Abstract
Obesity has escalated into a pandemic over the past few decades. In turn, research efforts have sought to elucidate the molecular mechanisms underlying the regulation of energy balance. A host of endogenous mediators regulate appetite and metabolism, and thereby control both short- and long-term energy balance. These mediators, which include gut, pancreatic and adipose neuropeptides, have been targeted in the development of anti-obesity pharmacotherapy, with the goal of amplifying anorexigenic and lipolytic signaling or blocking orexigenic and lipogenic signaling. This article presents the efficacy and safety of these anti-obesity drugs.
Keywords: appetite control, neuropeptides, obesity, pharmacotherapy
The unmet clinical need
Obesity is now a global pandemic [1]. Worldwide, more than 1 billion adults are overweight (BMI >25 kg/m2), while 300 million adults are obese (BMI >30 kg/m2) [2]. In the USA, 65% of adults are overweight, and 32.2% are obese [3,4]. This obese population has doubled in only 20 years [5]. Moreover, obesity rates in children have achieved epidemic levels in developed countries and continue to grow worldwide [6,7]. Obesity is associated with striking comorbidities, including cancer, coronary artery disease, hypertension, liver/biliary disease, obstructive sleep apnea, osteoarthritis, stroke and Type 2 diabetes. In that context, life expectancy with chronic obesity is significantly shortened [8]. Notably, chronic obesity is associated with higher rates of health problems and healthcare costs compared with smoking or alcohol abuse [9]. Currently, up to US$100 billion of annual healthcare expenses in the USA can be ascribed to obesity. Within the next 15 years, 20% of US national health-care costs will reflect the care of chronic diseases related to obesity [10].
Therapeutic approaches to the obese patient
Medical treatment for obese patients has largely focused on complications and comorbidities, such as diabetes, hypertension and hyper-lipidemia. Studies have shown, however, that targeting the underlying disease through weight loss and lifestyle modifications are effective in combating chronic comorbidities, such as cardiovascular disease and diabetes. Lifestyle modification programs and clinical intervention have succeeded in driving approximately a 10% weight reduction within 6 months [11]. In that regard, even a modest loss of weight (<10%) significantly improves blood pressure, cholesterol levels and glycemic control [12,13]. Unfortunately, patients enrolled in lifestyle modification programs typically regain about 35% of their lost weight within 1 year following treatment, while >50% of patients return to their baseline weight in fewer than 5 years [14,15].
Bariatric surgery generates the most rapid and sustained weight loss. Through gastric banding, gastric bypass or sleeve gastrectomy, bariatric surgery achieves long-term weight loss in patients, reduces their risk for obesity-related comorbidities and thereby improves their lifestyle [16,17]. Unfortunately, this treatment is associated with a number of principal adverse effects and, consequently, is recommended only for patients who are morbidly obese (BMI >40 kg/m2) or suffering serious comorbidities. Therefore, bariatric surgery is an unlikely treatment for the millions of obese patients worldwide [16,18]. However, clinical trials are underway for transoral gastroplasty, in which the stomach is stapled or sutured to reduce its capacity via oral insertion of flexible devices, thereby eliminating surgical incisions [19].
Anti-obesity pharmacotherapy may represent the means by which overweight and obese patients can safely achieve long-term weight reduction. For example, clinical trials have demonstrated that the weight-loss drug orlistat (Alli® [GlaxoSmithKline]; Xenical® [Roche]), which is a lipase inhibitor and prevents some of the fat in food from being absorbed, can produce weight loss that can be sustained for up to 2 years [20–22]. Those observations notwithstanding, the history of anti-obesity pharmacotherapy is characterized by setbacks and dilemmas over safety, efficacy, abuse and adverse effects. For example, the potentially fatal adverse effects of valvular heart disease and pulmonary hypertension of the anti-obesity medication fenfluramine–phentermine (Fen–Phen) led to its withdrawal and legal damages of more than US$13 billion [23]. In fact, with the withdrawal of sibutramine (Meridia®; Abbott) from the market after 13 years owing to cardiovascular risks, orlistat has recently become the only drug that is US FDA approved for long-term use in weight management [24–28].
Anti-obesity drug development research has focused on the regulation of appetite and energy consumption. Endocannabinoid signaling and monoamine neurotransmission are involved in this regulation, but many centrally-acting drugs directed at these pathways induce major cardiovascular and psychological adverse effects. Hormonal regulators, including neuropeptides from gut, pancreatic and adipose tissue, serve as endogenous mediators of energy balance. By targeting these pathways, drug development programs aim to minimize central or peripheral adverse effects, while driving appetite reduction and weight loss. This article discusses the efficacy and safety of these anti-obesity pharmacotherapeutics.
Central appetite regulation
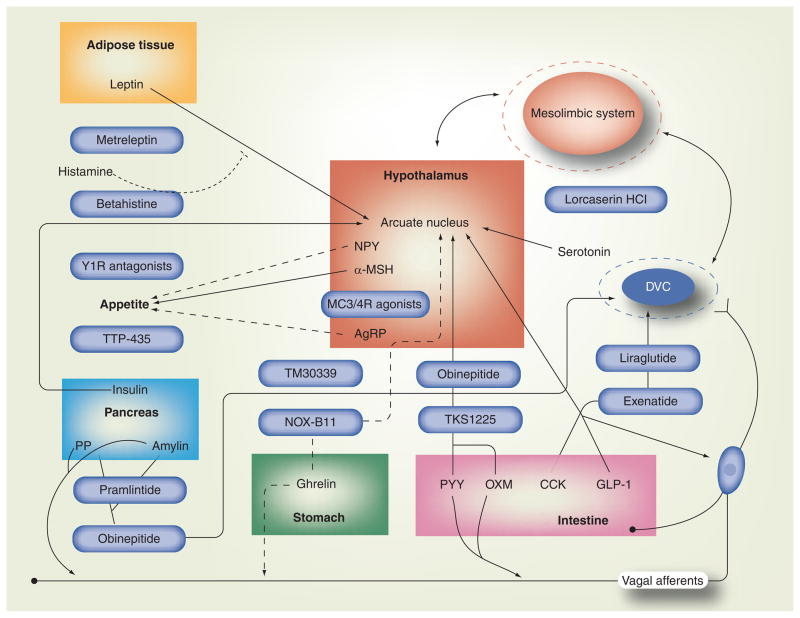
The hypothalamus integrates neurohormonal signaling from gut and adipose tissue. Specific hypothalamic nuclei, including the dorsomedial nucleus (DMN), paraventricular nucleus (PVN) and ventromedial nucleus (VMN), serve as control centers for appetite. In animals, lateral hypothalamic lesions produce anorexia while VMN lesions produce hyperphagia [29–33]. In that context, more recent studies have demonstrated that appetite reflects the integration of orexigenic and anorexigenic signals from numerous hypothalamic nuclei and tissues outside the CNS (Figure 1).
Figure 1. Satiety regulation by endogenous hormones and therapeutic intervention.
Appetite is regulated by integrated neuronal circuits between the hypothalamus, mesolimbic system and DVC. Endogenous hormones regulate appetite by directly signaling to the arcuate nucleus in the hypothalamus or indirectly to the DVC in the brainstem, which then communicates with the hypothalamus. Appetite is also affected by reward mechanisms predominately regulated by neuronal signaling in the mesolimbic system, which has projections to the hypothalamus. Secretion of appetite-stimulating neurohormones, NPY and AgRP, is activated by ghrelin and inhibited by leptin, insulin, GLP-1, OXM and PYY. The α-MSH released from POMC/CART neurons in the arcuate nucleus is appetite-suppressing. Leptin, GLP-1, OXM and serotonin act on POMC/CART neurons to promote α-MSH-mediated suppression of appetite. CCK, GLP-1, PP and amylin induce satiety by activating appetite-suppressing neurons in the DVC directly or indirectly through vagal afferents. Hormones are color-coded by origin. Drugs targeting specific pathways are represented by blue capsules. Solid lines: appetite-suppressing; dashed lines: appetite-stimulating.
α-MSH: α-melanocyte-stimulating hormone; AgRP: Agouti-related protein; CART: Cocaine- and amphetamine-regulated transcript; CCK: Cholecystokinin; DVC: Dorsal vagal complex; GLP-1: Glucagon-like peptide-1; NPY: Neuropeptide Y; OXM: Oxyntomodulin; POMC: Pro-opiomelanocortin; PP: Pancreatic polypeptide; PYY: Peptide YY.
The arcuate nucleus (ARC) is the major target for peripheral hormones that regulate appetite. The ARC is located at the base of the hypothalamus, outside the blood–brain barrier. As it is surrounded by a permeable barrier, the ARC is accessible to circulating hormones [34]. The ARC contains two distinct neuronal subtypes that are critical for appetite regulation:
Those expressing the neuropeptides pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART);
Those expressing neuropeptide Y (NPY) and Agouti-related protein (AgRP).
Pro-opiomelanocortin/CART-expressing neurons suppress appetite, while NPY/AgRP neurons stimulate appetite. It is the balance between these neuronal signals that regulates energy homeostasis [34–36].
Pro-opiomelanocortin is a precursor polypeptide that is cleaved to yield several hormones, including melanocortins such as α-melanocyte stimulating hormone (α-MSH). Melanocortins control appetite by activating the melanocortin 3 (MC3R) and melanocortin 4 (MC4R) receptors on second-order neurons. Intracerebroventricular (ICV) administration of MC3R and MC4R agonists reduces food intake, while antagonist administration produces hyperphagia [37]. Elimination of MC4R expression in transgenic mouse models produces overeating and obesity [38]. Furthermore, polymorphisms of MC4R are associated with obesity [39].
Cocaine- and amphetamine-regulated transcript is coexpressed with α-MSH and has similar anorexigenic properties. Fasting produces a significant decline in CART expression. ICV administration of CART peptide suppresses feeding, while ICV administration of antiserum to CART stimulates food intake [40]. Some reports, however, have demonstrated that CART injection into specific hypothalamic nuclei produces an orexigenic phenotype [41], indicating that CART’s effects depend on signaling location.
Neuropeptide Y belongs to the pancreatic polypeptide family, which activates the seven-transmembrane G protein-coupled receptors (GPCRs) Y1–Y6 [42]. NPY is a potent orexigenic neuro-peptide. Its expression is controlled by nutritional status, and mRNA levels are increased during fasting and reduced following food intake [43,44]. Signaling pathways mediating NPY effects have yet to be definitively characterized. Nevertheless, Y1- and Y5-receptor activation appears to stimulate appetite [45], while Y2- and Y4-receptor activation suppresses food intake via presynaptic inhibition of NPY release [46].
