Abstract
Background:
Anti-angiogenic therapy with bevacizumab (an anti-vascular endothelial growth factor (VEGF) antibody) predominantly targets immature blood vessels. Bevacizumab has shown a survival benefit in non-small cell lung carcinoma (NSCLC) and has recently been demonstrated to be safe in patients with brain metastases. However, it is not known whether bevacizumab is effective against brain metastases or whether metastases are representative of their primary in terms of VEGF expression, hypoxia, proliferation and vascular phenotype. The aim of this study was to evaluate these factors in a series of matched primary NSCLCs and brain metastases.
Methods and Results:
Immunohistochemistry showed strong correlation of carbonic anhydrase 9 expression (a marker of hypoxia) in primary and secondary cancers (P=0.0002). However, the proliferation index, VEGF expression, microvessel density and the proportion of mature vessels were discordant between primary and secondary cancers. The mean proportion of mature vessels was 63.2% higher in the brain metastases than the primary tumours (P=0.004). Moreover, the vascular pattern of the primary tumour was not representative of the metastasis.
Conclusions:
Brain metastases have a significantly higher proportion of mature vasculature, suggesting that they may be refractory to anti-VEGF therapy. These findings may have implications for clinical trials and biomarker studies evaluating anti-angiogenic agents in brain metastases.
Keywords: vascular, angiogenesis, non-small cell lung carcinoma, brain, metastases
Angiogenesis, the development of new blood vessels, is a feature common to many carcinomas and has been proposed as a hallmark of cancer (Hanahan and Weinberg, 2000). However, morphological assessment of lung carcinomas has revealed that they may be subdivided into angiogenic and relatively non-angiogenic tumours (Pezzella et al, 1997); the latter co-opt pre-existing vasculature to support an alveolar pattern of growth. Nevertheless, the majority of non-small cell lung carcinomas (NSCLCs) are angiogenic and express vascular endothelial growth factor-A (VEGF) (Jubb et al, 2004), the predominant pro-angiogenic ligand that is exploited by tumours (Kerbel, 2008). Animal models (Kim et al, 1993; Huang et al, 2004; Mancuso et al, 2006) and human studies (Willett et al, 2004) have shown that blocking VEGF signalling results in tumour shrinkage, associated with the pruning of angiogenic (proliferative, leaky and immature (i.e., without associated pericytes)) blood vessels. This observation led to the development of a humanised anti-VEGF monoclonal antibody, bevacizumab, which has shown an improved progression-free survival benefit in numerous tumour types (Hurwitz et al, 2004; Miller et al, 2007; Rini et al, 2010).
In recurrent or advanced NSCLC, the addition of bevacizumab to carboplatin and paclitaxel chemotherapy (E4599 study) resulted in a 2-month improvement in median overall survival (Sandler et al, 2006). When combined with cisplatin and gemcitabine (AVAiL study) in NSCLC, bevacizumab showed no improvement in overall survival, but demonstrated improved progression-free survival (Reck et al, 2010). The E4599 study excluded patients with brain metastases due to concerns that bevacizumab might exacerbate the incidence of tumour-associated brain haemorrhage (Sandler et al, 2006). Several analyses now suggest that bevacizumab is safe in patients with brain metastases (Socinski et al, 2009; Besse et al, 2010), but there is no evidence regarding the efficacy of bevacizumab in this indication. Indeed, there is little published on the vascular phenotype, VEGF expression, hypoxia and proliferation of NSCLC brain metastases, which may inform efficacy.
To date, no valid biomarkers of the survival benefit of bevacizumab have been identified. Objective response rates have been shown not to predict benefit from bevacizumab in colorectal cancer (Grothey et al, 2008), and there are concerns that changes in the MRI appearance of glioblastoma following bevacizumab treatment do not predict clinical benefit (Gerstner et al, 2010). Efforts to identify novel in situ biomarkers of efficacy have focused on available tissue samples from primary tumour resections (Ince et al, 2005; Jubb et al, 2006), to make inferences concerning the treatment of metastatic disease. However, while there is evidence to support this for metastatic colorectal cancer (Kuramochi et al, 2006), there is little evidence to suppose that this is a valid approach to inform the biology of NSCLC brain metastases.
