Abstract
Three new bianthraquinone derivatives, alterporriol K (1), L (2) and M (3), along with six known compounds were obtained from extracts of the endophytic fungus Alternaria sp. ZJ9-6B, isolated from the mangrove Aegiceras corniculatum collected in the South China Sea. Their structures were elucidated by one- and two-dimensional NMR spectroscopy, MS data analysis and circular dichroism measurements. Compounds 1, 2 and 3 were first isolated alterporriols with a C-2–C-2′ linkage. The crystallographic data of tetrahydroaltersolanol B (7) was reported for the first time. In the primary bioassays, alterporriol K and L exhibited moderate cytotoxic activity towards MDA-MB-435 and MCF-7 cells with IC50 values ranging from 13.1 to 29.1 μM.
Keywords: endophytic fungus, Alternaria sp., bianthraquinone, alterporriol, cytotoxicity
1. Introduction
A wide variety of anthraquinone derivatives isolated from plants, animals and marine fungi have served as candidates for various therapeutic uses [1–3]. Anthraquinones inhibit the proliferation of human breast, colon and lung cancer cells [4]. They also displayed inhibitory ability towards protein kinase, NADH oxidase, quinone reductase and calmodulin [5–8]. Several laboratories have investigated anthraquinones as antibacterial agents [9].
The alterporriol family of bianthraquinone derivatives were first reported from Alternaria porri by Suemitsu et al. in 1984 [10]. Over the last 27 years, nine additional alterporriols have been reported from fungi. All the alterporriols except alterporriols G-J were described from Alternaria sp. [5,11–13]. In terms of the underlying monomers, alterporriols can occur as either homodimers or heterodimers. With regard to the coupling positions of the monomers, alterporriols A, B, D, E, I and J feature a C-5–C-5′ linkage, alterporriol C shows a C-1–C-7′ connection, and G and H possess a C-7–C-5′ linkage [5,11–13].
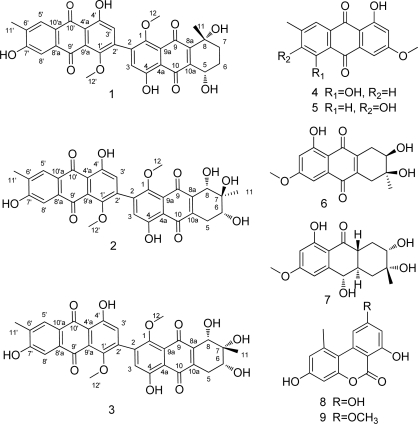
As part of our ongoing program to search for new bioactive natural products from the South China Sea [14–16], an endophytic fungus Alternaria sp. ZJ9-6B has been isolated from the fruit of the marine mangrove Aegiceras corniculatum in Zhanjiang, Guangdong, China. Chemical investigation of this fungus led to the isolation of nine metabolites, including three new anthraquinone derivatives 1–3 and six known compounds 4–9 (Figure 1). It is interesting that compounds 1–3 all possess dimeric structures with a C-2–C-2′ linkage. In this report, we describe the isolation, structural elucidation and biological activity of these new metabolites.
Figure 1.
Structures of 1–9 isolated from Alternaria sp. ZJ9-6B.
2. Results and Discussion
The methanol extract of the dried mycelium was subjected to a combination of column chromatography on silica gel, Sephadex LH-20 and C18 reversed phase silica gel.
