the yoga group reporting about one session a week at home.
FIG. 1.
Flow chart of subject recruitment through completion. GABA, γ-aminobutyric acid.
Table 1.
Demographics and Study Participation
| |
Yoga group (n = 19) |
Walking group (n = 15) |
|
|
|
|---|---|---|---|---|---|
| Factor | Mean ± SD | Mean ± SD | t | df | p |
| Age | 23.9 ± 3.0 | 25.6 ± 4.9 | −1.25 | 32 | 0.22 |
| Female | 11 (58%) | 11 (73%) | – | – | 0.48 |
| Single | 18 (95%) | 12 (80%) | – | – | 0.30 |
| Years of education | 16.4 ± 1.1 | 16.7 ± 1.4 | −0.74 | 32 | 0.46 |
| Possible drinking days | 91.6 ± 6.9 | 89.3 ± 15.8 | 0.56 | 32 | 0.58 |
| Actual drinking days | 14.8 ± 15.5 | 8.7 ± 7.4 | 1.41 | 32 | 0.17 |
| Heavy drinking days | 0.4 ± 0.6 | 0.4 ± 0.7 | 0.09 | 32 | 0.93 |
| Very heavy drinking days | 0.0 ± 0.0 | 0.0 ± 0.0 | – | 32 | – |
| Total drinks | 23.8 ± 22.4 | 13.9 ± 11.3 | 1.57 | 32 | 0.13 |
| Average drinks/possible drinking days | 0.3 ± 0.2 | 0.2 ± 0.1 | 1.41 | 32 | 0.17 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 2.43 | 32 | 0.02 |
| Weight (kg) | 68.2 ± 12.5 | 65.6 ± 10.9 | 0.64 | 32 | 0.53 |
| Body–mass index | 22.5 ± 2.9 | 23.8 ± 2.7 | −1.33 | 32 | 0.19 |
| PARs (during 12-week intervention) | 11.3 ± 10.6 | 24.8 ± 22.90 | 2.35 | 32 | 0.02 |
| PARs week prior to scan 2 | 8.5 ± 16.1 | 19.5 ± 24.9 | 1.57 | 32 | 0.13 |
| Attendance (of 36) | 23.4 ± 7.5 | 24.8 ± 6.2 | 0.59 | 32 | 0.56 |
| Home yoga sessions/week | 0.9 ± 1.0 | − | − | − |
SD, standard deviation; df, degrees of freedom; PAR, Physical Activity Recall.
Analysis of mood and anxiety scales and GABA levels
The following analyses were done with statistically significant findings reported in the tables: (1) a GEE model for changes in mood and anxiety scores for each group at weeks 0, 4, 8, and 12; (2) tonic changes in GABA levels were assessed over the course of the intervention by subtracting Scan 1 from Scan 2 values, while acute changes associated with the intrascan session were assessed by subtracting Scan 2 from Scan 3 values; (3) tonic and acute changes in GABA levels; (4) correlations of mood and anxiety scores with GABA levels for each scan; (5) correlations of tonic (Scan 2–1) and acute (Scan 3–2) changes in mood and anxiety scores with tonic and acute changes in GABA levels.
In the three “positive” subscales of EIFI (Positive Engagement, Revitalization, and Tranquility), an increase in score indicates improved mood. In the two “negative” scales, the STAI-State and EIFI-Physical Exhaustion, an increase in score indicates increased anxiety and physical exhaustion, respectively. Inverse associations (-beta), with the negative scales, indicate decreased anxiety in the within-group analysis and greater decrease in anxiety for the yoga group in the between-group analysis.
In the GEE analysis, the yoga group showed increased scores over the course of the intervention in the three positive EIFI subscales (Positive Engagement, Revitalization, and Tranquility), while the walking group showed an increase in the EIFI-Revitalization subscale. The yoga group showed a decrease in the STAI-State score, indicating decreased anxiety. The between-group analysis showed the yoga group to have greater increases in all positive scales and greater decreases in all negative scales compared with the walking group, indicating improved mood, decreased anxiety and decreased exhaustion (Table 2).
Table 2.