In summary, a greater understanding of the vascular phenotype of NSCLC brain metastases may better inform the use of anti-angiogenic agents in this indication. The aim of this study was to assess the relative expression of VEGF, hypoxia, proliferation, microvessel density, vascular pattern and vascular maturity in a series of matched primary NSCLCs and brain metastases.
MATERIALS AND METHODS
Tissue
Formalin-fixed paraffin-embedded blocks for 15 cases of primary NSCLC with matched resected brain metastases were retrieved from the pathology archives at the Catholic University Hospital, Rome. Ethical approval for this research was granted by the local research ethics committees.
Immunohistochemistry
Characterisation of the antibodies and immunohistochemical methods for VEGF (Turley et al, 1998), CA9 (Jubb et al, 2009), Ki67 (Sington et al, 2007), CD34 (Kuzu et al, 1992) and CD34 and smooth muscle actin (SMA) (double stain; QBEnd/10 and 1A4; Dako, Ely, UK) (Patel et al, 2006) have been described in detail elsewhere.
Scoring
The maximum intensity of VEGF expression in >10% of tumour cells was recorded on a semiquantitative scale from 0 (no expression) to 3 (very strong expression). The percentage of tumour cells with membranous expression of CA9 was estimated by a pathologist. The percentage of tumour cells with nuclear positivity for Ki67 was counted by a pathologist. Microvessel density analysis was performed using the Chalkley method; at low power ( × 40), five vascular hotspots in CD34-labelled sections were selected. Microvessel density was assessed by counting the number of CD34-labelled vessels that overlapped with dots on a 25-point Chalkley eyepiece graticule in each high-power field ( × 200) (Fox et al, 1995). The five counts were then added together to provide the density score. Vascular maturity is defined by the coverage of endothelial cells (CD34 positive) by pericytes (SMA positive) and was calculated by counting the proportion of CD34-labelled vessels that were surrounded, at least in part, by SMA-labelled cells from 10 high-power fields ( × 400). Each case was classified according to the predominant vascular pattern (alveolar, basal, diffuse or papillary) described elsewhere (Pezzella et al, 1997). All scoring was performed blind to the patients’ identities.
Statistical analyses
Student's t-test was used to assess the difference between subsets of continuous data and Spearman's correlation coefficient was used to assess covariance. The χ2 test was used to evaluate associations between categorical data and Cohen's κ statistic was used to determine agreement.
RESULTS
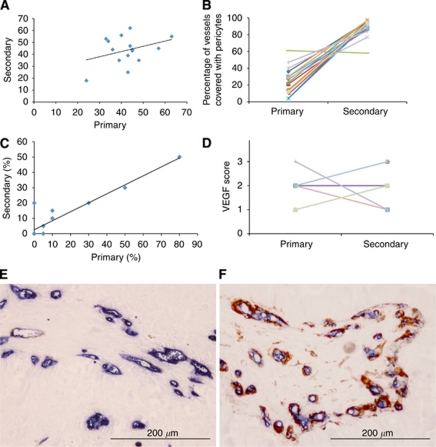
Chalkley counts in primary NSCLCs and brain metastases were not significantly correlated (r=0.148, P=0.60) (Table 1; Figure 1). Although, mean Chalkley counts were similar in primary and secondary cancers (42.5 vs 43.5, respectively, P=0.77). The proportion of mature vessels was on average 63.2% greater in brain metastases than their matched primary NSCLCs (mean 25.7% vs 88.9%, respectively, P=0.004) (Table 1; Figure 1). The proportions of mature vessels in primary and secondary cancers were not significantly correlated (r=−0.46, P=0.09). Differences were also observed in vascular patterns, with a predominance of alveolar, basal and diffuse patterns in primary tumours and a predominance of diffuse and papillary patterns in secondary tumours (Table 1).