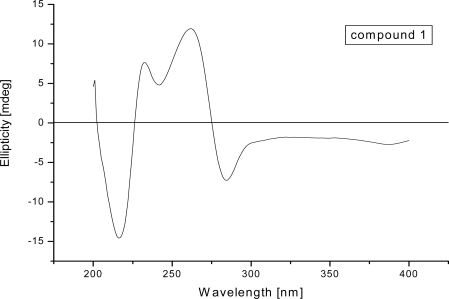
Compound 1 was isolated as a red amorphous powder. HR-EIMS at m/z = 586.1471 [M]+ indicated the molecular formula C32H26O11 (calcd. for C32H26O11, 586.1470). Compound 1 exhibited strong optical rotation (c = 1.0, MeOH) which indicated the possibility of an asymmetric centre and/or axial chirality (Figure 2). The IR spectrum (KBr) exhibited a weak shoulder at 1652 cm−1 and an intense band at 1638 cm−1 for carbonyl groups. The UV spectrum displayed bands at 224, 280 and 437 nm, suggesting a quinonoid chromophore. The 1H NMR spectrum (Table 1) showed a pair of chelated hydroxyl resonances (δH = 13.61 and 13.15 ppm), four aromatic protons (δH = 7.67, 7.55, 6.92 and 6.88 ppm), two methoxyl protons (δH = 3.68 and 3.66 ppm), two singlet methyls (δH = 2.18 and 1.07 ppm), two methylene protons (δH = 2.53 and 2.72 ppm, δH = 2.20 and 2.34 ppm), and oxygenated methine (δH = 3.51 ppm). The 13C NMR spectrum displayed four carbonyl signals (δC = 183.6, 187.8, 181.1 and 186.7 ppm), twenty signs of aromatic carbons, one quaternary carbon (δC = 69.0 ppm), one methine (δC = 70.1 ppm) and two methylenes (δC = 29.1 and 36.1 ppm). These data implied that compound 1 possessed a bianthranquinone scaffold, including an anthraquinone unit and a tetrahydroanthraquinone unit (Figure 1) [5,13]. The unsubstituted carbons for two aromatic rings of the anthraquinone unit were located at C-8′ (δC = 130.3 ppm; δH = 7.67 ppm, d, J = 0.8 Hz), C-5′ (δC = 110.5 ppm; δH = 7.55 ppm, d, J = 0.8 Hz) and C-3′ (δC = 103.8 ppm; δH = 6.921 ppm, s) by the HMBC correlations (Figure 3). In the tetrahydroanthraquinone unit, one aromatic proton at H-3 (δH = 6.88 ppm, s) and the protons in the alicyclic ring, including one oxygenated methine H-5 (δH = 3.51 ppm, ddd, J = 5.4, 5.5, 12.5 Hz) and two methylene protons H-6 (δH = 2.53 and 2.72 ppm) and H-7 (δH = 2.20 and 2.34 ppm) were observed.
Figure 2.
CD Spectra of 1. Recorded in MeOH at amibient temperature.
Table 1.
| Atom | δC (ppm) | δH (ppm) (multiplicity, J(Hz)) | COSYb | HMBCb | NOESY |
|---|---|---|---|---|---|
| 1 | 164.1 | ||||
| 2 | 123.3 | ||||
| 3 | 103.7 | 6.88 (s) | C-1, 2, 2′, 4, 4a, 10 | ||
| 4 | 163.8 | ||||
| 4a | 108.6 | ||||
| 5 | 70.1 | 3.51 (ddd, 5.5, 5.5, 12.5) | H-6a, 6b, 5-OH | C-6, 7, 8a, 11 | H-11 |
| 6 | 29.1 | a: 2.53 (m) | H-5, 7a, 7b | C-5, 8, 10a | H-11 |
| b: 2.72 (m) | H-5, 6a, 7a, 7b | C-5, 8, 10a, 11 | |||
| 7 | 36.1 | a: 2.31 (m) | H-6a, 6b, 7b | C-5, 8, 8a, 11 | |
| b: 2.35 (m) | H-6a, 6b, 7a | C-5, 8, 8a, 11 | H-11 | ||
| 8 | 69.0 | ||||
| 8a | 141.6 | ||||
| 9 | 183.6 | ||||
| 9a | 128.9 | ||||
| 10 | 187.8 | ||||
| 10a | 143.2 | ||||
| 11 | 25.2 | 1.07 (s) | C-5, 7, 8 | ||
| 12 | 56.7 | 3.66 (s) | C-1, 3 | ||
| 4-OH | 13.15 (s) | C-3, 4, 4a | |||
| 5-OH | 4.69 (d, 5.5) | H-5 | C-5, 6, 8 | ||
| 8-OH | 4.30 (s) | C-5, 7, 8, 11 | |||
| 1′ | 164.7 | ||||
| 2′ | 122.5 | ||||
| 3′ | 103.8 | 6.92 (s) | C-2, 2′, 4′, 4′a, 10′ | ||
| 4′ | 165.0 | ||||
| 4′a | 110.0 | ||||
| 5′ | 110.5 | 7.55 (d, 0.8) | C-6′, 7′, 8′a, 10′, 10′a, 11′ | ||
| 6′ | 125.2 | ||||
| 7′ | 161.3 | ||||
| 8′ | 130.3 | 7.67 (d, 0.8) | C-7′, 8′a, 9′, 10′a, 11′ | ||
| 8′a | 132.4 | ||||
| 9′ | 181.1 | ||||
| 9′a | 130.7 | ||||
| 10′ | 186.7 | ||||
| 10′a | 132.2 | ||||
| 11′ | 16.1 | 2.18 (s) | C-5′, 7′, 8′ | ||
| 12′ | 56.8 | 3.69 (s) | C-1′ | ||
| 4′-OH | 13.63 (s) | C-3′, 4′, 4′a | |||
| 7′-OH | 8.11 (s) |
Measured at 500 MHz (for 1H) and 125 MHz (for 13C);
For the HMBC and COSY spectra, see the Supporting Information.