Generalized Estimated Equations Analysis of Mood and Anxiety Scales During Interventions
| |
Yoga group |
Walking group |
Difference |
||||||
|---|---|---|---|---|---|---|---|---|---|
| beta | z | p | beta | z | p | beta | z | p | |
| EIFI-Positive Engagement | 0.792 | 2.50 | 0.01 | −0.450 | −0.93 | 0.35 | 1.424 | 2.58 | 0.01 |
| EIFI-Revitalization | 3.069 | 4.46 | <0.001 | 1.771 | 2.01 | 0.04 | 2.359 | 2.15 | 0.03 |
| EIFI-Tranquility | 1.923 | 3.10 | 0.002 | −0.226 | −0.28 | 0.78 | 2.394 | 2.42 | 0.02 |
| EIFI-Physical Exhaustion | −1.343 | −1.86 | 0.06 | 0.931 | 0.99 | 0.32 | −2.275 | −1.95 | 0.05 |
| STAI-State | −4.517 | −2.11 | 0.04 | 2.415 | 1.05 | 0.30 | −6.088 | −1.93 | 0.05 |
EIFI, Exercise-Induced Feeling Inventory; STAI, State–Trait Anxiety Inventory.
Tonic changes in mean mood scores showed significant increases in the yoga group for Revitalization (1.8 ± 2.5, t = 3.21, df = 18, p = 0.005). Acute changes in mean scores indicated significant increases in the yoga group for Revitalization (2.5 ± 2.7, t = 4.12, df = 18, p < 0.001) and Tranquility (2.0 ± 1.8, t = 4.77, df = 18, p < 0.001), and significant decrease in the STAI-State (−5.2 ± 5.5, t = −4.05, df = 17, p < 0.001). There were no significant tonic or acute changes detected in the walking group.
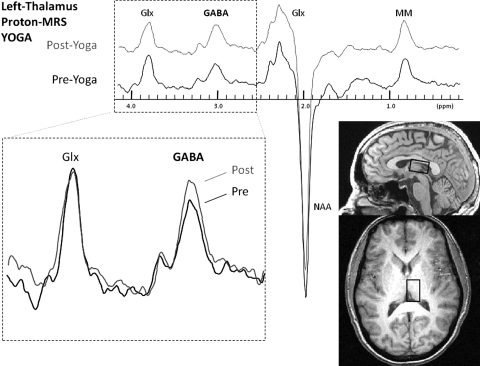
Analysis of the gray matter/white matter ratio in the thalamic voxel was done for each of the three scans. There was no difference in the average gray matter/white matter ratio among the three scans for the thalamus (F = 0.11, df = 2, p = 0.90), indicating consistent repositioning of the voxel over the same anatomical region for each scan. There was no difference between the yoga and walking group in baseline GABA levels (t = 0.73, df = 32, p = 0.47). There were no significant changes in tonic GABA levels in either group. There was a nearly significant increase in acute thalamic GABA levels in the yoga group (0.009 ± 0.019, t = 1.80, df = 17, p = 0.09) (Fig. 2 and Table 3).
FIG. 2.
Spectral data showing an increase in the area of the edited γ-aminobutyric acid (GABA) resonance doublet at 3.00 parts per million (ppm) from scan 2 to scan 3 for the left thalamic voxel in the yoga group. Glx, glutamate and glutamine complex; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate; MM, macromolecules.
Table 3.
Tonic and Acute Changes in Thalamic Gamma-Aminobutyric Acid Levels
| Tonic | Scan 1 | Scan 2 | (Scan 2–Scan 1) | t | df | p |
|---|---|---|---|---|---|---|
| Yoga group | 0.065 ± 0.020 | 0.061 ± 0.021 | −0.004 ± 0.017 | −1.01 | 18 | 0.33 |
| Walking group | 0.059 ± 0.023 | 0.060 ± 0.014 | 0.000 ± 0.020 | 0.04 | 14 | 0.97 |
| Acute | Scan 2 | Scan 3 | (Scan 3–Scan 2) | t | df | p |
|---|---|---|---|---|---|---|
| Yoga group | 0.058 ± 0.018 | 0.067 ± 0.019 | 0.009 ± 0.020 | 1.80 | 17 | 0.09 |
| Walking group | 0.060 ± 0.014 | 0.059 ± 0.019 | −0.000 ± 0.017 | −0.10 | 14 | 0.92 |
df, degrees of freedom.
