Abstract
Physical activity is a known modifiable lifestyle means for reducing postmenopausal breast cancer risk, but the biologic mechanisms are not well understood. Metabolic factors may be involved. In this study, we aimed to determine the effects of exercise on insulin resistance (IR) indicators, IGF1, and adipokines in postmenopausal women. The Alberta Physical Activity and Breast Cancer Prevention Trial was a two-armed randomized controlled trial in postmenopausal, inactive, cancer-free women. A year-long aerobic exercise intervention of 225 min/week (n=160) was compared with a control group asked to maintain usual activity levels (n=160). Baseline, 6- and 12-month serum levels of insulin, glucose, IGF1, IGF-binding protein 3 (IGFBP3), adiponectin, and leptin were assayed, and after data collection, homeostasis model assessment of IR (HOMA-IR) scores were calculated. Intention-to-treat analyses were performed using linear mixed models. The treatment effect ratio (TER) of exercisers to controls was calculated. Data were available on 308 (96.3%) women at 6 months and 310 (96.9%) women at 12 months. Across the study period, statistically significant reductions in insulin (TER=0.87, 95% confidence interval (95% CI)=0.81–0.93), HOMA-IR (TER=0.86, 95% CI=0.80–0.93), and leptin (TER=0.82, 95% CI=0.78–0.87), and an increase in the adiponectin/leptin ratio (TER=1.21, 95% CI=1.13–1.28) were observed in the exercise group compared with the control group. No significant differences were observed for glucose, IGF1, IGFBP3, adiponectin or the IGF1/IGFBP3 ratio. Previously inactive postmenopausal women who engaged in a moderate-to-vigorous intensity exercise program experienced changes in insulin, HOMA-IR, leptin, and adiponectin/leptin that might decrease the risk for postmenopausal breast cancer.
Introduction
Physical activity reduces the risk of postmenopausal breast cancer (Monninkhof et al. 2007, World Cancer Research Fund and the American Institute for Cancer Research 2007, Friedenreich & Cust 2008, Lynch et al. 2011), but the biologic mechanisms underlying this association are not well understood. Insulin (Osborne et al. 1976, Chappell et al. 2001) insulin-like growth factor 1 (IGF1 and its binding proteins; Yu & Rohan 2000, Lann & LeRoith 2008) leptin (Catalano et al. 2003, Geisler et al. 2007, Cirillo et al. 2008), and adiponectin (Brakenhielm et al. 2004, Dieudonne et al. 2006, Arditi et al. 2007) are metabolic factors hypothesized to mediate the association between physical activity and postmenopausal breast cancer risk (Neilson et al. 2009).
Differing levels of evidence support roles for these proposed biomarkers in breast cancer, but the greatest pool of epidemiologic data in postmenopausal women exists for insulin (Gunter et al. 2009, Kabat et al. 2009, Neilson et al. 2009) and IGF1 and its binding proteins (Key et al. 2010). The link between insulin and breast cancer is now sufficiently convincing that metformin, a pharmacologic agent that improves insulin sensitivity, is being considered for breast cancer prevention and treatment (Gonzalez-Angulo & Meric-Bernstam 2010) with clinical trials underway (Cazzaniga et al. 2009, Goodwin et al. 2009). An association between high circulating IGF1 and breast cancer risk has been demonstrated fairly consistently among premenopausal, but not postmenopausal, women (Fletcher et al. 2005) and associations with IGF-binding protein 3 (IGFBP3) have been inconsistent (Renehan et al. 2006). Nevertheless, a recent pooled analysis found a significantly increased risk of breast cancer with elevated IGF1 and IGFBP3 in postmenopausal women (Key et al. 2010).