Agouti-related protein (AgRP) expression is localized to the ARC [47]. AgRP is a selective inverse agonist of MC3R and MC4R and a powerful appetite stimulant. AgRP levels rise during fasting and decline following food intake [43]. ICV administration of AgRP stimulates food intake [48,49]. Mice overexpressing AgRP develop hyperphagia and obesity [50].
From the ARC, these first-order POMC/CART and NPY/AgRP neurons project to second-order neurons in several hypothalamic nuclei, including the VMN, DMN, PVN and the lateral hypothalamic area. These second-order neurons subsequently project to such areas as the caudal brainstem, cortex and limbic system, and thereby act to process and integrate feeding signals. Lesions in these hypothalamic nuclei result in hyperphagia (PVN, DMN and VMN) or hypophagia, and demonstrate the significance of these second-order neurons in generating hunger and satiety responses [29–33].
Second-order neurons in these hypothalamic nuclei are crucial to regulation of feeding, and express potent chemical mediators themselves to serve this function. Corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH) are anorexigenic peptides expressed in the PVN. NPY/AgRP signaling downregulates expression of CRH and TRH, and α-MSH signaling, in turn, upregulates their expression [51–53]. Melanin-concentrating hormone (MCH) is expressed in the lateral and perifornical hypothalamus. As a potent orexigenic neuropeptide, MCH levels rise during fasting and stimulate appetite with ICV administration [54]. Orexin A and B are a pair of neuropeptides that also are expressly found in the lateral hypothalamus and perifornical areas, and function to stimulate appetite [34]. Brain-derived neurotrophic factor (BDNF) has a wide tissue distribution, but has a notably high expression level in the VMN. In rodents, central BDNF administration produces a decline in weight and appetite [55]. In humans, a mutation in NTRK2, which encodes TrkB, the BDNF receptor, causes hyperphagia and obesity [56]. Activation of MC4R upregulates BDNF expression and signaling [57], which suggests that central melanocortins may curtail appetite by activating downstream BDNF effectors.
Extensive reciprocal circuits between the hypothalamus and brainstem relay information regarding feeding status. Of note, the dorsal vagal complex (DVC) receives signals from peripheral satiety hormones and vagal afferents from the GI tract [58,59]. In addition, dopamine reward pathways play a role in feeding. Mice deficient in dopamine exhibit hypophagia, and recover with dopamine replacement in the caudate putamen and nucleus accumbens [60]. Furthermore, opioid signaling pathways regulate feeding behavior. In mice, deficiency in the opioid receptor ligands β-endorphin and enkephalin diminish food-seeking behavior [61]. Finally, the endocannabinoid system, notably through central CB1 receptors, stimulates appetite and promotes lipogenesis in the hypothalamus, mesolimbic system and periphery [62]. In the following section, we will discuss the anti-obesity drugs that the pharmaceutical industry has in various stages of clinical development; Table 1 lists the prominent identifying and distinguishing features of these pharmacotherapeutics.
Table 1.
Therapeutic intervention targeting central appetite regulation signaling.
| Drug | Company | Clinical trial status | Mechanism of action | Effects | Ref. |
|---|---|---|---|---|---|
| TTP-435 | TransTech Pharma | Phase II | AgRP inhibitor, increasing MC3/4R signaling | Reduced food intake, bodyweight and insulin levels in animal models | [301,318] |
| RM-493 | Rhythm | Preclinical | MC3/4R agonist, mimicking the effect of α-MSH | Reduced food intake, bodyweight and insulin resistance in preclinical studies | [319] |
| Spiegelmer® (NOX-B11) | Pfizer | Preclinical | Bind to and inhibit ghrelin | Reduced food intake and c-Fos induction in arcuate nucleus in rats and mice. However, other fasting-related signaling might mask the loss of the ghrelin effect | [201, 320] |
| GI 181771X | GlaxoSmithKine | Terminated | CCK-A agonist, mimicking the effect of CCK | Clinical trials failed | [82] |
| Sitagliptin (MK-0431) | Merck | Approved for diabetes | DDP-IV inhibitor, increasing GLP-1 signaling | Reduced weight gain and potentiated the secretion of insulin † Side effects include rare nausea, common cold-like symptoms and pancreatitis |
[97,321] |
| Vildagliptin (LAF237) | Novartis | Phase III | DDP-IV inhibitor, increasing GLP-1 signaling | Reduced hyperglycemia † Skin lesions and kidney impairment in animal models. Additional safety data from clinical trials are required |
[97,322] |
| Pramlintide | Amylin | Approved for diabetes | Amylin analog | Decreased blood glucose level and bodyweight † Side effects include nausea, hypoglycemia, vomiting, headache, abdominal pain and fatigue |
[62,323] |
| Exenatide | Amylin/Eli Lilly | Approved for diabetes | GLP1R agonist, GLP-1 mimicking | Decreased blood glucose level and bodyweight † Side effects include gastrointestinal symptoms, acute pancreatitis, dizziness and headache. May increase risks of sulfonylurea-induced hypoglycemia and thryroid cancer |
[97,98,100, 304,305,324] |
| Liraglutide (NN2211) | Novo Nordisk | Approved for diabetes | GLP1R agonist, GLP-1 mimicking | Maintained normal blood glucose and bodyweight † Side effects including increased risks of C-cell carcinoma and thyroid C-cell focal hyperplasia were observed in rats and mice |
[97,303, 325–327] |
| NN9924 | Novo Nordisk | Phase I | GLP1R agonist, GLP-1 mimicking | Variant of liraglutide | [328] |
| TKS1225 | Thiakis/Wyeth/Pfizer | Phase I | GLP1R agonist, OXM mimicking | Reduced insulin resistance, appetite and bodyweight preclinical models | [329,330] |
| Histalean (Betahistine) | OBEcure Ltd. | Phase II | H1 receptor agonist H3 receptor antagonist |
Weight loss and decreased appetite † Side effects include headache and nausea |
[331,332] |
| SCH-497079 | Schering–Plough | Phase II | Histamine receptor antagonist | No study results posted | [333,334] |
| Metreleptin | Amylin/Takeda | Phase III | Leptin receptor agonist | Weight loss † Side effects include nausea and injection site adverse events |
[310,311,335] |
| BMS-830216 | Bristol-Myers Squibb | Phase I/II | MCH receptor antagonist | The Phase I/II studies is anticipated to be completed in 2011. No study results posted | [302,336] |
| ALB-127158 | AMRI | Phase I | MCH1 antagonist | The Phase I study is anticipated to be completed in 2011. No study results posted | [337] |
| Qnexa® (phentermine/topiramate) | Vivus | Phase III | Norepinephrine-releasing agent GABA receptor activator |
Weight loss Currently under review for approval. Possible side effects include suicidal thoughts, heart palpitations, memory lapses and birth defects |
[338] |
| Empatic™ (zonisamide SR/bupropion SR) | Orexigen | Phase II | Norepinephrine/dopamine reuptake inhibitor GABA receptor activator |
Weight loss and reduced metabolic syndrome † Side effects include birth defects. Possible side effects in cognitive function, thoughts of suicide or depression were not significantly different from placebo |
[339] |
| Contrave® (bupropion SR/naltrexone SR) | Orexigen | Rejected for approval in February 2011 | Norepinephrine/dopamine reuptake inhibitor GABA receptor activator |
Weight loss, decreased appetite and reduced metabolic syndrome † Side effects include headaches and constipation. Concerns are cardiovascular safety and psychological issues |
[340,341] |
| Taranabant (MK-0364) | Merck | Phase III terminated | Cannabinoid receptor inverse agonist | Study stopped due to high level of control side effects, mainy depression and anxiety | [202,342] |
| Lorcaserin HCI | Eisai | Rejected for approval in October 2010 | Selective 5HT2c receptor agonist | Limited weight loss efficiacy and possible increase in cancer risk † Side effects include headache, infection, sinusitis, nausea, depression, anxiety and suicidal thoughts. Possible concerns of cancer risk |
[199,316,343] |
| Tesofensine (NS2330) | NeuroSearch | Phase III | Serotonin-norepinephrine/dopamine reuptake inhibitor | Weight loss † Side effects include dry mouth, headache, nausea, insomnia, diarrhea and constipation |
[344,345] |
| TM30339 | 7TM Pharma | Phase I/II | Y4R agonist in DVC, Y2R agonist in arcuate nucleus | Weight loss and reduced metabolic syndrome. The study is ongoing and no study results posted | [309,346] |
| Obinepitide | 7TM Pharma | Phase II | Y4R agonist in DVC, Y2R agonist in arcuate nucleus | Weight loss and reduced metabolic syndrome. No study results posted | [308,347] |
| PYY (3-36) | Merck | Phase II | Y4R agonist in DVC, Y2R agonist in arcuate nucleus | The Phase II trial does not meet weight loss end point | [42,94,112– 118, 348] |
| MK-0557 | Merck | Phase II | Y5 receptor antagonist, NPY blocker | No significant weight loss compared with placebo | [65,349] |
| Velneperit (S-2367) | Shionogi USA | Phase II | Y5 receptor antagonist, NPY blocker | Reduced bodyweight † Side effects include nasopharyngitis, upper respiratory infection, sinusitis and headache |
[350,351] |
Adverse effect(s) of the drug.
α-MSH: α-melanocyte stimulating hormone; AgRP: Agouti-related protein; CCK: Cholecystokinin; DPP-IV: Dipeptidyl peptidase IV; DVC: Dorsal vagal complex; GABA: γ-aminobutyric acid; GLP-1: Glucagon-like peptide 1; GPL1R: Glucagon-like peptide 1 receptor; MC3/4R: Melanocortin 3/4 receptor; MCH: Melanin-concentrating hormone; NPY: Neuropeptide Y; OXM: Oxyntomodulin; Y2R: Neuropeptide Y receptor subtype 2.
Antagonists of central neuropeptide signaling
Neuropeptide Y
Neuropeptide Y regulates feeding through Y1- and Y5-receptors. NPY signaling inhibition reduces food intake and bodyweight in mice [63,64]. In patients, the NPY receptor antagonist MK-0557 (Merck; 1 mg/day) induced modest weight loss over the initial 12-week period of administration. Following 52 weeks of therapy, MK-0557 produced a statistically significant, but not clinically meaningful (less than 3 kg), weight loss [65].