Table 1. Scoring frequencies.
| Case |
VEGF intensity score (0 to 3)
|
CA9 (% positive)
|
Ki67 (% positive)
|
Chalkley count (n)
|
Vascular maturity (% mature)
|
Vascular pattern
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Lung | Brain | Lung | Brain | Lung | Brain | Lung | Brain | Lung | Brain | Lung | Brain |
| 1 | 2 | 2 | 0 | 20 | 10 | 50 | 34 | 51 | 36 | 96 | Basal | Papillary |
| 2 | 2 | 1 | 0 | 0 | 50 | 50 | 45 | 43 | 21 | 88 | Basal | Basal |
| 3 | 2 | 3 | 0 | 0 | 10 | 30 | 39 | 35 | 23 | 97 | Basal | Papillary |
| 4 | 2 | 3 | 0 | 0 | 0 | 15 | 44 | 62 | 4 | 97 | Alveolar | Papillary |
| 5 | 2 | 2 | 30 | 20 | 60 | 80 | 44 | 47 | 4 | 93 | Diffuse | Basal |
| 6 | 1 | 2 | 5 | 0 | 20 | 15 | 45 | 44 | 14 | 95 | Alveolar | Diffuse |
| 7 | 2 | 3 | 0 | 0 | 40 | 30 | 33 | 53 | 27 | 94 | Basal | Basal |
| 8 | 2 | 2 | 10 | 15 | 0 | 70 | 24 | 18 | 9 | 58 | Diffuse | Diffuse |
| 9 | 2 | 2 | 0 | 0 | 40 | 40 | 57 | 45 | 61 | 86 | Papillary | Papillary |
| 10 | 2 | 1 | 0 | 0 | 0 | 20 | 40 | 56 | 30 | 88 | Alveolar | Diffuse |
| 11 | 1 | 2 | 30 | 20 | 10 | 5 | 43 | 25 | 30 | 97 | Diffuse | Diffuse |
| 12 | 2 | 3 | 80 | 50 | 30 | 10 | 63 | 55 | 28 | 77 | Alveolar | Papillary |
| 13 | 2 | 1 | 50 | 30 | 5 | 40 | 43 | 39 | 40 | 89 | Basal | Diffuse |
| 14 | 1 | 2 | 5 | 5 | 5 | 40 | 48 | 35 | 12 | 94 | Diffuse | Papillary |
| 15 | 3 | 1 | 0 | 0 | 10 | 40 | 36 | 44 | 47 | 84 | Basal | Diffuse |
| Median | 2 | 2 | 0 | 0 | 10 | 40 | 43 | 44 | 27 | 93 | NA | NA |
| IQR | 2–2 | 1.5–2.5 | 0–20 | 0–20 | 5–35 | 17.5–45 | 37.5–45 | 37–52 | 13–33 | 87–95.5 | NA | NA |
Abbreviations: CA9=carbonic anhydrase 9; IQR=interquartile range; NA=not applicable; VEGF=vascular endothelial growth factor.
Figure 1.
(A) A scatter plot of CD34 Chalkley counts in matched primary (lung) and secondary (brain) cancers. (B) A line plot showing differences in the percentage of blood vessels covered by pericytes in matched primary and secondary cancers. (C) A scatter plot of the percentage of matched primary and secondary cancers positive for carbonic anhydrase 9. (D) A line plot showing differences in the VEGF score in matched primary and secondary cancers. (E, F) Double-labelled immunohistochemistry for CD34 (blue) and smooth muscle actin (brown) in a matched primary (E) and secondary (F) cancer.
The percentage of CA9-positive tumour cells was similar to that previously reported (Giatromanolaki et al, 2001) and was closely correlated between primary NSCLCs and brain metastases (r=0.825, P=0.0002), suggesting that there were similar levels of hypoxia in matched cases from this series (Table 1; Figure 1). The mean percentage of cells positive for CA9 was not statistically significantly different in primary and secondary cancers (14.0 vs 10.7, respectively, P=0.27). Vascular endothelial growth factor expression was seen in all primary and secondary NSCLCs; however, there was no agreement between the scores in matched pairs (κ=−0.25) and the association was not statistically significant (P=0.15) (Table 1; Figure 1). The proliferative fraction (percentage of Ki67-labelled cells) was also not significantly correlated between primary and secondary cancers (r=0.179, P=0.524), though the mean Ki67 was significantly higher in the brain metastases than the primary lung cancers (35.7% vs 19.3%, respectively, P=0.018) (Table 1).