Leptin and adiponectin are adipokines that relate strongly to obesity and insulin resistance (IR; Pittas et al. 2004, Dyck et al. 2006) but their roles in breast cancer are unclear. While it is biologically plausible that leptin increases postmenopausal breast cancer risk, epidemiological findings have been inconsistent (Neilson et al. 2009, Wu et al. 2009, Maccio et al. 2010). In contrast, most studies in postmenopausal women have shown significant inverse associations between adiponectin and breast cancer risk (Neilson et al. 2009). Emerging research suggests that the ratio of adiponectin:leptin (A/L or conversely, L/A) provides a surrogate measure of IR (Finucane et al. 2009), and perhaps also acts as an indicator of postmenopausal breast cancer risk, which may be more sensitive than either marker on its own (Cleary et al. 2009, Jarde et al. 2009).
Since all of these proposed biomarkers are related to IR and obesity, we hypothesized that their levels could be modified with sustained physical activity. Only a few studies have addressed this question previously via randomized controlled trials (RCTs) on postmenopausal women (Neilson et al. 2009), including one RCT that focused on breast cancer (Frank et al. 2005). A need for additional tightly controlled, long-term intervention trials to examine the impact of exercise on proposed biomarkers for breast cancer risk has been widely recognized (McTiernan et al. 1999, Friedenreich 2001, IARC Working Group 2002, Kushi et al. 2006, Physical Activity Guidelines Advisory Committee 2008). In response to this recognized need for more research, the Alberta Physical Activity and Breast Cancer Prevention (ALPHA) Trial was a 12-month exercise intervention trial designed to improve the understanding of the biologic mechanisms mediating the association between physical activity and postmenopausal breast cancer risk. We previously reported that the intervention significantly decreased adiposity (Friedenreich et al. 2010b) and circulating estradiol and increased sex hormone-binding globulin (Friedenreich et al. 2010a), which were primary outcomes of the trial. Here, we report on metabolic factors as secondary outcomes, namely, insulin, glucose, homeostasis model assessment of IR (HOMA-IR), IGF1, IGFBP3, IGF1/IGFBP3 ratio, leptin, adiponectin, and A/L ratio.
Materials and methods
The design and methods for the ALPHA Trial have been described (Friedenreich et al. 2010a). In brief, this study was an efficacy trial designed as a two-centred, two-armed RCT conducted in postmenopausal women living in Calgary and Edmonton, Alberta, Canada. The study and protocol were approved by the Research Ethics Boards at the Alberta Cancer Board and the Universities of Calgary and Alberta. All participants provided written informed consent.
Participants and randomization
We recruited women from the general population through targeted mailings to participants in the Alberta Breast Screening Program, posters and brochures distributed to family physicians, and through media campaigns. Specific eligibility criteria included: age 50–74 years; postmenopausal; no previous cancer diagnosis; no major co-morbidities; acceptable heart and lung function assessed by a fitness test; inactive (<90 min/week of exercise or, if 90–120 min/week, having a VO2max level <34 ml/kg per min); physician clearance for unrestricted physical activity; normal fasting levels of glucose, serum cholesterol, TSH, alanine aminotransferase; body mass index (BMI) 22–40 kg/m2; non-smoker; <14 drinks of alcohol/week; no medications or exogenous hormones that might influence estrogen metabolism; and not currently in a weight loss program or planning to undertake the one.
A stratified (on center and BMI), blocked randomization was achieved using a random number program in S-plus written by the study biostatistician (R F B). Numbered, sealed envelopes were opened by the Study Coordinator at the time of randomization. Outcome assessments were blinded to group assignment.
Intervention
The exercise prescription was aerobic exercise for at least 45 min, 5 days/week for 1 year. At least three sessions/week were facility-based with on-site exercise trainers, and the remaining sessions were home-based. Participants wore heart rate monitors (Polar A3) to maintain workout intensity at the target of 70–80% of heart rate reserve. The prescription ramped up over the first 3 months and was maintained for the final 9 months. Adherence was monitored through weekly participant- and trainer-administered exercise logs. Controls were asked to maintain their inactive lifestyle. All participants were instructed not to change their usual diet.