Other appetite modulators
The AgRP inhibitor TTP-435 (TransTech Pharma), in Phase II trials, antagonizes the activity of AgRP with no effect on MC4R alone or in the presence of α-MSH [301]. In addition, the MCH-1 receptor antagonist BMS-830216 (Bristol-Myers Squibb) is in Phase I/II clinical testing [302]. The MC4R agonist MK-0493 (Merck) induced a reduction in food intake and minimized weight gain in rodents with diet-induced obesity. However, in Phase II clinical trials, MK-0493 demonstrated only statistically insignificant weight loss relative to placebo in both a 12-week fixed-dose study as well as an 18-week stepped-titration study [66]. As MC4R agonism has been a promising target of anti-obesity pharmacotherapy, drug development studies continue to search for selective small agonists for MC4R. Deficiency of MC4R signaling due to over 150 different mutations is a well-characterized genetic basis of obesity, and potential therapeutics including exogenous agonists and molecular chaperones continue to be investigated in preclinical studies [67]. However, given the extensive and complex activity of melanocortins in not only energy homeostasis but also cardiovascular and sexual function, significant concerns remain over the potential adverse effects of long-term MC4R agonism, including hypertension and sexual arousal [68]. While neuromedin U (NMU) originally looked promising as an anorexigenic, inducing the release of CRH by neurons of the PVN [69], administration of NMU does not reduce food intake or weight [70], and diet-induced obese rats are relatively insensitive to the effects of NMU [71].
Therapeutic approaches to regulating food intake
Intestinal peptides
Cholecystokinin
Cholecystokinin (CCK) is produced by I cells in the duodenum and jejunum, and serves a variety of functions, including that of a neurotransmitter in the CNS [72]. CCK secretion by the gut is stimulated by foods high in fat and protein, and aids digestion [73]. The CCK receptor, a seven-transmembrane GPCR, exists as two different isoforms, CCK-A and CCK-B. CCK-A is expressed in the pyloric sphincter and in vagal afferents. It appears to be responsible for the effect of CCK signaling on satiety, and CCK-A agonists suppress appetite [74,75]. In rodents, deficiency of CCK-A or receptor blockade produces hyperphagia and obesity [76]. In humans also, treatment with the CCK-A receptor antagonist, loxiglumide, drives increased caloric intake [77]. However, targeted antagonism of CCK-B appears to have no effect on CCK-mediated appetite reduction [78]. With chronic administration, tolerance to CCK develops [79], eliminating its ability to reduce weight [80]. Moreover, intermittent CCK reduces the size, but increases the frequency, of meals [81]. In that context, the selective CCK-A agonist GI 181771X (GlaxoSmithKline) failed to induce weight loss in Phase II trials [82]. However, CCK potentiates appetite and weight reduction by leptin [83], suggesting that combination therapy may have utility. It is noteworthy that chronic CCK administration in animals produced pancreatitis [84,85], suggesting limited utility of this therapeutic approach in humans.
Glucagon-like peptide 1
Glucagon-like peptide 1 (GPL-1) is produced by enteroendocrine L cells in the ileum and proximal colon. It is generated after processing of preproglucagon by prohormone convertase-1 [86]. Its secretion is stimulated by nutrients and neural and endocrine factors [87] after ingestion of a meal, particularly one rich in fat and carbohydrates [88]. The GLP-1 receptor (GLP-1R) is a GPCR expressed in the heart, kidney, lung, pancreas, CNS and PNS, including the nucleus tractus solitarius (NTS) of the DVC, and the ARC [86]. GLP-1 is an incretin, which are gut hormones that increase secretion of insulin by pancreatic β-cells [87]. GLP-1 is anorexigenic. Its administration induces satiety and weight loss in animals and humans [89–92]. In rats, GLP-1 effectiveness was lost after monosodium glutamate-induced lesions in the ARC [93], subdiaphragmatic vagotomy and transection of brainstem–hypothalamic connections [94], suggesting that hypothalamic and vagal signaling are essential for mediating the satiating effects of GLP-1. Moreover, GLP-1 antagonizes the orexigenic effects of NPY [95].
Glucagon-like peptide 1 has a characteristic short circulating half-life, reflecting rapid proteolysis by dipeptidyl peptidase IV (DPP-IV) [96], limiting its therapeutic utility in patients. This pharmacokinetic limitation has been abrogated by employing two different approaches. GLP-1 analogues resistant to DPP-IV degradation have been developed, including liraglutide (Victoza®; Novo Nordisk) and exenatide (Byetta®; Amylin/Eli Lilly), comprising exendin-4, a peptide extracted from the salivary gland of the Heloderma suspectum(gila monster). Furthermore, orally active DPP-IV inhibitors, vildagliptin (Novartis) and sitagliptin (Januvia®; Merck) have been developed. A meta-analysis examining these approaches in Type 2 diabetic patients revealed that, compared with patients receiving insulin therapy, patients receiving GLP-1 analogues lost an average of 4.76 kg [97]. Indeed, weight loss induced by GLP-1 analogues was dose dependent, progressive and did not plateau by 30 weeks. Further to this, more weight was lost in patients receiving exenatide compared with liraglutide [97]. Moreover, DPP-IV inhibitors were less effective in inducing weight loss compared with GLP-1 analogues [97]. Similarly, in nondiabetic obese individuals, liraglutide induced a mean of approximately 6.0 kg in weight loss and >35% of the subjects treated with the highest dose achieved a reduction of ≥10% baseline weight [303]. As a result, liraglutide was approved by the EMA in 2009 and the US FDA in 2010 for the treatment of Type 2 diabetes, and is in trials to win FDA approval for the treatment of obesity. Recently, long-acting exenatide (exenatide-LAR) was shown to improve bodyweight during a 15-week trial in diabetic patients [98]. Following 2 years of weekly treatment, exenatide-LAR induced average reductions in bodyweight of 5.8 lbs [304]. To improve convenience and patient compliance, nasal and transdermal formulations of exenatide are being developed [305]. In that context, oral formulations of GLP-1 are being developed, and oral administration induces a rapid dose-dependent rise in circulating peptides, which are active [99]. With respect to adverse events, severe hypoglycemia was rare, but mild-to-moderate hypoglycemia was more than twice as common in patients receiving GLP-1 analogues [97]. In patients receiving exenatide, nausea and vomiting were the most common adverse effects [97]. Mild-to-moderate nausea was also the most common adverse effect reported with weekly treatment of exenatide-LAR [98]. Furthermore, exenatide should not be used in patients with renal disease, and kidney function should be monitored in patients receiving this medication [100].
Oxyntomodulin
Oxyntomodulin (OXM), a product of the gut, is also produced from processing of preproglucagon. OXM is secreted post-prandially along with GLP-1 by the L cells of the colon. It demonstrates weak activity as an incretin, but induces potent appetite suppression. In rats, ICV or intraperitoneal administration of OXM produced diminished feeding and weight gain [101,102]. In humans, intravenous infusion of OXM suppressed appetite and feeding, without a significant change in circulating insulin levels [103]. OXM may mediate its effects via activation of central GLP-1Rs. The anorectic effects of OXM are abolished both in GLP-1R knock out mice [104] and with administration of the GLP-1R antagonist exendin (9–39), even with concomitant ICV OXM administration or OXM injection into the PVN [101]. Another OXM receptor, however, may exist: compared with GLP-1, OXM binds GLP-1R with 100-fold lower affinity, but exerts the same degree of anorexia at equimolar concentrations [102]. Furthermore, intra-ARC administration of exendin (9–39) blocks the anorectic effects of OXM, but has no effect on GLP-1 activity [102]. Perhaps OXM stimulates ARC neurons directly, while GLP-1 does so indirectly through connections with the brainstem. The role of OXM in the ARC may be to stimulate POMC neurons, as the incubation of POMC neurons with OXM ex vivo stimulates α-MSH release [102]. Intravenous infusion of OXM (3.0 pmol/kg/min) reduced food intake by 19% compared with saline-infused subjects. Furthermore, OXM infusion reduced 12-h food intake by 11%, without affecting 24-h food consumption [103]. Subcutaneous injection of OXM (three-times daily, 30 min before each meal for 4 weeks) significantly reduced food consumption at the beginning and end of the 4-week trial, inducing an average weight loss of 2.3 kg [105]. In overweight and obese subjects, OXM administered before meals increased activity-related energy expenditure by 26% and total energy expenditure by 9.5% in addition to reducing food consumption [106]. Inducing an increase in physical activity is noteworthy, as weight loss can be achieved by promoting greater energy expenditure than energy intake. Many stimulants are well-characterized drugs that effectively increase physical activity, suppress appetite and promote weight loss. However, stimulants are also well known to pose serious risks such as addiction, hypertension and cardiovascular damage. Therefore, OXM might represent a safer means of stimulating an increase in energy expenditure. However, as injections of OXM are required to induce weight loss, this is considered to be a barrier to therapy. Regarding adverse effects, this agent rarely induced mild nausea [105,106].
Peptide YY
Peptide YY (PYY) belongs, along with NPY, to the pancreatic polypeptide family, which bind to the GPCRs Y1–Y6 [42]. However, in contrast to NPY, PYY is potently anorexigenic. PYY is expressed throughout the small intestine, with the highest concentration found in L cells of the terminal ileum and colon, which secrete the peptide in response to a meal [107]. PYY stimulates gastrointestinal absorption of fluids and electrolytes [108], reduces gastric and pancreatic secretions and delays gastric emptying [109]. In rodents, administration of PYY induces a dose-dependent decrease in food intake [110–112]. PYY-deficient mice display hyperphagia and obesity [113]. Obese humans and rodents have lower circulating levels of postprandial PYY compared with lean controls [114]. Notably, however, obese subjects achieve a progressive rise back to normal plasma PYY levels following bariatric surgery. This phenomenon has been implicated in the success of bariatric surgery in producing long-term weight loss. Regarding PYY, obesity engenders a state of deficiency rather than resistance, which is the converse of obesity’s effects on leptin. Thus, PYY replacement therapy is an attractive concept for treatment. PYY circulates as two major forms: PYY1–36 and PYY3–36. The more common PYY3–36 exhibits high affinity for Y2R, and some affinity for the Y1R and Y5R [42]. Peripheral PYY administration induces appetite suppression by activating Y2R in the ARC. ICV administration, however, stimulates food intake, presumably due to PYY activation of orexigenic Y1R and Y5R in second-order neurons of the PVN [115]. Therefore, PYY conceivably suppresses appetite by activating presynaptic Y2R, which inhibits the activity of NPY/AgRP neurons. Vagal afferent signaling, too, is implicated, as bilateral subdiaphragmatic vagotomy or transecting brainstem–hypothalamic connections attenuates the anorectic effects of PYY [94]. Continuous infusion of PYY in healthy subjects reduced hunger and caloric intake by 36% [112], and obese patients behaved similarly [116]. Indeed, infusion of PYY reduced food consumption in a dose-dependent manner, with a maximum inhibition of 35% [117]. Unfortunately, continuous intravenous infusion is not a tractable approach for weight-loss therapy, and an intranasal formulation of PYY (Nastech/Merck) was ineffective in inducing weight loss [118]. Moreover, PYY produces nausea and vomiting in a dose-dependent manner, limiting its therapeutic utility in appetite suppression [117,118].