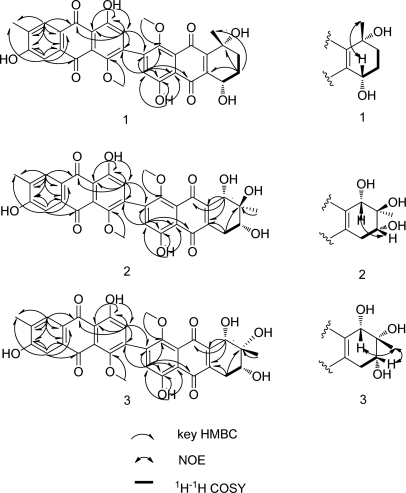
Figure 3.
Key HMBC, NOE and 1H-1H COSY correlations of 1–3.
A contiguous sequence of coupled signals from H-5 to H-7 in the 1H-1H COSY spectrum combined with the HMBC correlations from H-5 to C-6, C-7 and C-8a, from H-6 to C-5, C-8, and C-10a, and from H-7 to C-5, C-8, C-8a, C-9 and C-11, established the substructure of the cyclohexene ring (Figure 3).
HMBC correlations from each of 4-OH (δH = 13.15 ppm) and 4′-OH (δH = 13.63 ppm) to C-4, C-3, C-4a and to C-4′, C-3′, C-4′a, respectively, indicated their location at C-4 and C-4′ of the bianthraquinone scaffold.
The presence of a bond connecting C-2 and C-2′ was suggested by the absence of two sets of ortho-coupled doublets and the presence of two singlets H-3 (δH = 6.88 ppm, s) and H-3′ (δH = 6.92 ppm, s). Moreover, HMBC correlations of H-3 with C-1, C-2, C-2′, C-4, C-4a and C-10, and of H-3′ with C-1′, C-2, C-2′, C-4′, C-4′a and C-10′, respectively (Figure 3) provided evidence for C-2–C-2′ linkage of 1. The HMBC spectrum showed correlations of the methoxyl proton (δH = 3.66 ppm, s, 3H, H-12) with C-1 and C-3. Likewise, correlations were observed from methoxyl proton (δH = 3.69 ppm, s, 3H, H-12′) to C-1′. These allowed us to assign two methoxyl groups to C-1 and C-1′.
The relative configuration of the chiral centers of C-5 and C-8 were deduced by 2D 1H-1H NOESY experiments (Figure 3) and the analysis of 1H-1H coupling constants (Table 1). A NOESY correlation between CH3-11 (δH = 1.07 ppm, s) and H-5 suggested that they were on the same side of the cyclohexene ring. The axial position of H-5 was confirmed by coupling constant JH-5,H-6a = 12.5 Hz, indicating CH3-11 to also have an axial orientation. Therefore, compound 1 was determined as (5S*,8R*)-4,4′,5,7′,8-pentahydroxy-1,1′-dimethoxy-6′,8-dimethyl-5,6,7,8-tetrahydro-[2,2′-bianthracene]-9,9′,10,10′-tetraone. We propose the trivial name alterporriol K.
Compounds 2 and 3 were obtained as a mixture after separating with Sephadex LH-20 chromatography. Upon HPLC analysis, the mixture exhibited two partially overlapped peaks (area ratio ca. 4:1). Then compounds 2 and 3 were isolated with re-separation by preparative HPLC, respectively. Compound 2 was a red amorphous powder, (c = 1.0, MeOH). The HR-ESI-TOF-MS exhibited a peak at m/z = 601.1340 [M – H]− indicating a molecular formula of C32H26O12 (calcd. for C32H25O12, 601.1346). Comparison of the 1H and 13C NMR spectral data of 2 (Table 2) with that of 1 showed a close structural relationship between both compounds, except for the presence of an additional oxymethine group proton (δH = 4.03, d, J = 6.7 Hz, H-8) and an additional hydroxyl group signal (δH = 2.17 ppm, s, 6-OH) and the absence of the methylene signals corresponding to H-7 in the 1H NMR spectrum of 2. The substructure of the cyclohexene ring was established by HMBC correlations from H-8 to C-6, C-7, C-8a, C-9, C-10a and C-11 and 1H-1H COSY correlations between H-5 and H-6 (Figure 3). NOE difference and analysis of 1H-1H coupling constants (Table 2) enabled the relative of configuration of 2 to be deduced. In the NOE experiment, when the methyl signal CH3-11 (δH = 1.16, s) was irradiated, no enhancement of signals H-6 and H-8 was observed. Meanwhile, irradiation of H-8 caused an enhancement of H-6. These data suggested that CH3-11 with H-6 and H-8 was trans-configuration in the cyclohexene ring (Figure 3). A large coupling constant for H-5a/H-6 (JH-5a,H-6 = 10.0 Hz) indicated an axial location for H-6, likewise suggesting an axial location for H-8. Thus, the relative configuration at C-6, C-7, and C-8 was 6S*, 7R* and 8R*. Compound 2 was finally defined as (6S*,7R*,8R*)-4,4′,6,7,7′,8-hexahydroxy-1,1′-dimethoxy-6′,7-dimethyl-5,6,7,8-tetrahydro-[2,2′-bianthracene]-9,9′,10,10′-tetraone, named as alterporriol L.