Covariate information
Demographic characteristics, medical and reproductive history were obtained from a self-administered baseline questionnaire; all other covariate measures were taken at baseline and 12 months. Dietary intake of the previous year was assessed using the National Cancer Institute's Diet History Questionnaire previously adapted for use in Canadian populations (Csizmadi et al. 2007) and physical activity was assessed using the past year total physical activity questionnaire (Friedenreich et al. 2006). Fitness was assessed using a modified Balke treadmill protocol to estimate maximum oxygen consumption (VO2max) from submaximal exercise intensities. Duplicate anthropometric measurements were taken using standardized methods. Total body fat was assessed using whole body dual X-ray absorptiometry scans (Friedenreich et al. 2010b). Percent body fat was calculated as 100%×(fat mass/(fat mass+lean mass)).
Blood collection and hormone assays
Blood was collected after a minimum 10 h fast at baseline (60 ml), 6 and 12 months (40 ml), and medications taken in the past 24 h were recorded. Participants were asked not to exercise for 24 h pre-blood draw. All blood samples were collected, processed, and stored within 12 h of collection, then shipped and stored in −86 °C freezers until assay. Analyses were conducted by the Reproductive Endocrine Research Laboratory at the University of Southern California (FZS), a laboratory with well-established protocols and quality control procedures.
Each participant's samples from the three time points were included in a single batch, but the order was randomized. Each batch had an equal number of samples from exercisers and controls with similar randomization dates and two pooled quality control samples. The quality control serum pool consisted of blood drawn from women who were interested in the study, postmenopausal, and not taking hormone replacement therapy, but deemed ineligible after blood draw for reasons unrelated to the metabolic factors of interest. Additional repeat blood samples from volunteers were included to assess within-subject, between-time variability. Laboratory personnel were blinded to subject and quality control sample identity.
Insulin, IGF1 and IGFBP3 were measured by direct chemiluminescent immunoassay using the Immulite Analyzer (Siemens Medical Solutions Diagnostics, Malvern, PA, USA); intra-assay coefficients of variation (CV) were 3, 2 and 7%, and inter-assay CV were 3, 4 and 7% respectively. Glucose was measured using the oxygen rate method, employing an oxygen electrode on a Beckman glucose analyzer (Model DT60II, Vitros System, Johnson & Johnson, Rochester, NY, USA); the intra-assay CV was 8% and the inter-assay CV was 13%. A surrogate measure of whole body insulin sensitivity and β-cell function, HOMA-IR, was calculated as: fasting glucose (mM)×fasting insulin (μIU/ml)/22.5 (Hosker et al. 1985, Matthews et al. 1985, Bonora et al. 1989, Levy et al. 1991, Rudenski et al. 1991, Turner et al. 1993). Leptin and total adiponectin were measured by RIA (Millipore, St Charles, MO, USA); intra-assay and inter-assay CV were 6 and 5%, and 8 and 12% respectively.
Sample size
Sample size calculations were based on the primary estrogen and adiposity outcomes for which power ≥80% was available (Friedenreich et al. 2010a). For IR outcomes, using the same approach for sample size estimation as for the primary outcomes, namely the normal theory formula for comparing means of two independent samples with α=0.05 (two-sided; Rosner 1986) and s.d. from the Physical Activity for Total Health trial, (Irwin et al. 2003, McTiernan et al. 2004) we had power >95% to detect changes of 10–20%.
Statistical analysis
The primary analysis assessed the intervention effect based on assigned group at randomization regardless of adherence for participants with complete data at baseline and end of study (intent-to-treat). Skewness in these biomarkers was corrected using natural logarithm transformations. Intervention effects were evaluated with general linear models considering IR indicators, IGF1, IGFBP3, leptin, and adiponectin measures at 6 and 12 months as repeated measures.