Ghrelin
Ghrelin is the only known circulating orexigenic hormone. Ghrelin is cleaved from preproghrelin and is mainly produced in the gastric fundus. It has been reported to stimulate the release of growth hormone by activating the growth hormone secretagogue receptor (GHS-R) [119]. As ghrelin deficiency does not translate into defective growth in mice, however, its physiological relevance on growth hormone release is unclear [120]. Ghrelin plays a role in energy balance. In rodents, ICV or peripheral administration induces a dose-dependent increase in food intake and bodyweight [121,122]. Ghrelin also regulates long-term energy homeostasis. Obese patients display reduced circulating ghrelin levels and anorexic patients display exaggerated circulating ghrelin levels. Weight gain correlates with a decline in ghrelin levels [123–125]. The receptor GHS-R1a is expressed throughout the CNS, notably within certain hypothalamic nuclei, the pituitary gland and the hippocampus. GHS-R1a is also expressed, albeit at lower levels, in the adrenal glands, heart, pancreas, spleen and thyroid [126,127]. Ghrelin is believed to induce hunger and feeding by activating NPY/AgRP neurons in the ARC. Vagal stimulation is also important. In rats with mechanical or chemical disruption of vagal signaling, ghrelin administration fails to stimulate feeding or activate NPY-expressing neurons [128]. In fact, ghrelin appears to function at several sites. Ghrelin induces food intake when injected into other CNS sites expressing GHS-R, including the mesolimbic reward pathway, the hippocampus and the dorsal raphe nucleus [129,130]. A variety of therapeutic approaches to blocking ghrelin’s action are being explored as a strategy for treating obesity. A first-generation ghrelin vaccine, CYT009-GhrQb, was discontinued because patients did not lose weight, even though they showed a strong ghrelin antibody response [306]. The next generation of ghrelin vaccine has been developed that decreases feeding, adiposity and bodyweight in rodents [131]. Furthermore, a ghrelin-neutralizing RNA Spiegelmer®, NOX-B11 (NOXXON Pharma Ag), which is an aptamer that binds to and inactivates ghrelin, blocked the orexigenic activity of exogenous ghrelin administration but did not alter food intake in rats [132]. Furthermore, ghrelin antagonists, produced by Elixir Pharmaceuticals, are in preclinical testing [307]. Moreover, ghrelin O-acyltransferase (GOAT) is a membrane-bound enzyme that adds octanoate to ghrelin, which is required for receptor binding, and inhibition of GOAT may be an effective strategy to inhibit ghrelin activity [133].
Pancreatic hormones
Pancreatic polypeptide
Pancreatic polypeptide (PP) is homologous to PYY, possibly originating as a duplication of the PYY gene [134]. PP levels in the circulation rise after eating, increasing proportionally to caloric intake, and remain elevated for up to 6 h [135]. PP secretion is induced by vagal stimulation and peripheral hormones, including ghrelin [136]. PP administration to obese mice decreases appetite [137] and repeated administration limits their weight gain [138]. PP administration to lean mice also suppresses feeding associated with delayed gastric emptying [138]. Similarly, overexpression of PP in transgenic mice suppresses eating, gastric emptying and weight gain [139]. Moreover, fasting- and food-induced PP levels are lower in obese patients [140], while PP responses are exaggerated in patients with anorexia nervosa [141]. PP binds with highest affinity to the Y4- and Y5-receptors [42]. As with PYY, the route of PP administration affects its impact on appetite. In rats, ICV administration of PP increases feeding [142], reflecting activation of orexigenic Y5R [64]. By contrast, peripheral administration of radiolabeled PP reveals significant accumulation in the area postrema (AP) of the DVC, which expresses Y4-receptors [143,144]. Vagotomy abolishes PP’s anorectic effects in mice [138]. Peripheral administration of PP increases vagal activity and induces changes in the levels of hypothalamic neuropeptides, including decreasing NPY and orexin, and increasing the anorexigenic peptide, urocortin [138]. In healthy subjects, intravenous infusion of PP (10 pmol/kg/min) reduced appetite and caloric intake by 22%, an effect that was sustained over 24 h [145]. PP has a short half-life, and extended-duration formulations of Y2R or Y4R agonists may be more efficacious in long-term appetite control and weight loss [146]. Obinepitide (7TM Pharma), a Y2/Y4-receptor agonist, and TM30339 (7TM Pharma), a selective Y4-receptor agonist, are in Phase I/II clinical trials [308,309].
Amylin
Amylin, or islet amyloid polypeptide, is secreted with insulin by β-cells [147], and patients with Type 1 diabetes are deficient in both hormones. Like insulin, fasting plasma levels of amylin are low and increase in response to eating [148,149]. Amylin regulates post-prandial glucose levels together with insulin. Beyond glucose homeostasis, amylin has anorectic characteristics, and ICV administration reduced food intake in rodents, while constant infusion over 10 days reduced feeding and adiposity [150]. Conversely, pharmacologic antagonism of amylin signaling increased rodent appetite and adiposity [151], and amylin-deficient mice gain excess weight [152,153]. Amylin is homologous to calcitonin gene-related peptide, calcitonin and adrenomedullin [154,155], and amylin receptors appear when calcitonin receptors are coexpressed with receptor activity-modifying proteins [156,157]. Amylin receptors are expressed in selective regions of brain, including the AP [158]. The effects of amylin on gastric emptying and appetite are attenuated by vagotomy or injury of the AP/NTS, suggesting that vagal signaling is essential in mediating the appetite suppression induced by amylin [159–161]. Pramlintide, a synthetic amylin analogue (Symlin®; Amylin) [162] has a pharmacokinetic and pharmaco-dynamic profile that is similar tothat of amylin [163]. Pramlintide is approved to treat diabetes and, unlike traditional diabetic medications, elicits weight loss in diabetic patients. Thus, subcutaneous injection of pramlintide with meals reduced bodyweight over 52 weeks of treatment in Type 2 diabetic patients [164,165]. Similarly, pramlintide produced bodyweight reductions in Type 1 diabetic patients [166]. Finally, a pooled post hoc analysis in Type 2 diabetic subjects demonstrated that pramlintide, at 120 μg twice daily or 150 μg three-times daily, induced an average weight loss of 2.6 kg over 52 weeks of therapy [167]. The only adverse effects associated with pramlintide were a transient increase in mild-to-moderate nausea and headache [164–167].
Adipose tissue hormones
Leptin
Leptin, an adipose tissue-derived hormone, has been labeled the ‘obese gene’ (ob), since mice harboring mutations develop morbid obesity [168]. Administration of leptin to ob/ob mice decreases consumption, increases energy expenditure and is associated with a 30% decrease in weight following 2 weeks of therapy [169,170]. Similarly, congenital leptin deficiency in humans manifests as early-onset obesity, which is treated with leptin replacement [171,172]. Circulating levels of leptin reflect both the degree of adiposity [173] and the feeding state [174]. Typically, obese patients exhibit elevated circulating leptin levels [173], which can be confirmed in rodent models of obesity. Elevated circulating leptin in obesity reflects leptin receptor resistance. Leptin receptors, members of the gp130 family of cytokine receptors, are expressed in the hypothalamus [175]. Leptin receptors activate janus kinase [176], which, in turn, activates signal transducer and activator of transcription-3, increasing the expression of POMC, while reciprocally suppressing the expression of AgRP [177]. Similarly, activated janus kinase phosphorylates insulin receptor substrate proteins, stimulating the phosphoinositide 3-kinase pathway, which also suppresses NPY and AgRP, while increasing POMC. Furthermore, 5′-AMP-activated protein kinase, an energy-sensing protein, which is active in low energy states and stimulates feeding, is inhibited in multiple areas of the hypothalamus by leptin receptor activation [178]. Leptin regulation of appetite is specifically related to signaling in the ARC. NPY/AgRP and POMC/CART neurons in the hypothalamus express leptin receptors [179,180] and ICV leptin fails to reduce food intake in rats if the ARC is damaged [181]. Indeed, leptin inhibits NPY/AgRP signaling and downregulates the expression of these neuropeptides, while it upregulates POMC expression and stimulates POMC/CART signaling in the ARC [182–184]. Targeting leptin as an therapeutic endocrine approach to obesity and weight loss has been disappointing, probably reflecting receptor resistance in obesity [185].
Adiponectin
Adiponectin is secreted from adipose tissue into the bloodstream and is very abundant in plasma relative to many hormones. Adiponectin promotes insulin sensitivity and the survival of pancreatic β-cells and cardiomyocytes [186]. Similar to leptin, it acts in the brain to mediate weight loss. However, it has yet to enter clinical trials.
Combination therapy with pramlintide & leptin
The ability of amylin to reduce appetite and weight in obese rats is potentiated by coadministration of leptin. This effect is specific to amylin, and synergy is not observed with other peptides, including PYY and GLP-1/exendin-4 analogues. This synergy appears to reflect the ability of amylin to restore leptin receptor signaling in the hypothalamus in the setting of obesity [187]. In overweight and obese patients, coadministration of pramlintide and leptin by subcutaneous injection twice daily produced approximately 13 kg of weight loss, while monotherapy with either agent only resulted in approximately 8 kg of loss. Importantly, patients on combination therapy continued to lose weight, while those on mono-therapy achieved a plateau over the duration of the study [187]. In a Phase II study, overweight and obese individuals treated with combination therapy twice daily lost approximately 11% of bodyweight, which was significantly greater than patients receiving either agent alone (approximately 5.0%) [310]. In a continuation of this study, patients receiving cotherapy exhibited sustained weight loss, while those receiving placebo regained almost all their weight [311]. Based on these results, pramlintide/leptin cotherapy is advancing into Phase III trials.