Table 2.
| Actom |
2 |
3 |
||||
|---|---|---|---|---|---|---|
| δC (ppm) | δH (ppm) (multiplicity, J (Hz)) | HMBC | δC (ppm) | δH (ppm) (multiplicity, J (Hz)) | HMBC | |
| 1 | 164.3 | 164.5 | ||||
| 2 | 123.2 | 123.5 | ||||
| 3 | 103.6 | 6.90 (s) | C-1, 2, 2′, 4, 4a, 10 | 103.4 | 6.92 (s) | C-1, 2, 2′, 4, 4a, 10 |
| 4 | 163.8 | 163.9 | ||||
| 4a | 108.8 | 108.8 | ||||
| 5 | 28.8 | a: 2.33 (dd, 19.5, 10.0) | C-6, 8a, 10a | 28.8 | a: 2.33 (dd, 19.3, 9.9) | C-6, 8a, 10a |
| b: 2.79 (dd, 19.5, 6.0) | C-6, 7, 8a, 10a | b: 2.78 (dd, 19.3, 5.9) | C-6, 7, 8a, 10, 10a | |||
| 6 | 66.7 | 3.70 c | 66.6 | 3.69 c | ||
| 7 | 71.9 | 71.9 | ||||
| 8 | 69.0 | 4.03 (d, 6.7) | C-6, 7, 9, 8a, 10a | 69.0 | 4.06 (d, 5.7) | |
| 8a | 142.6 | 142.6 | ||||
| 9 | 183.3 | 183.1 | ||||
| 9a | 128.9 | 128.3 | ||||
| 10 | 188.4 | 188.3 | ||||
| 10a | 143.7 | 143.6 | ||||
| 11 | 21.8 | 1.16 (3H, s) | C-6, 7, 8 | 21.8 | 1.16 (3H, s) | C-6, 7, 8 |
| 12 | 56.7 | 3.68 (3H, s) | C-1 | 56.7 | 3.68 (3H, s) | C-1 |
| 4-OH | 13.11 (s) | C-3, 4, 4a | 13.13 (s) | C-3, 4, 4a | ||
| 6-OH | 2.17 (s) | 2.16 (s) | ||||
| 7-OH | 4.25 (s) | C-7, 8 | 4.48 (s) | |||
| 8-OH | 5.42 (d, 6.7) | C-6, 7, 8 | 5.27 (d, 5.7) | |||
| 1′ | 164.4 | 164.9 | ||||
| 2′ | 122.3 | 122.5 | ||||
| 3′ | 104.0 | 6.92(s) | C-2, 2′, 4′, 4′a, 10′ | 103.5 | 6.89 (s) | C-1′, 2, 2′, 4′, 4′a,10′ |
| 4′ | 164.9 | 165.1 | ||||
| 4′a | 110.0 | 110.0 | ||||
| 5′ | 110.5 | 7.55 (s) | C-6′, 7′, 8′a, 10′, 10′a, 11′ | 110.7 | 7.51 (s) | C-6′, 7′, 8′a, 10′, 10′a, 11′ |
| 6′ | 125.0 | 125.2 | ||||
| 7′ | 161.6 | 162.8 | ||||
| 8′ | 130.2 | 7.68 (s) | C-7′, 8′a, 9′, 10′a, 11′ | 130.1 | 7.65 (s) | C-7′, 8′a, 9′, 10′a, 11′ |
| 8′a | 132.4 | 132.4 | ||||
| 9′ | 181.1 | 180.6 | ||||
| 9′a | 131.2 | 130.7 | ||||
| 10′ | 186.8 | 186.9 | ||||
| 10′a | 132.3 | 132.4 | ||||
| 11′ | 16.1 | 2.19 (s) | C-5′, 6′, 7′, 8′, 10′a | 16.3 | 2.18 (s) | C-5′, 6′, 7′, 8′ |
| 12′ | 56.7 | 3.68 (s) | C-1′ | 56.8 | 3.71 (s) | C-1′ |
| 4′-OH | 13.61 (s) | C-3′, 4′, 9′a | 13.70 (s) | C-3′, 4′, 9′a | ||
| 7′-OH | 7.65 (s) | C-7′, 8′a, 9′, 10′, 11′ | 7.67 (s) | C-5′, 7′, 8′a, 11′ | ||
Measured at 500 MHz (for 1H) and 125 MHz (for 13C);
For the HMBC and COSY spectra, see the Supporting Information;
Overlapped by methoxyl signal.