Whether thalamic GABA levels correlate with improved mood or decreased anxiety is a general question, independent of group assignment. Therefore, the correlation of mood and anxiety scores with GABA levels was done for the whole group. There were significant positive correlations of GABA levels with Revitalization and Tranquility scores and a negative correlation with the STAI-State scores (Table 4).
Table 4.
Significant Correlations of Thalamic Gamma-Aminobutyric Acid Levels with Mood and Anxiety Scores
| Scan(s) | rho | t | df | p | |
|---|---|---|---|---|---|
| Correlation of thalamic GABA levels with mood and anxiety scores | |||||
| Whole group | |||||
| Revitalization | 1 | 0.38 | 2.32 | 32 | 0.03 |
| Tranquility | 1 | 0.50 | 3.27 | 32 | 0.003 |
| Tranquility | 2 | 0.47 | 3.01 | 32 | 0.005 |
| STAI-S | 2 | −0.35 | 2.11 | 32 | 0.04 |
| STAI-S | 3 | −0.41 | 2.46 | 30 | 0.01 |
| Correlation of changes in thalamic GABA levels with changes in mood and anxiety scores | |||||
| Yoga group | |||||
| Revitalization | 2-1 | 0.78 | 5.14 | 17 | 0.001 |
| Tranquility | 2-1 | 0.50 | 2.38 | 17 | 0.03 |
| STAI-S | 2-1 | −0.50 | 2.38 | 17 | 0.03 |
| Tranquility | 3-2 | 0.56 | 2.70 | 16 | 0.01 |
GABA, γ-aminobutyric acid; df, degrees of freedom; STAI, State–Trait Anxiety, Inventory.
In the yoga group, for the tonic condition, there were positive correlations of changes in mood and anxiety scores with changes in mean GABA levels for Revitalization and Tranquility scores and a negative correlation with the STAI-State, while in the acute condition there were positive correlations with Tranquility scores (Table 4).
Women (n = 22) used an approved method of birth control and had negative urine pregnancy tests prior to each imaging session. Because decreases in GABA levels occur during the follicular phase, women were scheduled for scanning in the nonluteal stage defined by a serum progesterone <3.0 ng/mL.27 All women, except 1 using hormonal contraception and assumed to be in the nonluteal stage, had progesterone levels drawn before each imaging session. Serum progesterone levels were <2.0 ng/mL for all but four samples. Two (2) women had levels <5.2 ng/mL for imaging session I. For imaging session II, serum progesterone levels were 20.2 ng/mL for 1 subject in the walking group, and 17.3 ng/mL for one subject in the yoga group, consistent with the luteal phase that is associated with higher GABA levels. Increases in GABA levels due to the luteal phase during imaging session II would have made tonic increases easier to detect, yet there was no significant increase in tonic GABA levels for either group. In the previous study, in the yoga group, menstrual stage did not affect increases in GABA levels. Accordingly, the luteal levels of progesterone for imaging session II should not have affected the detection of acute changes.
Discussion
In this study, the yoga intervention was associated with greater improvements in mood and decreases in anxiety in the tonic, acute and intervention analyses compared to the metabolically matched walking intervention, suggesting that the effect of yoga on mood and anxiety is not solely due to the metabolic demands of the activity. In our prior study, significant acute increases in brain GABA levels immediately after a yoga session were recorded. The current study found near-significant acute increases, but stable tonic levels during the 12-week yoga intervention. These observations are consistent with a time-limited effect of the yoga intervention on thalamic GABA levels. The lack of tonic changes in this study, and the lack of baseline differences between the experienced yoga practitioners and controls in the prior study, suggest that tonic GABA levels are stable in subjects screened to exclude low GABA states.