Oleoyl-estrone
Oleoyl-estrone (OE) is packaged in lipoproteins derived from adipose tissue for secretion in the circulation. Like leptin, OE levels are associated with adiposity [188], but in contrast to leptin, obese patients exhibit reduced circulating OE. OE induces dose-dependent decreases in appetite and weight [189] with a preservation of body protein and wasting of fat stores in rodents [190]. Moreover, weight loss is maintained for 26 days following 2 weeks of constant OE infusion in lean rats, while obese rats regain weight immediately following cessation of OE infusion, reflecting a deficient leptinergic system [191]. Importantly, oral administration of OE induced loss of adipose tissue associated with a decrease in food intake, without changing the metabolic rate [189,192]. Although the underlying mechanism of action is not yet clear, the loss of weight can be sufficiently explained owing to the decrease in food consumption [193]. These observations underscore the principal advantage of OE, which is oral bioavailability, in contrast to peptide hormones, which require intravenous or subcutaneous administration. In humans, oral OE (150–300 μmol/day) administered to morbidly obese patients over ten consecutive 21-day trial periods followed by 2-month recovery periods induced a weight loss of 38.5 kg over 27 months [194]. While these data were promising, subsequent randomized clinical trials failed to demonstrate significant placebo-adjusted weight loss in obese patients [312].
Modulators of monoamine neurotransmission
Monoamine neurotransmitters, such as norepinephrine, serotonin and dopamine, are involved in regulating an array of neuronal functions, including appetite control [195]. Drugs that target monoamine neurotransmitter levels are effective in generating weight loss in patients. However, because of the variety of neuronal pathways that utilize these neurotransmitters, these drugs carry risks of addiction, tolerance, hypertension and cardiovascular adverse effects [196].
Bupropion is a dopamine and norepinephrine reuptake inhibitor, and is used as an antidepressant and a smoking cessation aid. Naltrexone is an opioid receptor antagonist and is used in treating opiate and alcohol addiction. The combination of the two drugs, marketed as Contrave® (Orexigen® Therapeutics), tries to synergize their mechanisms of action: bupropion stimulates hypothalamic POMC neurons and downstream α-MSH neurons, both anorexigenic, while naltrexone blocks the autoinhibition of the POMC neurons by endogenous β-endorphins [197]. Phase III clinical trials have demonstrated that patients on a diet and exercise program achieved greater weight loss over 56 weeks with bupropion/naltrexone (6.1 kg) than with placebo (1.4 kg) [313]. However, in February 2011, the FDA rejected approval of the bupropion/naltrexone combination due to concerns over potential cardiovascular risks [314].
Phentermine is a norepinephrine reuptake inhibitor. Topiramate is an antiepileptic and anticonvulsant. Individually, phentermine and topiramate have demonstrated efficacy in weight loss. However, the combination of topiramate and phentermine, marketed as Qnexa® (Vivus), was rejected by the FDA as a weight-loss drug due to concerns over adverse effects, including suicidal thoughts, heart palpitations, memory lapses and birth defects [315].
5-hydroxytryptamine receptor subtype 2C (5HT2C) binds serotonin and acts in the regulation of feeding behavior, among other roles [198]. Lorcaserin (ADP-356; Arena) is a selective 5HT2C agonist that proved to be effective in inducing weight loss in Phase II/III testing [199]. However, the FDA rejected approval for lorcaserin owing to marginal efficacy in weight loss and the risk of breast tumors in female rats [316].
Expert commentary
A host of technical, institutional and economic issues pose grave challenges to the development and deployment of safe and effective anti-obesity medications. The morbidity and mortality risks accompanying anti-obesity medications have figured prominently in the development of therapeutic agents in this field. Demonstrated safety appears as elusive a goal as it is undeniably a prerequisite for physicians who would prescribe these drugs for their patients, especially in light of the established safety and efficacy of many of the drugs used to treat the comorbidities of obesity. In addition, current drug formulations are often delivered via injection, which represents a serious impediment to patient compliance. Developing orally active drugs is essential to success in this field. Furthermore, most physicians prefer to limit pharmacological therapy to comorbidities, and address obesity through lifestyle modification programs, only employing anti-obesity drugs as a last resort. In that model, patients struggle unsuccessfully for months or years to achieve and maintain adequate weight loss. Earlier administration of anti-obesity pharmacotherapy could provide significant benefit in the reduction of both weight and the risk for the development of comorbidities. Therefore, educating both patients and physicians about the safety and efficacy of these new drugs will be paramount.
Regulatory guidelines for anti-obesity therapy represent a significant obstacle to developing drugs for this application. The FDA mandates that weight control by new drugs must be demonstrated over 1 year to classify a product as efficacious. These efficacy guidelines suggest that: placebo-subtracted weight loss induced by the drug must be ≥ 5%; and the percentage of drug-treated subjects losing ≥ 5% of baseline bodyweight must be ≥ 35% and double the percentage from the placebo-treated subjects. Moreover, at least 3000 subjects must be assigned to the experimental drug with no fewer than 1500 subjects assigned to placebo for a 1-year period to satisfy safety concerns [200,317]. These regulatory guidelines promote drug safety and efficacy and are therefore essential for the responsible and worthwhile development of pharmacotherapy. They nonetheless demand an enormous investment of time and resources from biopharmaceutical companies, and have contributed to the wane of approved anti-obesity drugs that are currently available to physicians and their patients.
Beyond regulatory considerations, there are also financial barriers for patients to consider anti-obesity therapy. Indeed, obesity is not classified as a disease itself, a position propagated by the FDA and its regulatory guidelines. Unfortunately, this position provides insurance companies with a basis to consider anti-obesity drugs with cosmetic procedures as exclusions, and decline patients reimbursement for anti-obesity medications. Thus, patients without an indication for another comorbid condition may have to pay out-of-pocket for anti-obesity therapy. Such costs could represent a major obstacle to patient care, especially in low-income populations with disproportionately high obesity rates. In that context, a 1-month supply of orlistat, for example, costs approximately US$120–140, a major hurdle for patients of low economic status.
Five-year view
Anti-obesity pharmacotherapeutics, leveraging a variety of pathophysiological mechanisms, are in preclinical and clinical development, with several showing great promise to be superior alternatives to orlistat. In the context of the pandemic into which obesity has evolved, recent efforts have focused on the development of combination therapeutics for the treatment of obesity, and based on the positive results achieved with these agents and the effectiveness of combination drug therapy in treating a variety of other pathologies, new combinations of anti-obesity drugs can be expected. Agents that target gut, pancreatic and adipose hormone and neuropeptide signaling will also continue to be developed. Furthermore, new delivery methods, including oral, intranasal and transdermal formulations, will make these drugs more attractive to patients and physicians. In addition, a better understanding of how the body regulates appetite will probably result in the discovery of new therapeutic targets. For example, an obstacle such as obesity-related leptin resistance may be circumvented as we further define mechanisms by which central leptin resistance develops in obesity. Despite this progress, however, the aforementioned scientific, regulatory and economic hurdles must be overcome to permit the rapid entry of anti-obesity pharmacotherapeutics into mainstream clinical care.
Key issues
Obesity has evolved into a global pandemic associated with comorbidities including Type 2 diabetes and cardiovascular disease. The health and economic impacts of chronic obesity exceed those of smoking or alcohol abuse.
Safe and effective therapies to treat obesity and induce long-term weight loss represent an urgent unmet clinical need. Bariatric surgery is reserved for morbidly obese patients and those with serious comorbidities, while the sole anti-obesity drug that is US FDA-approved for long-term use, orlistat, has limited efficacy associated with substantial gastrointestinal side effects.
Elucidating central and peripheral mechanisms regulating appetite has produced anti-obesity drug development programs targeting these pathways.
Supplementation of hormonal regulators of appetite (glucagon-like peptide-1, oxyntomodulin, peptide YY, pancreatic polypeptide and amylin) reduces appetite associated with weight loss. Unfortunately, these regulatory peptides require administration by injection, which is a major drawback.
New delivery methods for these peptide hormones, including oral, intranasal and transdermal formulations, will make these drugs more attractive to patients and physicians. Orally active drugs targeting hormone and neuropeptide receptors are in early development.