Compound 3, obtained as a red amorphous power, (c = 1.0, MeOH), had the molecular formula C32H26O12, as determined by HR-ESI-TOF-MS at m/z = 601.1340 [M – H]− (calcd. for C32H25O12, 601.1346). The MS analysis revealed 3 to be an isomer of 2. The UV and IR absorptions, 1H and 13C NMR data, HMBC and 1H-1H COSY correlations (Figure 3 and Table 2) of 3 were almost identical with those of 2, suggesting the two compounds to have identical carbon skeletons. However, in contrast with 2 in the NOE experiment of 3, irradiation of the methyl signal CH3-11 (δH = 1.16 ppm, s) resulted in obvious enhancements of the signals for both H-6 and H-8. The coupling constant for H-5a/H-6 was measured to be JH-5a,H-6 = 9.9 Hz suggesting axial locations for H-6 and, by extension, H-8. These data suggested that 2 and 3 are epimers at C-7. Therefore compound 3 was elucidated as (6S*,7S*,8R*)-4,4′,6,7,7′,8-hexahydroxy-1,1′-dimethoxy-6′,7-dimethyl-5,6,7,8-tetrahydro-[2,2′-bianthracene]-9,9′,10,10′-tetraone, named alterporriol M.
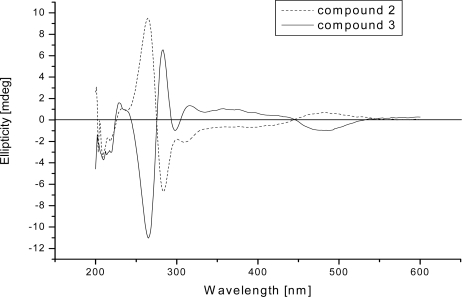
An interesting feature of the isolated bianthquinones is their optical activity. Ab initio calculations of CD spectra for several phenylanthraquinones with chiral axes have recently been reported [17,18]. Specific optical rotation of compounds 2 and 3 showed and −30, respectively. CD spectra of 2 and 3 recorded in methanol showed a near quasi-mirror image pattern (Figure 4). In the case of 2, the CD spectrum consists of a small positive band at 480 nm, followed by two moderate negative bands in the 300–400 nm regions, and a stronger negative band centered at 220 nm, then a stronger positive band at 264 nm. For 3, the sequence of bands is similar but their signs are inverted. The CD spectra of 2 and 3 reflect contributions from a chiral axis and three chiral centers. The chiral axis occurring within the chromophore is expected to dominate the observed CD spectrum [18,19] (Figure 4).The chiral center C-7 is expected to have a much smaller contribution to the observed CD spectrum. With these results, compounds 2 and 3 were deduced as two diastereomers and atropisomers [20].
Figure 4.
CD Spectra of 2 and 3. Recorded in MeOH at amibient temperature.
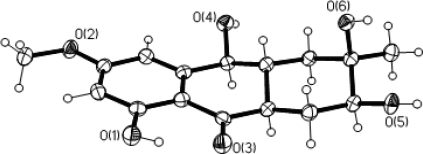
The known compounds were identified as physcion (4), marcrospin (5), dactylariol (6), tetrahydroaltersolanol B (7), alternariol (AOH) (8) and alternariol methyl ether (AME) (9) by spectral analyses and comparison with reported literature data, respectively [21–24]. The structure of 7 was confirmed by X-ray diffraction analysis (Figure 5) and its crystallographic data was reported for the first time.
Figure 5.
X-ray crystal structure of 7.
Compounds 1 and 2 were evaluated for their cytotoxicity against human breast cancer cell lines MDA-MB-435 and MCF-7 by MTT assay. Compound 1 had an IC50 value of 26.97 μM against MDA-MB-435 and 29.11 μM against MCF-7 cells, respectively. Compound 2 showed activities against MDA-MB-435 (IC50 = 13.11 μM) and MCF-7 (IC50 = 20.04 μM). No biological studies were performed for compound 3 due to the limited yield.