Specifically, we used the following general linear model:
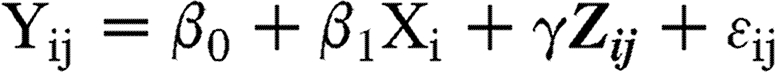 |
where, for ith subject, Yij is a log-transformed biomarker measure at jth time (j=1 for 6 months and j=2 for 12 months), Xi is an indicator variable for the exercise intervention arm (0 for control and 1 for exercise intervention), Zij is a vector of adjustment variables including the baseline biomarker value, time, and change in the total energy intake, and (ϵi1, ϵi2) is a potentially correlated error vector that followed a bivariate Gaussian distribution with a mean of 0. Of the parameters β0, β1, and γ (associated with the intercept, exercise intervention, and adjustment variables respectively), β1 was our main interest, representing the adjusted difference in the mean log biomarker measure between the exercise intervention group and the control group over the two follow-up time points, adjusting for the baseline biomarker value, time, and change in the total energy intake. We referred to the exponential of β1 as the treatment effect ratio (TER), as it is a ratio of adjusted geometric means of the biomarker for the exercise intervention group over the control group.
Secondary analyses examined whether or not the effect of exercise on the metabolic factors varied by adherence and body composition change from baseline to 12 months. Spearman's rank correlation coefficients were estimated to relate changes in metabolic factors to changes in percent body fat in exercisers. We classified exercise adherence into three categories pre-defined by public health guidelines: <150, 150–225, and >225 min/week (Warburton et al. 2007, Physical Activity Guidelines Advisory Committee 2008). All statistical tests were two-sided with a significance level set at 0.05. Statistical analyses were performed using SAS Software (Version 9.1; SAS Institute, Inc., Cary, NC, USA).
Results
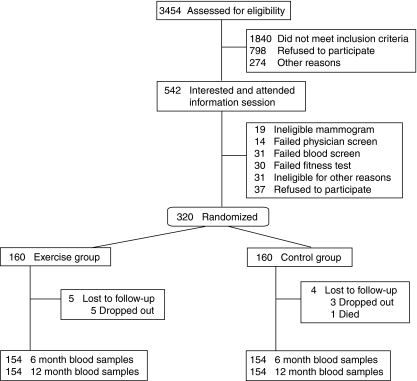
A total of 3454 women were assessed for eligibility; 320 women were randomized and nine were lost to follow-up post-randomization. The primary analysis included 154 exercisers and 154 controls with 6-month blood samples and 154 exercisers and 156 controls with 12-month blood samples (Fig. 1). Recruitment began in May 2003 and was completed in June 2006. Exercisers and controls were similar at baseline with respect to age, body composition, and proposed biomarker concentrations (Table 1). Baseline leptin concentrations were high relative to normal weight women (Mahabir et al. 2007) and ∼80% of women were overweight or obese (BMI≥25).
Figure 1.
Recruitment, randomization, and follow-up of participants in the Alberta Physical Activity and Breast Cancer Prevention Trial 2003–2006.
Table 1.