Substantial scientific, regulatory and economic barriers to developing anti-obesity pharmacotherapeutics still remain.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Support was provided by NIH grants R01 CA75123, R01 CA95026, RC1 CA75123 and P30 CA56036 and from Targeted Diagnostic and Therapeutics, Inc. (Scott A Waldman). Michael A Valentino is the recipient of a predoctoral fellowship from the Pharmaceutical Research and Manufacturers of America Foundation. Francheska Colon-Gonzalez is the recipient of a post-doctoral fellowship from the Pharmaceutical Research and Manufacturers of America Foundation Foundation. Scott A Waldman is the Samuel MV Hamilton Endowed Professor. Scott A Waldman is also the Chair of the Data Safety Monitoring Board for the C-Cure Trial sponsored by Cardio3 Biosciences; and the Chair (uncompensated) of the Scientific Advisory Board to Targeted Diagnostics and Therapeutics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263(4):336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 2.Campanini B, editor. WHO. Reducing Risks, Promoting Healthy Life. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 5.Daniels J. Obesity: America’s epidemic. Am J Nurs. 2006;106(1):40–49. doi: 10.1097/00000446-200601000-00028. quiz 49–50. [DOI] [PubMed] [Google Scholar]
- 6.Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. Nutr J. 2005;4:24. doi: 10.1186/1475-2891-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 8.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(Suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 9.Sturm R. The effects of obesity, smoking, and drinking on medical problems and costs. Health Affairs. 2002;21(2):245–253. doi: 10.1377/hlthaff.21.2.245. [DOI] [PubMed] [Google Scholar]
- 10.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Miller ER, 3rd, Erlinger TP, Young DR, et al. Results of the diet, exercise, and weight loss intervention trial (DEW-IT) Hypertension. 2002;40(5):612–618. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes. 1992;16(6):397–415. [PubMed] [Google Scholar]
- 14.Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diabetes Obes. 2009;16(5):347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 15.Perri M, Corsica J. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden T, Stunkard A, editors. Handbook of Obesity Treatment. Guilford Press; NY, USA: 2002. pp. 357–379. [Google Scholar]
- 16.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. J Am Med Assoc. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 17•.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. Along with [16], this article demonstrates that obese patients who undergo bariatric surgery experience both weight loss and improvements of their obesity-associated comorbidities. [DOI] [PubMed] [Google Scholar]
- 18.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142(7):547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 19.Cote GA, Edmundowicz SA. Emerging technology: endoluminal treatment of obesity. Gastrointest Endosc. 2009;70(5):991–999. doi: 10.1016/j.gie.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352(9123):167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 21.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. J Am Med Assoc. 1999;281(3):235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 22.James WPT, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet. 2000;356(Suppl):2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 23.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 24.Diets, drugs and surgery for weight loss. Treatment Guidelines from the Medical Letter. 2008;6(68):23–28. [PubMed] [Google Scholar]
- 25.Bays HE. Current and investigational anti-obesity agents and obesity therapeutic treatment targets. Obes Res. 2004;12(8):1197–1211. doi: 10.1038/oby.2004.151. [DOI] [PubMed] [Google Scholar]
- 26.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369(9555):71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 27.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: current and future pharmacological treatments. Ann Rev Pharmacol Toxicol. 2007;47:565–592. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- 29.Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 30.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 31.Sclafani A. Neural pathways involved in the ventromedial hypothalamic lesion syndrome in the rat. J Comp Physiol Psychol. 1971;77(1):70–96. doi: 10.1037/h0031574. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A, Kirchgessner A. The role of the medial hypothalamus in the control of food intake: an update. Feeding Behav. 1986:27–66. [Google Scholar]
- 33.Stellar E. The physiology of motivation. Psychol Rev. 1954;61(1):5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 34.Wynne K, Stanley S, McGowan B, Bloom SR. Appetite control. J Endocrinol. 2005;184(2):291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 36•.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909–1914. doi: 10.1126/science.1109951. Along with [35], this review describes neural and endocrine signaling by which energy balance is regulated. [DOI] [PubMed] [Google Scholar]
- 37.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortlnergic neurons in feeding and the Agouti obesity syndrome. Nature. 1997;385(6612):165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 38.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 39.Argyropoulos G, Rankinen T, Neufeld DR, et al. A polymorphism in the human Agouti-related protein is associated with late-onset obesity. J Clin Endocrinol Metab. 2002;87(9):4198–4202. doi: 10.1210/jc.2002-011834. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 41.Abbott CR, Rossi M, Wren AM, et al. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology. 2001;142(8):3457–3463. doi: 10.1210/endo.142.8.8304. [DOI] [PubMed] [Google Scholar]
- 42.Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65(3):165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 43.Swart I, Jahng JW, Overton JM, Houpt TA. Hypothalamic NPY, AgRP, and POMC mRNA responses to leptin and refeeding in mice. Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R1020–R1026. doi: 10.1152/ajpregu.00501.2001. [DOI] [PubMed] [Google Scholar]
- 44.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of AgRP and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 45.Inui A. Neuropeptide Y feeding receptors: are multiple subtypes involved? Trends Pharmacol Sci. 1999;20(2):43–46. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- 46.King PJ, Widdowson PS, Doods HN, Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J Neurochem. 1999;73(2):641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. [DOI] [PubMed] [Google Scholar]
- 47.Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to Agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 48.Rossi M, Kim MS, Morgan DGA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 49.Small CJ, Kim MS, Stanley SA, et al. Effects of chronic central nervous system administration of Agouti-related protein in pair-fed animals. Diabetes. 2001;50(2):248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- 50.Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by Agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 51.Fekete C, Légrádi G, Mihály E, et al. α-melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000;20(4):1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fekete C, Sarkar S, Rand WM, et al. Agouti-related protein (AgRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AgRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology. 2002;143(10):3846–3853. doi: 10.1210/en.2002-220338. [DOI] [PubMed] [Google Scholar]
- 53.Sarkar S, Lechan RM. Central administration of neuropeptide Y reduces α-melanocyte-stimulating hormone-induced cyclic adenosine 5′-monophosphate response element binding protein (CREB) Endocrinology. 2003;144(1):281–291. doi: 10.1210/en.2002-220675. [DOI] [PubMed] [Google Scholar]
- 54.Qu D, Ludwig DS, Gammeltoft S, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 55.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131(2):229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 56.Yeo GSH, Connie Hung CC, Rochford J, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7(11):1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 57.Xu B, Goulding EH, Zang K, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricardo JA, Tongju Koh E. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 59.Ter Horst GJ, De Boer P, Luiten PGM, Van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 60.Szczypka MS, Kwok K, Brot MD, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30(3):819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 61.Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking β-endorphin and enkephalin. J Neurosci. 2002;22(18):8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8(5):585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- 63.Mashiko S, Moriya R, Ishihara A, et al. Synergistic interaction between neuropeptide Y1 and Y5 receptor pathways in regulation of energy homeostasis. Eur J Pharmacol. 2009;615(1–3):113–117. doi: 10.1016/j.ejphar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 64.Kanatani A, Mashiko S, Murai N, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: Comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141(3):1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- 65.Erondu N, Gantz I, Musser B, et al. Neuropeptide Y5 receptor antagonism does not induce clinically meaningful weight loss in overweight and obese adults. Cell Metabol. 2006;4(4):275–282. doi: 10.1016/j.cmet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Krishna R, Gumbiner B, Stevens C, et al. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: energy intake predicts lack of weight loss efficacy. Clin Pharmacol Ther. 2009;86(6):659–666. doi: 10.1038/clpt.2009.167. [DOI] [PubMed] [Google Scholar]
- 67.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31(4):506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov. 2008;7(4):307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell J, Maguire J, Davenport A. Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. Br J Pharmacol. 2009;158(1):87–103. doi: 10.1111/j.1476-5381.2009.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson EL, Murphy KG, Todd JF, et al. Chronic administration of NMU into the paraventricular nucleus stimulates the HPA axis but does not influence food intake or body weight. Biochem Biophys Res Commun. 2004;323(1):65–71. doi: 10.1016/j.bbrc.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 71.Novak CM, Zhang M, Levine JA. Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity. Brain Res. 2007;1169(1):57–68. doi: 10.1016/j.brainres.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16(10):858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 73.Crawley JN. Biological actions of cholecystokinin. Peptides. 1994;15(4):731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 74.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol. 1997;272(4 Pt 2):R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 75.Moran TH, Shnayder L, Hostetler AM, McHugh PR. Pylorectomy reduces the satiety action of cholecystokinin. Am J Physiol. 1988;255(6):R1059–R1063. doi: 10.1152/ajpregu.1988.255.6.R1059. [DOI] [PubMed] [Google Scholar]
- 76.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beglinger C, Degen L, Matzinger D, D’Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol. 2001;280(4):R1149–1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- 78.Asin KE, Bednarz L. Differential effects of CCK-JMV-180 on food intake in rats and mice. Pharmacol Biochem Behav. 1992;42(2):291–295. doi: 10.1016/0091-3057(92)90529-o. [DOI] [PubMed] [Google Scholar]
- 79.Crawley JN, Beinfeld MC. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature. 1983;302(5910):703–706. doi: 10.1038/302703a0. [DOI] [PubMed] [Google Scholar]
- 80.Lukaszewski L, Praissman M. Effect of continuous infusions of CCK-8 on food intake and body and pancreatic weights in rats. Am J Physiol. 1988;254(1):R17–R22. doi: 10.1152/ajpregu.1988.254.1.R17. [DOI] [PubMed] [Google Scholar]
- 81.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984;15(5):R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- 82.Fong TM. Advances in anti-obesity therapeutics. Expert Opin Investig Drugs. 2005;14(3):243–250. doi: 10.1517/13543784.14.3.243. [DOI] [PubMed] [Google Scholar]
- 83.Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol. 1999;276(4 Pt 2):R1038–R1045. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- 84.Plusczyk T, Westermann S, Rathgeb D, Feifel G. Acute pancreatitis in rats: effects of sodium taurocholate, CCK-8, and Sec on pancreatic microcirculation. Am J Physiol. 1997;272(2 Pt 1):G310–G320. doi: 10.1152/ajpgi.1997.272.2.G310. [DOI] [PubMed] [Google Scholar]