3. Experimental Section
3.1. General
Column chromatography (CC) was performed using silica gel (200–300 mesh, Qingdao marine Chemical). The HPLC system consisted of a Waters 2010 series. A mini ODS column (250 × 10 mm, 10 μm particle size) was used. Melting points were determined on an X-4 micro-melting point apparatus and were uncorrected. Circular dichroism was measured on a Schmidt Haensch Polartronic HH W5 polarimeter and was uncorrected. UV spectra were measured on a Shimadzu UV-2501 PC spectrophotometer. IR spectra were measured on a Bruker EQUINOX55 spectrophotometer. 1H and 13C NMR data were recorded on a Varian Inova 500 NB and a Burker AVANCE 400 spectrometer, respectively (TMS as internal standard). EIMS were on a Thermo DSQ EI-mass spectrometer. LC/MS data were acquired using an Applied Biosystems/MDS Sciex and ESI source. HR-EIMS were measured on a Thermo MAT95XP High Resolution mass spectrometer. HR-ESIMS were measured on a Schimadzu LCMS-IT-TOF.
3.2. Strain Isolation, Taxonomic Classification and Endophyte Fermentation
The fungus Alternaria sp. ZJ9-6B was isolated from the fruit of a mangrove tree Aegiceras Corniculatum collected in Zhanjiang Mangrove, Guangdong province, P.R. China, in 2008. A voucher specimen (registration number: ZJ9-6B) has been deposited in the Natural Products Laboratory and the Department of Applied Chemistry, Sun Yat-sen University, China. It was identified according to a molecular biological protocol by DNA amplification and sequencing of the ITS region as described previously with an ITS sequence GenBank ID: HM 754629. The fungal strain was cultivated in potato dextrose broth (PDB) medium (20 g of dextrose and 3 g of crude sea salt in 1 L of potato infusion). Starter cultures were maintained on cornmeal seawater agar. Plugs of agar supporting mycelia growth were cut and transferred aseptically into a 500 mL Erlenmeyer flask containing 250 mL of liquid medium, and incubated at 28 °C on a rotary shaker for 5–7 days. The mycelium was aseptically transferred into 1000 mL Erlenmeyer flasks containing 500 mL PDB medium and incubated at 28 ± 1 °C for 30 days under stationary conditions.
3.3. Extraction and Separation of Metabolites
The cultures (200 L) were separated into mycelium and filtrate. The dried mycelium (724 g) was extracted with methanol (6 L × 4) to give 151.6 g of a crude extract. The crude extract was subjected to silica gel CC using gradient elution with petroleum-ether (PE) and ethyl acetate (EA) mixture (v/v, 95:5–0:100) to give six fractions (A–F). Fraction C (27.1 g) was purified by silica CC with a PE-EA mixture (v/v, 85/15, 4 L) to give three subfractions C-1 (8.7 g), C-2 (5.1 g) and C-3 (3.5 g). These three subfractions were further purified by silica gel CC with a PE-EA mixture to give 4 (161 mg), 5 (67 mg), 8 (19 mg) and 9 (28 mg). Fraction D (13.5 g) was isolated by silica gel CC with PE-EA (v/v, 75:25, 3 L) to give two subfractions D-1 (2.7 g) and D-2 (3.6 g). These two subfractions D-1 and D-2 were further purified by Sephadex LH-20 gel CC with CHCl3-MeOH (65:35, v/v) as a mobile phase and by preparative HPLC with an ODS column (10 × 250 mm), eluting with MeOH-H2O (68:32, v/v) to give 1 (11 mg), 2 (18.4 mg), 3 (3 mg), 6 (13 mg) and 7 (21 mg).
Alterporriol K: Red powder. (c = 1.0, MeOH). UV (MeOH): λmax (log ε) = 279.80 (1.52), 223.80 (1.96) nm. IR (KBr): νmax = 3448, 2966, 2855, 1652, 1638, 1595, 1464, 1430, 1388, 1289, 1208, 1111, 1072, 973, 933, 842, 621 cm−1. For 1H, 13C and 2D NMR spectroscopic data, see Table 1. HR-EIMS: calcd. for C32H26O10, 586.1470; found m/z = 586.1471 [M]+.