DISCUSSION
This is the first report comparing VEGF expression, CA9 expression, proliferation, microvessel density, vascular pattern and vascular maturity in matched primary NSCLCs and brain metastases. The data show that brain metastases have a significantly greater proliferation rate and vascular maturity than their matched primaries. Neither the proliferation rate, vascular maturity, expression of VEGF, vascular pattern nor microvessel density could be predicted in the brain metastasis from examination of the primary NSCLC. Only CA9 expression showed a strong correlation between primary and secondary NSCLCs. These observations are important for the following reasons:
First, anti-VEGF therapies were thought to increase the risk of cerebral haemorrhage in patients with brain metastases, but recent data suggest that bevacizumab is safe in this indication. Our data provide a biological explanation for this observation, showing that the vasculature of brain metastases is stable, and (extrapolating from observations in rectal cancer) (Willett et al, 2004) is unlikely to regress following anti-VEGF therapy, minimising the risk of associated haemorrhage.
Second, mature vasculature is less sensitive to anti-VEGF therapy, suggesting that while patients with primary NSCLCs may benefit from anti-VEGF therapy, those with brain metastases may not. Moreover, preclinical data suggest that when endothelial regression is seen in mature vessels targeted by anti-VEGF therapy, a pericyte scaffold remains and permits rapid re-angiogenesis following cessation of therapy (Mancuso et al, 2006). Thus, a rebound effect may be expected if anti-VEGF therapy is used in brain metastases and continuous, rather than intermittent, therapy might show greater efficacy. No efficacy data exist for bevacizumab in brain metastases, but Socinski et al (2009) have stated that ‘patients with treated brain metastases will likely derive similar benefit from bevacizumab as patients without brain metastases’ without any biological, pathological or clinical evidence. The data herein are an important counterbalance to this claim.
Third, non-angiogenic NSCLCs (Pezzella et al, 1997) that show an alveolar pattern of growth may metastasize, and do not merely co-opt existing vasculature in the brain, but instead develop a more angiogenic phenotype with haphazardly arranged vessels. Moreover, even non-angiogenic, alveolar tumours express VEGF, are under the influence of hypoxia and are supported by an often immature, dense vasculature. Therefore, one may expect non-angiogenic tumours to potentially respond to anti-VEGF therapy at their site of origin.
Finally, retrospective analyses of archived primary tumours from trials of anti-VEGF therapy have thus far failed to identify a biomarker of efficacy (Jubb and Harris, 2010). The data presented herein suggest that the vascular phenotypes of primary and secondary cancers are very different. Therefore, when evaluating anti-angiogenic treatments in the metastatic setting, biomarker studies should also be conducted on metastases and not the primary tumours.
The limitations of this study include the sample size, which is small in statistical terms, but it is large for a series of matched brain metastases, provides informative data and reports novel findings. Furthermore, while one may make inferences regarding the applicability of these observations to anti-VEGF therapy using preclinical data (Huang et al, 2004; Mancuso et al, 2006) and clinical data from other tumours (Willett et al, 2004), only serial biopsies of brain metastases from patients receiving bevacizumab will provide a definitive answer. To date, the two reported series of NSCLC brain metastases treated with bevacizumab in the literature are small, n=36 (Besse et al, 2010) and n=115 (Socinski et al, 2009) and provide no histopathological data. Moreover, it is unlikely that clinicians would want to subject terminally ill patients to repeated invasive procedures to provide such material.
In conclusion, brain metastases of NSCLCs have a vascular phenotype that is distinct from the primary tumours. This has implications for the use of anti-VEGF therapy in this setting and should be taken into consideration when designing clinical trials to assess the efficacy of such drugs and biomarkers that may predict benefit.
Acknowledgments
We thank all patients and clinicians who provided material for this research. Dr Adrian Jubb is the recipient of a Career Development Fellowship from the Pathological Society of Great Britain and Ireland. This study was supported by Cancer Research UK, Pathological Society of Great Britain and Ireland and the NIHR Biomedical Research Centre, Oxford.