- 85.Pandol SJ, Periskic S, Gukovsky I, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117(3):706–716. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 86.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 88.Brubaker PL. The glucagon-like peptides: Pleiotropic regulators of nutrient homeostasis. Ann NY Acad Sci. 2006;1070:10–26. doi: 10.1196/annals.1317.006. [DOI] [PubMed] [Google Scholar]
- 89.Meeran K, O’Shea D, Edwards CMB, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology. 1999;140(1):244–250. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- 90.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 91.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Näslund E, King N, Mansten S, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91(3):439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 93.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide 1(7–36) amide’s central inhibition of feeding and peripheral inhibition of are abolished by neonatal monosodium glutamate treatment. Diabetes. 1998;47(4):530–537. doi: 10.2337/diabetes.47.4.530. [DOI] [PubMed] [Google Scholar]
- 94.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY 3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem–hypothalamic pathway. Brain Res. 2005;1044(1):127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Furuse M, Matsumoto M, Mori R, Sugahara K, Kano K, Hasegawa S. Influence of fasting and neuropeptide Y on the suppressive food intake induced by intracerebroventricular injection of glucagon-like peptide-1 in the neonatal chick. Brain Res. 1997;764(1–2):289–292. doi: 10.1016/s0006-8993(97)00623-9. [DOI] [PubMed] [Google Scholar]
- 96.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214(3):829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 97•.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in Type 2 diabetes: systematic review and meta-analysis. J Am Med Assoc. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194. This meta-analysis demonstrates that incretin therapy is an effective treatment option for diabetes. [DOI] [PubMed] [Google Scholar]
- 98.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with Type 2 diabetes. Diabetes Care. 2007;30(6):1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 99.Beglinger C, Poller B, Arbit E, et al. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3–36: a proof-of-concept study in healthy subjects. Clin Pharmacol Ther. 2008;84(4):468–474. doi: 10.1038/clpt.2008.35. [DOI] [PubMed] [Google Scholar]
- 100.Gallwitz B. Benefit–risk assessment of exenatide in the therapy of Type 2 diabetes mellitus. Drug Saf. 2010;33(2):87–100. doi: 10.2165/11319130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 101.Dakin CL, Gunn I, Small CJ, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 102.Dakin CL, Small CJ, Batterham RL, et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145(6):2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 103.Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88(10):4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 104.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 105.Wynne K, Park AJ, Small CJ, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 106.Wynne K, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes. 2006;30(12):1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 107.Adrian TE, Ferri GL, Bacarese-Hamilton AJ. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 108.Liu CD, Aloia T, Adrian TE, et al. Peptide YY: A potential proabsorptive hormone for the treatment of malabsorptive disorders. Am Surg. 1996;62(3):232–236. [PubMed] [Google Scholar]
- 109.Adrian TE, Savage AP, Sagor GR. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89(3):494–499. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 110.Adams SH, Won WB, Schonhoff SE, Leiter AB, Paterniti JR., Jr Effects of peptide YY[3–36] on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology. 2004;145(11):4967–4975. doi: 10.1210/en.2004-0518. [DOI] [PubMed] [Google Scholar]
- 111.Chelikani PK, Haver AC, Heidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology. 2005;146(2):879–888. doi: 10.1210/en.2004-1138. [DOI] [PubMed] [Google Scholar]
- 112•.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. Demonstrates that peripheral peptide YY reduces food intake and weight gain via hypothalamic signaling. [DOI] [PubMed] [Google Scholar]
- 113.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4(3):223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 114.Le Roux CW, Batterham RL, Aylwin SJB, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 115.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132(6):2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 116.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 117.Degen L, Oesch S, Casanova M, et al. Effect of peptide YY3–36 on food intake in humans. Gastroenterology. 2005;129(5):1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Gantz I, Erondu N, Mallick M, et al. Efficacy and safety of intranasal peptide YY3–36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007;92(5):1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- 119.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 120.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23(22):7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121•.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. Demonstrates that ghrelin stimulates appetite and induces weight gain. [DOI] [PubMed] [Google Scholar]
- 122.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(7–12):2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 123.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 124.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145(5):669–673. [PubMed] [Google Scholar]
- 125.Tolle V, Kadem M, Bluet-Pajot MT, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88(1):109–116. doi: 10.1210/jc.2002-020645. [DOI] [PubMed] [Google Scholar]
- 126.Guan XM, Yu H, Palyha OC, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48(1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 127.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 128.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 129.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 130.Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, De Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313(3):635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 131.Zorrilla EP, Iwasaki S, Moss JA, et al. Vaccination against weight gain. Proc Natl Acad Sci USA. 2006;103(35):13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moran TH, Dailey MJ. Gut peptides: targets for anti-obesity drug development? Endocrinology. 2009;150(6):2526–2530. doi: 10.1210/en.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA. 2008;105(31):10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hort Y, Baker E, Sutherland GR, Shine J, Herzog H. Gene duplication of the human peptide YY gene (PYY ) generated the pancreatic polypeptide gene (PPY ) on chromosome 17q21.1. Genomics. 1995;26(1):77–83. doi: 10.1016/0888-7543(95)80085-z. [DOI] [PubMed] [Google Scholar]
- 135.Adrian TE, Bloom SR, Bryant MG. Distribution and release of human pancreatic polypeptide. Gut. 1976;17(12):940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Malaisse-Lagae F, Carpentier JL, Patel YC. Pancreatic polypeptide: a possible role in the regulation of food intake in the mouse. Hypothesis Experientia. 1977;33(7):915–917. doi: 10.1007/BF01951279. [DOI] [PubMed] [Google Scholar]
- 138.Asakawa A, Inui A, Yuzuriha H, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124(5):1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- 139.Ueno N, Inui A, Iwamoto M, et al. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117(6):1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 140.Reinehr T, Enriori PJ, Harz K, Cowley MA, Roth CL. Pancreatic polypeptide in obese children before and after weight loss. Int J Obes. 2006;30(10):1476–1481. doi: 10.1038/sj.ijo.0803393. [DOI] [PubMed] [Google Scholar]
- 141.Fujimoto S, Inui A, Kiyota N, et al. Increased cholecystokinin and pancreatic polypeptide responses to a fat-rich meal in patients with restrictive but not bulimic anorexia nervosa. Biol Psychiatry. 1997;41(10):1068–1070. doi: 10.1016/S0006-3223(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 142.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115(1):427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 143.Larsen PJ, Kristensen P. The neuropeptide Y (Y4) receptor is highly expressed in neurones of the rat dorsal vagal complex. Brain Res Mol Brain Res. 1997;48(1):1–6. doi: 10.1016/s0169-328x(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 144.Whitcomb DC, Taylor IL, Vigna SR. Characterization of saturable binding sites for circulating pancreatic polypeptide in rat brain. Am J Physiol. 1990;259(4 Pt 1):G687–G691. doi: 10.1152/ajpgi.1990.259.4.G687. [DOI] [PubMed] [Google Scholar]
- 145.Batterham RL, Le Roux CW, Cohen MA, et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88(8):3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 146.Adrian TE, Greenberg GR, Besterman HS, Bloom SR. Pharmacokinetics of pancreatic polypeptide in man. Gut. 1978;19(10):907–909. doi: 10.1136/gut.19.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pittner RA, Albrandt K, Beaumont K, et al. Molecular physiology of amylin. J Cell Biochem. 1994;55(Suppl):19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- 148.Koda JE, Fineman M, Rink TJ, Dailey GE, Muchmore DB, Linarelli LG. Amylin concentrations and glucose control. Lancet. 1992;339(8802):1179–1180. doi: 10.1016/0140-6736(92)90785-2. [DOI] [PubMed] [Google Scholar]
- 149.Koda JE, Fineman MS, Kolterman OG, Caro JF. 24 hour plasma amylin profiles are elevated in IGT subjects vs. normal controls. Diabetes. 1995;44(Suppl 1):238A. [Google Scholar]
- 150.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000;141(2):850–853. doi: 10.1210/endo.141.2.7378. [DOI] [PubMed] [Google Scholar]
- 151.Rushing PA, Hagan MM, Seeley RJ, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142(11):5035–5038. doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- 152.Gebre-Medhin S, Mulder H, Pekny M, et al. IAPP (amylin) null mutant mice; plasma levels of insulin and glucose, body weight and pain responses. Diabetologia. 1997;40:A26. [Google Scholar]
- 153.Devine E, Young AA. Weight gain in male and female mice with amylin gene knockout. Diabetes. 1998;47(Suppl 1):A317. [Google Scholar]
- 154.Cooper GJS. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr Rev. 1994;15(2):163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- 155.Young AA, Wang MW, Gedulin B, Rink TJ, Pittner R, Beaumont K. Diabetogenic effects of salmon calcitonin are attributable to amylin-like activity. Metabolism. 1995;44(12):1581–1589. doi: 10.1016/0026-0495(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 156.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 157.Muff R, Bühlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140(6):2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- 158.Young A, Moore C, Herich J, Beaumont K. Neuroendocrine actions of amylin. In: Poyner D, Marshall I, Brain SD, editors. The CGRP Family: Calcitonin Gene-Related Peptide (CGRP), Amylin, and Adrenomedullin. Lands Bioscience; TX, USA: 2000. pp. 91–102. [Google Scholar]
- 159.Jodka C, Green D, Young A, Gedulin B. Amylin modulation of gastric emptying in rats depends upon an intact vagus nerve. Diabetes. 1996;45(Suppl 2):A235. [Google Scholar]
- 160.Edwards GL, Gedulin BR, Jodka C, Dilts RP, Miller CC, Young A. Area postrema (AP)-lesions block the regulation of gastric emptying by amylin. Neurogastroenterol Motil. 1998;10(4):26. [Google Scholar]
- 161.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes. 2001;25(7):1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 162.Edelman SV, Weyer C. Unresolved challenges with insulin therapy in Type 1 and Type 2 diabetes: potential benefit of replacing amylin, a second β-cell hormone. Diabetes Technol Ther. 2002;4(2):175–189. doi: 10.1089/15209150260007390. [DOI] [PubMed] [Google Scholar]
- 163.Young AA, Vine W, Gedulin BR, et al. Preclinical pharmacology of pramlintide in the rat: comparisons with human and rat amylin. Drug Dev Res. 1996;37(4):231–248. [Google Scholar]
- 164.Ratner RE, Want LL, Fineman MS, et al. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with Type 2 diabetes. Diabetes Technol Ther. 2002;4(1):51–61. doi: 10.1089/15209150252924094. [DOI] [PubMed] [Google Scholar]