The whole group analysis of the correlations between mood and anxiety scales taken before each scan and the GABA levels obtained from those scans demonstrated significant positive correlations with the positive scales of Tranquility and Revitalization and a negative correlation with the STAI-State. To our knowledge, this is the first study to report a positive correlation between thalamic GABA levels and improved mood or decreased anxiety. The correlations between group changes in mood and anxiety scores and changes in GABA levels in the tonic and acute conditions suggest that increases in thalamic GABA levels are associated with improved mood and decreased anxiety. This is the first study to show that a behavioral intervention (i.e., yoga) is associated with a positive correlation between changes in thalamic GABA levels and improvements in mood and anxiety.
The use of METs controlled for metabolic demands of each intervention and allowed the activity level of the 2 groups (outside of the intervention) to be compared. The significantly greater level of physical activity outside the intervention in the walking group compared to the yoga group was not expected. The higher level of outside activity in the walking group could have contributed to the finding of smaller changes in mood and anxiety in the walking group, as the intervention may not have been a great enough physical challenge given the greater level of outside activity.
The small sample size in the yoga group where only a nearly significant increase in thalamic GABA levels was detected was a limitation, which was offset by the findings of significant correlations between GABA levels and mood and anxiety scores. In our previous study, the acute increase in whole-slab GABA levels in experienced yoga practitioners was 27% (published) and a 26% increase in thalamic GABA levels (unpublished), compared to a 13% thalamic increase seen in the current study of yoga-naïve subjects who were trained for 12 weeks.8 This suggests that while subjects can be trained to practice yoga in a relatively short time with a measurable effect, the associated change in GABA levels may increase with experience.
The effect of the yoga intervention on GABA levels may be due to the ability of yoga practices to increase parasympathetic nervous system (PNS) activity.28–30 In a study comparing Iyengar yoga to a walking control, the Iyengar yoga group showed greater increases in PNS activity.31 Yoga techniques and vagal nerve stimulation (VNS) increase PNS tone by stimulating vagal afferents.1,32 Studies suggest that the antiepileptic effects of VNS are largely mediated by widespread release of GABA.33 Accordingly, the practice of yoga through stimulation of vagal afferents may result in the increase of brain GABA levels as seen in the yoga group.
Conclusions
The 12-week yoga intervention was associated with greater improvements in mood and anxiety than a metabolically matched walking exercise. This is the first study to demonstrate that increased thalamic GABA levels are associated improved mood and decreased anxiety. It is also the first time that yoga postures have been associated with a positive correlation between acute increases in thalamic GABA levels and improvements in mean scores on mood and anxiety scales. Given that pharmacologic agents that increase the activity of the GABA system are prescribed to improve mood and decrease anxiety, the reported correlations are in the expected direction.34,35 The possible role of GABA in mediating the beneficial effects of yoga on mood and anxiety warrants further study.
Footnotes
This article was previously presented as a poster at the American Psychiatric Association Annual Meeting, San Francisco, CA in 2008.
Acknowledgments
We acknowledge the certified Iyengar yoga teachers without whom this project would not have been possible: Marysia Gensler, Annie Hoffman, Nancy Turnquist, and Lynnae LeBlanc. Funding for this study was provided through NIH grants: 1R21AT004015 (CCS), DAO15116 (PFR), R01 1AA 015923 (DAC), M01RR00533 and Ul1RR025771 (General Clinical Research Unit at Boston University Medical Center).
Disclosure Statement
Dr. Renshaw is a consultant for Novartis, Roche and Kyowa Hakko and received research support from GlaxoSmithKline and Roche. Dr. Ciraulo is a consultant for Novo Nordisk and received research support from Merck and Catalyst.