Alterporriol L: Red powder. (c = 1.0, MeOH). UV (MeOH): λmax (log ε) = 280.20 (1.24), 225.40 (1.61) nm. IR (KBr): νmax = 3440, 2926, 2857, 1592, 1462, 1430, 1389, 1288, 1208, 1109, 1070, 974, 927, 846, 793, 605 cm−1. For 1H, 13C and 2D NMR spectroscopic data, see Table 2. HR-ESIMS: calcd. for C32H25O12, 601.1346; found m/z = 601.1340 [M – H]−.
Alterporriol M: Red powder. (c = 1.0, MeOH). UV (MeOH): λmax (log ε) = 279.00 (1.33), 225.60 (1.77) nm. IR (KBr): νmax = 3450, 2928, 2027, 1638, 1463, 1389, 1280, 1207, 1111, 1046, 977, 929, 858, 618 cm−1. For 1H, 13C and 2D NMR spectroscopic data, see Table 2. ESIMS m/z = 601.1 [M – H]−. HR-ESIMS calcd. for C32H25O12, 601.1346; found m/z = 601.1340 [M – H]−.
Crystallographic data of 7: The X-ray diffraction data for 7 (Figure 5) was measured on an Xcalibur Nova 1000 CCD diffractometer (Mo Kα-radiation, graphite monochromator). Solvent for crystallization: methanol; Wavelength: 0.71073 Å; Temperature: 173 K; Empirical formula: C16H20O6; Crystal system: monoclinic; Space group C2 with a = 37.306(6) Å, b = 5.8251(10) Å, c = 7.9087(13) Å, α = 90.00°, β = 102.210(3)°, γ = 90.00°; V = 1679.8(5) Å3; Density: 1.397 g/cm3; Z = 2, F (000) = 756.0; Goodness-of-fit on F2: 1.087; R Indices (all data): R1 = 0.0399, wR2 = 0.1208. CCDC-751689 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-Mail: deposit@ccdc.cam.ac.uk).
3.4. Cytotoxic Assays
The cytotoxicity of compounds 1 and 2 were evaluated using human breast cancer cell lines MDA-MB-435 and MCF-7 by MTT assay. Cell viability was measured using the CellTiter 96 aqueous nonradioactive cell proliferation assay. Results were expressed as the mean value of triplicate data points.
4. Conclusions
Alternaria sp. ZJ9-6B is a prolific producer of bioactive metabolites. Nine compounds have been isolated from this fungal strain, including three new alterporriols. These three alterporriols all possess dimeric structures with a C-2–C-2′ linkage. In the primary bioassay, compounds 1 and 2 showed moderate cytotoxic activity against human breast cancer cell lines.
Supplementary Data
Acknowledgments
This work was supported by the 863 Foundation of China (2006AA09Z422, 2007AA), the National Natural Science Foundation of China (20772162, 20972197), the National Science Foundation of Guangdong Province, China (91510227501000055) and the Science & Technology Plan Project of Guangdong Province of China (2010B030600004).
Footnotes
Samples Availability: Available from the authors.
References
- 1.Ji XZ, Leslie G. Microbial transformation of amino- and hydroxyanthraquinones by Beauveria bassiana ATCC 7159. J. Nat. Prod. 2006;69:1525–1527. doi: 10.1021/np060339k. [DOI] [PubMed] [Google Scholar]
- 2.Gizachew A, Berhanu A. Bianthraquinones from the seeds of Senna multiglandulosa. Phytochemistry. 1996;41:919–921. [Google Scholar]
- 3.Zhang JY, Tao LY, Liang YJ, Chen LM, Mi YJ, Zheng LS, Wang F, She ZG, Lin YC, Kenneth KWT, Fu LW. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Mar Drugs. 2010;8:1469–1481. doi: 10.3390/md8041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert HC, Zhang YJ, Navindra PS, Muraleedharan GN. Inhibition of human tumor cell proliferation by novel anthraquinones from daylilies. Life Sci. 2004;74:1791–1799. doi: 10.1016/j.lfs.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Debbab A, Aly AH, Edrada-Ebel R, Wray V, Müller WEG, Totzke F, Zirrgiebel U, Schächtele C, Kubbutat MHG, Lin WH, et al. Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J Nat Prod. 2009;72:626–631. doi: 10.1021/np8004997. [DOI] [PubMed] [Google Scholar]
- 6.Haraguchi H, Abo T, Fukuda A, Okamura N, Yagi A. Mode of phytotoxic action of altersolanols. Phytochemistry. 1996;43:989–992. [Google Scholar]