Baseline characteristics of participants, Alberta physical activity, and Breast Cancer Prevention Trial 2003–2006, n=320a
| Exercisers (n=160) | Controls (n=160) | |
|---|---|---|
| Baseline characteristic | Mean±s.d. | Mean±s.d. |
| Age (years) | 61.2±5.4 | 60.6±5.7 |
| Body composition measurements | ||
| Body mass index (kg/m2) | 29.1±4.5 | 29.2±4.3 |
| Intra-abdominal fat area (cm2) | 101.4±55.4 | 103.2±56.0 |
| Total body fat (kg) | 30.9±8.2 | 31.3±8.6 |
| Percent body fat | 42.2±4.9 | 42.4±5.7 |
| N (%) | N (%) | |
|---|---|---|
| Education (>high school) | 112 (70) | 102 (64) |
| White race/ethnicity | 144 (91) | 145 (91) |
| Median (IQR) | Median (IQR) | |
|---|---|---|
| Insulin (μIU/ml) | 6.2 (3.8–9.5) | 5.7 (3.4–8.9) |
| Glucose (mM) | 5.4 (4.9–6.1) | 5.3 (4.9–5.8) |
| HOMA-IR | 1.4 (0.9–2.4) | 1.4 (0.8–2.3) |
| IGF1 (ng/ml) | 117 (95–138) | 119 (100–141) |
| IGFBP3 (μg/ml) | 4.0 (3.5–4.5) | 3.9 (3.4–4.5) |
| Leptin (ng/ml) | 19.2 (13.5–25.6) | 20.9 (12.8–30.1) |
| Adiponectin (μg/ml) | 11.5 (9.1–17.2) | 12.5 (9.6–16.1) |
| Adiponectin/leptin | 0.63 (0.38–1.07) | 0.64 (0.37–1.09) |
| IGF1/IGFBP3 | 29.5 (25.9–33.6) | 30.5 (25.9–40.0) |
IQR, interquartile range; HOMA-IR, homeostasis model assessment of insulin resistance=fasting glucose (mM)×fasting insulin (μIU/ml)/22.5; IGF1, insulin-like growth factor 1; IGFBP3, insulin-like growth factor-binding protein 3.
There were no statistically significant differences at baseline between exercisers and controls for these variables.
Adherence to our intervention has been previously reported (Friedenreich et al. 2010a). In brief, exercisers reported a larger 12-month increase in recreational activity than controls (20.2 vs 3.2 metabolic equivalent (MET)-hours/week, P<0.001). Sixteen controls (10%) reported increasing recreational activity levels by 20 MET-hours/week (equivalent to 200 min/week activity ≥6 MET-level (vigorous)) or more. Physical fitness, measured by VO2max, increased in exercisers versus controls (3.9 vs 0.7 ml/kg per min, P<0.001).
The main analyses showed an inverse effect of exercise on insulin, HOMA-IR, and leptin (P<0.001; Table 2) and a positive effect on the A/L ratio (P=0.001). The greatest changes in exercisers occurred in the first 6 months. Twelve-month percent changes relative to baseline were −10.3% for insulin, −11.4% for HOMA-IR, −18.9% for leptin, and +24.9% for the A/L ratio. There was no difference between exercisers and controls across 12 months for changes in glucose, IGF1, IGFBP3, IGF1/IGFBP3, or adiponectin.
Table 2.
Difference between exercisers and controls on concentrations of proposed biomarkers over 6 and 12 months from baseline
| Baseline | 6 months | 12 months | |||
|---|---|---|---|---|---|
| Geometric mean (95% CI)* | Geometric mean (95% CI) | Geometric mean (95% CI) | Treatment effect ratio of exercise/control (95% CI)a | Between-group P value | |
| Insulin (μIU/ml) | |||||
| Exercisersb | 6.1 (5.5 to 6.8) | 5.4 (4.8 to 5.9) | 5.3 (4.8 to 5.9) | 0.87 (0.81 to 0.93) | <0.001 |
| Controlsc | 5.7 (5.2 to 6.3) | 6.0 (5.4 to 6.7) | 5.9 (5.3 to 6.6) | ||
| Glucose (mM) | |||||
| Exercisers | 5.5 (5.4 to 5.7) | 5.5 (5.3 to 5.7) | 5.5 (5.3 to 5.6) | 0.99 (0.97 to 1.02) | 0.581 |