References
- 1.Khalsa S. Yoga as a therapeutic intervention: A bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269–285. [PubMed] [Google Scholar]
- 2.Craft LL. Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: A meta-analysis. J Sport Exercise Psychol. 1998;20:339–357. [Google Scholar]
- 3.Yardi N. Yoga for control of epilepsy. Seizure. 2001;10:7–12. doi: 10.1053/seiz.2000.0480. [DOI] [PubMed] [Google Scholar]
- 4.Brambilla P. Perez J. Barale F, et al. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 5.Houser CR. GABA neurons in seizure disorders: A review of immunocytochemical studies. Neurochem Res. 1991;16:295–308. doi: 10.1007/BF00966093. [DOI] [PubMed] [Google Scholar]
- 6.Breier A. Paul S. The GABAa/benzodiazepine receptor: Implications for the molecular basis of anxiety. J Psychiatr Res. 1990;24:91–104. doi: 10.1016/0022-3956(90)90040-w. [DOI] [PubMed] [Google Scholar]
- 7.Schatzberg AF. Nemeroff C. Textbook of Psychopharmacology. 2nd. Washington, DC: American Psychiatric Press; 1998. [Google Scholar]
- 8.Streeter CC. Jensen JE. Perlmutter RM, et al. Yoga Asana sessions increase brain GABA levels: A pilot study. J Altern Complement Med. 2007;13:419–426. doi: 10.1089/acm.2007.6338. [DOI] [PubMed] [Google Scholar]
- 9.Jorm AF. Christensen H. Griffiths KM, et al. Effectiveness of complementary and self-help treatments for depression. Med J Aust. 2002;176(suppl):S84–S96. doi: 10.5694/j.1326-5377.2002.tb04508.x. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF. Christensen H. Griffiths KM, et al. Effectiveness of complementary and self-help treatments for anxiety disorders. Med J Aust. 2004;181(7 suppl):S29–S46. doi: 10.5694/j.1326-5377.2004.tb06352.x. [DOI] [PubMed] [Google Scholar]
- 11.Lawlor D. Hopker S. The effectiveness of exercise as an intervention in the management of depression: Systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin Z. Canli T. Epperson CN. Effect of estrogen–serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 13.First MB. Gibbon M. Spitzer R, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- 14.Sobell L. Sobell M. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, editor; Allen J, editor. Measuring Alcohol Consumption. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- 15.Watson D. Clark L. Tellegen A. Development and validation of brief meaures of positive and negative affect: The PANAN scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 16.Gauvin L. Rejeski WJ. The exercise-induced feeling inventory: Development & initial validation. J Sport Exercise Psychol. 1993;15:403–423. [Google Scholar]
- 17.Spielberger CD. Gorsuch RL. Lushene R, et al. Manual for the State–Trait Anxiety Inventory (Form Y) Redwood City: Mind Garden; 1983. [Google Scholar]
- 18.Ainsworth BE. Haskell WL. Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 19.Blair S. Applegate W. Dunn A, et al. Activity Counseling Trail (ACT): Rationale, design, and methods. Med Sci Sport Exercise. 1998;30:1097–1106. doi: 10.1097/00005768-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Blair S. Haskell W. Ho P, et al. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 21.Silva M. Mehta S. Yoga: The Iyengar Way. New York: Alfred A. Knopf; 2001. [Google Scholar]
- 22.Craig AD. Interoception and Emotion. In: Lewis M, editor; Haviland-Jones JM, editor; Barrett LF, editor. Handbook of Emotions. 3rd. New York: The Guilford Press; 2008. pp. 272–288. [Google Scholar]
- 23.Geuze E. van Berckel BN. Lammertsma AA, et al. Reduced GABA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry. 2008;13:74–83. doi: 10.1038/sj.mp.4002054. [DOI] [PubMed] [Google Scholar]
- 24.Mescher M. Merkle H. Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY. Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.Zeger SL. Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 27.Epperson CN. Haga K. Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 28.Lee MS. Huh HJ. Kim BG, et al. Effects of Qi-training on heart rate variability. Am J Chin Med. 2002;30:463–470. doi: 10.1142/S0192415X02000491. [DOI] [PubMed] [Google Scholar]
- 29.Elliott R. Rubinsztein JS. Sahakian BJ, et al. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 30.Elliot S. Edmonson D. The New Science of Breath. 2nd. Allen, TX: Coherence Press; 2006. [Google Scholar]
- 31.Khattab K. Khattab AA. Ortak J, et al. Iyengar yoga increases cardiac parasympathetic nervous modulation among healthy yoga practitioners. Evid Based Complement Alternat Med. 2007;4:511–517. doi: 10.1093/ecam/nem087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemeroff CB. Mayberg HS. Krahl SE, et al. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 33.Woodbury DM. Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1990;31(suppl 2):S7–S19. doi: 10.1111/j.1528-1157.1990.tb05852.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanacora G. Mason GF. Rothman DL, et al. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 35.Sanacora G. Mason GF. Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]