- 7.Alison DP, Su BN, William JK, Douglas AK. An anthraquinone with potent quinone reductase-inducing activity and other constituents of the fruits of Morinda citrifolia (Noni) J Nat Prod. 2005;68:1720–1722. doi: 10.1021/np050383k. [DOI] [PubMed] [Google Scholar]
- 8.Sergio ML, Araceli PV, Rachel M. Natural products with calmodulin inhibitor properties. Phytochemistry. 2007;68:1882–1903. doi: 10.1016/j.phytochem.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Muhammad S, Mamona N, Muhammad S, Hidayat H, Yong S, Naheed R, Abdul J. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat Prod Rep. 2010;27:238–254. doi: 10.1039/b916096e. [DOI] [PubMed] [Google Scholar]
- 10.Suemitsu R, Sano T, Yamamoto M, Arimoto Y, Morimatsu F, Nabeshima T. Structural elucidation of alterporriol B, a novel metabolic pigment produced by Alternaria porri (Ellis) ciferri. Agric Biol Chem. 1984;48:2611–2613. [Google Scholar]
- 11.Suemitsu R, Ueshima T, Yamamoto T, Yanagawase S. Alterporriol C: A modified bianthraquinone from alternaria porri. Phytochemistry. 1988;27:3251–3254. [Google Scholar]
- 12.Suemitsu R, Horiuchi K, Kubota M, Okamatse T. Production of alterporriols, altersolanols and macrosporin by Alternaria porri and A Solani. Phytochemistry. 1990;29:1509–1511. [Google Scholar]
- 13.Pretsch A, Proksch P, Debbab A. Novel Anthraquinone Derivatives. Patent WO/2010/135759. Dec 2, 2010.
- 14.Wen L, Cai XL, Xu F, She ZG, Chan WL, Vrijimoed LLP, Jones EBG, Lin YC. Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the South China Sea. J Org Chem. 2009;74:1093–1098. doi: 10.1021/jo802096q. [DOI] [PubMed] [Google Scholar]
- 15.Yang JX, Xu F, Huang CH, Li J, She ZG, Pei Z, Lin YC. Metabolites from the mangrove endophytic fungus Phomopsis sp. (#zsu-H76) Eur J Org Chem. 2010;19:3692–3695. [Google Scholar]
- 16.Chen B, Wang DY, Ye Q, Li BG, Zhang GL. Anthranquinones from Gladiolus gandavensis. J Asian Nat Prod Res. 2005;7:197–204. doi: 10.1080/10286020310001653336. [DOI] [PubMed] [Google Scholar]
- 17.Bringmann G, Kraus J, Menche D, Messer K. Elucidation of the absolute configuration of knipholone and Knipholone anthrone by quantum chemical CD calculation. Tetrahedron. 1999;55:7563–7572. [Google Scholar]
- 18.Bringmann G, Menche D, Kraus J, Mühlbacher J, Peters K, Peters EM, Brun R, Bezabih M, Abegaz BM. Atropo-Enantioselective total synthesis of knipholone and related antiplasmodial phenylanthrquinones. J Org Chem. 2002;67:5595–5610. doi: 10.1021/jo020189s. [DOI] [PubMed] [Google Scholar]
- 19.Bringmann G, Mortimer JPA, Keller PA, Gresser MJ, Garner J, Breuning M. Atroposelective synthesis of axially chiral biaryl compounds. Angew Chem Int Ed. 2005;44:5384–5427. doi: 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]
- 20.Bringmann G, Mühlbacher J, Reichert M, Dreyer M, Kolz J, Speicher A. Stereochemistry of isoplagiochin C, a macrocyclic bisbibenzyl from liverworts. J Am Chem Soc. 2004;126:9283–9290. doi: 10.1021/ja0373162. [DOI] [PubMed] [Google Scholar]
- 21.Gizachew A, Berhanu A, Snatzke G, Duddeck H. Bianthraquinones and a spermidine alkaloid from Cassia floribunda. Phytochemistry. 1988;27:3255–3258. [Google Scholar]
- 22.Suemitsu R, Sakurai Y, Nakachi K, Miyoshi I, Kubota M, Ohnishi K. Alterporriol D and E, modified bianthraquinones from Alternaria porri (ellis) ciferri. Agric Biol Chem. 1989;53:1302–1304. [Google Scholar]
- 23.Stoessl A, Stothers JB. Tetrahydroaltersolanol B, a hexahydroxnthronol from Alternaria solani. Can J Chem. 1983;61:378–382. [Google Scholar]
- 24.An YH, Zhao TZ, Miao J, Liu GT, Zheng YZ, Xu YM, Van E, Robert L. Isolation, identification and mutagenicity of alternariol monomethyl ether. J Agric Food Chem. 1989;37:1341–1343. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.