Footnotes
Dr Adrian M Jubb received a speaker's honorarium in 2010 from Genentech Inc., which is developing anti-angiogenic therapies.
References
- Besse B, Lasserre SF, Compton P, Huang J, Augustus S, Rohr UP (2010) Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res 16: 269–278 [DOI] [PubMed] [Google Scholar]
- Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL (1995) Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol 177: 275–283 [DOI] [PubMed] [Google Scholar]
- Gerstner ER, Frosch MP, Batchelor TT (2010) Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol 28: e91–e93 [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61: 7992–7998 [PubMed] [Google Scholar]
- Grothey A, Hedrick EE, Mass RD, Sarkar S, Suzuki S, Ramanathan RK, Hurwitz HI, Goldberg RM, Sargent DJ (2008) Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol 26: 183–189 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Huang J, Soffer SZ, Kim ES, McCrudden KW, New T, Manley CA, Middlesworth W, O’Toole K, Yamashiro DJ, Kandel JJ (2004) Vascular remodeling marks tumors that recur during chronic suppression of angiogenesis. Mol Cancer Res 2: 36–42 [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, Hurwitz HI, Kabbinavar F, Novotny WF, Hillan KJ, Koeppen H (2005) Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst 97: 981–989 [DOI] [PubMed] [Google Scholar]
- Jubb AM, Harris AL (2010) Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11: 1172–1183 [DOI] [PubMed] [Google Scholar]
- Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD, Hillan KJ, Koeppen H (2006) Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24: 217–227 [DOI] [PubMed] [Google Scholar]
- Jubb AM, Pham TQ, Hanby AM, Frantz GD, Peale FV, Wu TD, Koeppen HW, Hillan KJ (2004) Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol 57: 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb AM, Turley H, Moeller HC, Steers G, Han C, Li JL, Leek R, Tan EY, Singh B, Mortensen NJ, Noguera-Troise I, Pezzella F, Gatter KC, Thurston G, Fox SB, Harris AL (2009) Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer 101: 1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358: 2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841–844 [DOI] [PubMed] [Google Scholar]
- Kuramochi H, Hayashi K, Uchida K, Miyakura S, Shimizu D, Vallbohmer D, Park S, Danenberg KD, Takasaki K, Danenberg PV (2006) Vascular endothelial growth factor messenger RNA expression level is preserved in liver metastases compared with corresponding primary colorectal cancer. Clin Cancer Res 12: 29–33 [DOI] [PubMed] [Google Scholar]
- Kuzu I, Bicknell R, Harris AL, Jones M, Gatter KC, Mason DY (1992) Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J Clin Pathol 45: 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O′Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116: 2610–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357: 2666–2676 [DOI] [PubMed] [Google Scholar]
- Patel NS, Dobbie MS, Rochester M, Steers G, Poulsom R, Le Monnier K, Cranston DW, Li JL, Harris AL (2006) Up-regulation of endothelial delta-like 4 expression correlates with vessel maturation in bladder cancer. Clin Cancer Res 12: 4836–4844 [DOI] [PubMed] [Google Scholar]
- Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M, Rilke F (1997) Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol 151: 1417–1423 [PMC free article] [PubMed] [Google Scholar]
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2010) Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 21: 1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2010) Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 28: 2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550 [DOI] [PubMed] [Google Scholar]
- Sington J, Giatromanolaki A, Campo L, Turley H, Pezzella F, Gatter KC (2007) BNIP3 expression in follicular lymphoma. Histopathology 50: 555–560 [DOI] [PubMed] [Google Scholar]
- Socinski MA, Langer CJ, Huang JE, Kolb MM, Compton P, Wang L, Akerley W (2009) Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol 27: 5255–5261 [DOI] [PubMed] [Google Scholar]
- Turley H, Scott PA, Watts VM, Bicknell R, Harris AL, Gatter KC (1998) Expression of VEGF in routinely fixed material using a new monoclonal antibody VG1. J Pathol 186: 313–318 [DOI] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]