- 165.Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with Type 2 diabetes. Diabetes Care. 2003;26(3):784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- 166.Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in Type 1 diabetes. Diabetes Care. 2002;25(4):724–730. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- 167.Maggs D, Shen L, Strobel S, Brown D, Kolterman O, Weyer C. Effect of pramlintide on A1C and body weight in insulin-treated African Americans and Hispanics with Type 2 diabetes: a pooled post hoc analysis. Metabolism. 2003;52(12):1638–1642. doi: 10.1016/j.metabol.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 168.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. Obes Res. 1996;4(1):91–95. doi: 10.1002/j.1550-8528.1996.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 169•.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. Illustrates that leptin induces weight loss and suppresses appetite. [DOI] [PubMed] [Google Scholar]
- 170.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 171.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 172.Farooqi IS, O’Rahilly S. Monogenic obesity in humans. Recent Prog Horm Res. 2005;59:409–424. doi: 10.1210/rp.59.1.409. [DOI] [PubMed] [Google Scholar]
- 173•.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. Reveals that obesity drives a compensatory increase in leptin expression due to the development of insensitivity. [DOI] [PubMed] [Google Scholar]
- 174.Ahlma RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 175.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 176.Münzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8(5):566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 177.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59(1):287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 178.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297(6):E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 180.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48(4):828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 181.Tang-Christensen M, Holst JJ, Hartmann B, Vrang N. The arcuate nucleus is pivotal in mediating the anorectic effects of centrally administered leptin. Neuroreport. 1999;10(6):1183–1187. doi: 10.1097/00001756-199904260-00005. [DOI] [PubMed] [Google Scholar]
- 182.Schwartz MW, Baskin DG, Bukowski TR, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45(4):531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 183.Stephens TW, Basinski M, Bristow PK, et al. The role of neuropeptide Y in the anti-obesity action of the obese gene product. Nature. 1995;377(6549):530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 184.Schwartz MW, Seeley RJ, Woods SC, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46(12):2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 185.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 186.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2010;17(1):55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187•.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105(20):7257–7262. doi: 10.1073/pnas.0706473105. Describes the amylin-induced restoration of leptin sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fernández-Real JM, Sanchis D, Ricart W, et al. Plasma oestrone-fatty acid ester levels are correlated with body fat mass in humans. Clin Endocrinol (Oxf) 1999;50(2):253–260. doi: 10.1046/j.1365-2265.1999.00669.x. [DOI] [PubMed] [Google Scholar]
- 189.Sanchis D, Balada F, Del Mar Grasa M, et al. Oleoyl-estrone induces the loss of body fat in rats. Int J Obes. 1996;20(6):588–594. [PubMed] [Google Scholar]
- 190.Sanchis D, Balada F, Picó C, et al. Rats receiving the slimming agent oleoyl-estrone in liposomes (Merlin-2) decrease food intake but maintain thermogenesis. Arch Physiol Biochem. 1997;105(7):663–672. doi: 10.1076/apab.105.7.663.11391. [DOI] [PubMed] [Google Scholar]
- 191.Adán C, Cabot C, Vilà R, et al. Oleoyl-estrone treatment affects the ponderostat setting differently in lean and obese Zucker rats. Int J Obes. 1999;23(4):366–373. doi: 10.1038/sj.ijo.0800828. [DOI] [PubMed] [Google Scholar]
- 192.Remesar X, Guijarro P, Torregrosa C, et al. Oral oleoyl-estrone induces the rapid loss of body fat in Zucker lean rats fed a hyperlipidic diet. Int J Obes. 2000;24(11):1405–1412. doi: 10.1038/sj.ijo.0801393. [DOI] [PubMed] [Google Scholar]
- 193.Strassburg S, Pfluger PT, Chaudhary N, et al. Action profile of the anti-obesity drug candidate oleoyl-estrone in rats. Obesity (Silver Spring) 2010;18(12):2260–2267. doi: 10.1038/oby.2010.53. [DOI] [PubMed] [Google Scholar]
- 194.Alemany M, Fernández-López JA, Petrobelli A, Granada M, Foz M, Remesar X. Weight loss in a patient with morbid obesity under treatment with oleoyl-estrone. Med Clin (Barc) 2003;121(13):496–499. doi: 10.1016/s0025-7753(03)74000-7. [DOI] [PubMed] [Google Scholar]
- 195.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 196.Sargent BJ, Moore NA. New central targets for the treatment of obesity. Br J Clin Pharmacol. 2009;68(6):852–860. doi: 10.1111/j.1365-2125.2009.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity. 2009;17(1):30–39. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 198.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and Type 2 diabetes in mice receptor with a mutated serotonin 5-HT2C gene. Nat Med. 1998;4(10):1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 199.Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR. Lorcaserin (APD356), a agonist, reduces body selective 5-HT2C weight in obese men and women. Obesity. 2009;17(3):494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- 200.Sonnenberg GE, Matfin G, Reinhardt RR. Drug treatments for obesity: where are we heading and how do we get there? Br J Diab Vasc Dis. 2007;7(3):111–118. [Google Scholar]
- 201.Becskei C, Bilik KU, Klussmann S, Jarosch F, Lutz TA, Riediger T. The anti-ghrelin Spiegelmer NOX-B11-3 blocks ghrelin- but not fasting-induced neuronal activation in the hypothalamic arcuate nucleus. J Neuroendocrinol. 2008;20(1):85–92. doi: 10.1111/j.1365-2826.2007.01619.x. [DOI] [PubMed] [Google Scholar]
- 202.Fong TM, Guan XM, Marsh DJ, et al. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2 -[[5-(trifluoromethyl)pyridin-2-yl]oxy] propanamide (MK-0364), in rodents. J Pharmacol Exp Ther. 2007;321(3):1013–1022. doi: 10.1124/jpet.106.118737. [DOI] [PubMed] [Google Scholar]
Websites
- 301.TransTech Pharma obesity product pipeline. www.ttpharma.com/TherapeuticAreas/MetabolicDiseases/Obesity/TTP435/tabid/126/Default.aspx.
- 302.Bristol-Myers Squibb metabolics clinical trial registry. http://ctr.bms.com/OneBmsCtd/InitTrialAction.do?linkname=Metabolics&type=pharma&sortby=default.
- 303.Novo Nordisk. Significant weight loss sustained in obese people treated with liraglutide for one year. www.novonordisk.com/science/Pipeline/liraglutide-press-releases.asp.
- 304.Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. Exenatide once weekly provided sustained improvements in glycemic control with weight loss over two years: DURATION-1 interim long-term data presented at ADA. 2009 www.amylin.com/assets/001/5097.pdf.
- 305.Amylin Pharmaceuticals, Inc research pipeline. www.amylin.com/research/pipeline/exenatide.htm.
- 306.Phase I/IIa clinical trial with obese individuals shows no effect of CYT009-GhrQb on weight loss. www.cytos.com/doc/Cytos_Press_E_061107.pdf.
- 307.Bloomberg Business Week: Elixir Pharmaceuticals, Inc. http://investing.businessweek.com/research/stocks/private/snapshot.asp?privcapId=2246762.
- 308.7TM Pharma: obinepitide. www.7tm.com/R-D/Metabolic_Disorders/Obinepitide.aspx.
- 309.7TM Pharma: TM30339. www.7tm.com/R-D/Metabolic_Disorders/TM30339.aspx.
- 310.Amylin Pharmaceuticals announces positive results from dose-ranging clinical study of pramlintide/metreleptin combination treatment for obesity. http://investors.amylin.com/phoenix.zhtml?c=101911&p=irol-newsArticle_pf&ID=1305954.
- 311.Amylin and Takeda announce decision to advance development of pramlintide/metreleptin combination treatment for obesity. www.takeda.com/press/article_35851.html.
- 312.Manhattan Pharmaceuticals announces results of Phase 2a studies for oral oleoyl-estrone. http://ir.manhattanpharma.com/releasedetail.cfm?ReleaseID=252872.
- 313.Orexigen® Therapeutics Announces Publication of COR-I Phase 3 Study of Contrave in Lancet. http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1454172&highlight=
- 314.FDA Issues Complete Response to New Drug Application for Contrave® for the Management of Obesity. http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1522207&highlight=
- 315.FDA issues complete response letter to VIVUS regarding new drug application for QNEXA®. http://ir.vivus.com/releasedetail.cfm?ReleaseID=524576.
- 316.Arena and Eisai provide update on lorcaserin FDA Advisory Committee Meeting. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=508298.
- 317.FDA. Guidance for industry developing products for weight management. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071612.pdf.
- 318.NIH Clinical Trials database: TTP435. http://clinicaltrials.gov/ct2/show/NCT00779519.
- 319.Rhythm Pharmaceuticals. www.rhythmtx.com/PROGRAMS/RM493.html.
- 320.Noxxon Pharma AG. Noxxon Pharma AG announces global strategic alliance with Pfizer NOX-B11 Spiegelmer® for obesity treatment licensed. http://145.253.103.53/noxxon16/downloads/pressrel/032306PRNOXXONPfizerAgreements.pdf.
- 321.NIH Clinical Trials database: Sitagliptin. http://clinicaltrials.gov/ct2/results?term=Sitagliptin.
- 322.NIH Clinical Trials database: Vildagliptin. http://clinicaltrials.gov/ct2/results?term=Vildagliptin.
- 323.NIH Clinical Trials database: Pramlintide. http://clinicaltrials.gov/ct2/results?term=Pramlintide.
- 324.NIH Clinical Trials database: Exenatide. http://clinicaltrials.gov/ct2/results?term=Exenatide.
- 325.Novo Nordisk receives US approval for Victoza® (liraglutide) for the treatment of Type 2 diabetes. 2010 January 26; www.novonordisk.com/press/sea/sea.asp?sNewsTypeGUID=&lMonth=&lYear=&sLanguageCode=&sSearchText=&sShowNew sItemGUID=7e58c0fd-2e96-4188-b201-a6 91ea2b4471&sShowLanguageCode=en-GB.
- 326.Questions and Answers – Safety requirements for victoza (liraglutide) www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm198543.htm.
- 327.NIH Clinical Trials database: Liraglutide. http://clinicaltrials.gov/ct2/results?term=Liraglutide.
- 328.NIH Clinical Trials database: NN9924. http://clinicaltrials.gov/ct2/results?term=NN9924.
- 329.Wyeth Pharmaceuticals acquires Thiakis Limited. www.drugs.com/news/wyeth-pharmaceuticals-acquires-thiakis-limited-15289.html.
- 330.BioCentury Online Intelligence: TKS-1225. www.biocentury.com/products/tks-1225.
- 331.MedicineNet: Betahistine – oral. www.medicinenet.com/betahistine-oral/article.htm.
- 332.NIH Clinical Trials database: Betahistine. http://clinicaltrials.gov/ct2/results?term=Betahistine.
- 333.SCH-497079 Clinical Trials. www.clinicaltrialssearch.org/22602-sch-497079-clinical-trials.html.
- 334.NIH Clinical Trials database: SCH-497079. http://clinicaltrials.gov/ct2/results?term=SCH-497079.
- 335.NIH Clinical Trials database: Metreleptin. http://clinicaltrials.gov/ct2/results?term=Metreleptin.
- 336.NIH Clinical Trials database: BMS-830216. http://clinicaltrials.gov/ct2/results?term=BMS-830216.
- 337.AMRI Pipeline: Obesity > MCH-1 Antagonists. www.amriglobal.com/pipeline/subpage.cfm?ID=17&subID=69.
- 338.NIH Clinical Trials database: Qnexa. http://clinicaltrials.gov/ct2/results?term=Qnexa.
- 339.Orexigen Therapeutics, Inc. Product Candidates. www.orexigen.com/candidates/candidates_empatic.php.
- 340.NIH Clinical Trials database: Contrave. http://clinicaltrials.gov/ct2/results?term=Contrave.
- 341.Orexigen Therapeutics, Inc. Product Candidates. www.orexigen.com/candidates/candidates_contrave.php.
- 342.NIH Clinical Trials database: MK-0364. http://clinicaltrials.gov/ct2/results?term=MK-0364.
- 343.NIH Clinical Trials database: Lorcaserin. http://clinicaltrials.gov/ct2/results?term=lorcaserin.
- 344.Informational guide to Tesofensine. www.tesofensine-information.com.
- 345.NIH Clinical Trials database: Tesofensine. http://clinicaltrials.gov/ct2/results?term=tesofensine.
- 346.NIH Clinical Trials database: TM30339. http://clinicaltrials.gov/ct2/results?term=TM30339.
- 347.Biocentury Online Intelligence: Obinepitide. www.biocentury.com/products/obinepitide.
- 348.NIH Clinical Trials database: PYY. http://clinicaltrials.gov/ct2/results?term=PYY.
- 349.NIH Clinical Trials database: MK-0557. http://clinicaltrials.gov/ct2/results?term=MK-0557.
- 350.NIH Clinical Trials database: Velneperit. http://clinicaltrials.gov/ct2/results?term=Velneperit+
- 351.Drug Information Online. www.drugs.com/clinical_trials/shionogi-announces-positive-top-line-efficacy-results-year-long-studies-velneperit-novel-npy-y5-6742.html.