| Controls | 5.5 (5.3 to 5.6) | 5.5 (5.4 to 5.7) | 5.5 (5.3 to 5.6) | ||
| HOMA-IR | |||||
| Exercisers | 1.5 (1.3 to 1.7) | 1.3 (1.2 to 1.5) | 1.3 (1.1 to 1.4) | 0.86 (0.80 to 0.93) | <0.001 |
| Controls | 1.4 (1.2 to 1.5) | 1.5 (1.3 to 1.6) | 1.4 (1.3 to 1.6) | ||
| IGF1 (ng/ml) | |||||
| Exercisers | 117 (112 to 123) | 114 (109 to 119) | 115 (110 to 121) | 1.00 (0.98 to 1.02) | 0.995 |
| Controls | 120 (114 to 126) | 116 (110 to 122) | 116 (111 to 122) | ||
| IGFBP3 (μg/ml) | |||||
| Exercisers | 3.9 (3.8 to 4.0) | 3.8 (3.7 to 4.0) | 3.8 (3.7 to 3.9) | 0.99 (0.97 to 1.00) | 0.163 |
| Controls | 3.9 (3.8 to 4.0) | 3.9 (3.7 to 4.0) | 3.9 (3.7 to 4.0) | ||
| IGF1/IGFBP3 | |||||
| Exercisers | 29.9 (28.9 to 31.0) | 29.6 (28.6 to 30.6) | 30.1 (29.1 to 31.2) | 1.01 (0.99 to 1.03) | 0.377 |
| Controls | 30.6 (29.3 to 31.9) | 29.9 (28.7 to 31.2) | 30.1 (28.9 to 31.3) | ||
| Leptin (ng/ml) | |||||
| Exercisers | 18.8 (17.3 to 20.4) | 14.8 (13.5 to 16.2) | 14.9 (13.5 to 16.5) | 0.82 (0.78 to 0.87) | <0.001 |
| Controls | 19.5 (17.7 to 21.4) | 18.5 (16.7 to 20.4) | 19.1 (17.4 to 21.1) | ||
| Adiponectin (μg/ml) | |||||
| Exercisers | 11.8 (10.9 to 12.9) | 11.7 (10.7 to 12.7) | 12.2 (11.3 to 13.3) | 0.99 (0.97 to 1.02) | 0.680 |
| Controls | 12.2 (11.4 to 13.1) | 12.3 (11.4 to 13.2) | 12.2 (11.4 to 13.1) | ||
| Adiponectin/leptin | |||||
| Exercisers | 0.63 (0.56 to 0.71) | 0.79 (0.69 to 0.90) | 0.82 (0.71 to 0.94) | 1.21 (1.13 to 1.28) | <0.001 |
| Controls | 0.63 (0.56 to 0.71) | 0.67 (0.59 to 0.75) | 0.64 (0.56 to 0.72) |
CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance; IGF1, insulin-like growth factor 1; IGFBP3, IGF-binding protein 3.
The treatment effect ratio was calculated from a general linear model for each biomarker outcome, estimating a parameter whose anti-logarithm corresponds to the ratio of adjusted geometric means of the biomarker for the exercise intervention group over the control group: this ratio was assumed to be common at 6 and 12 months post-randomization.
Exercise group: n, baseline=160, 6 month=154, 12 month=154.
Control group: n, baseline=160, 6 month=154, 12 month=156.
In secondary analyses, exercise duration was consistently associated with monotonic reductions in insulin, HOMA-IR, and leptin concentrations (Table 3) but not glucose, IGF1, IGFBP3, IGF1/IGFBP3, or adiponectin (data not shown). The A/L ratio increased with increasing weekly duration of exercise. The highest decreases of 16 and 30% were achieved with exercise >225 min/week for HOMA-IR and leptin, respectively, while the A/L ratio increased by 48% in this subgroup; also glucose decreased significantly (5.4%, P=0.013). No significant changes (P<0.05) occurred with exercise <150 min/week for any proposed biomarker.
Table 3.
Concentrations of proposed biomarkers at baseline and 12 months in controls and exercisers by three adherence levels
| Baseline | 12 months | |||||
|---|---|---|---|---|---|---|
| Geometric mean (95% CI) | Geometric mean (95% CI) | Ratio 12 months/baseline (95% CI)a | Percent changeb | P valuec | P for trendd | |
| Insulin (μIU/ml) | ||||||
| Controlse | 5.6 (5.1 to 6.2) | 5.9 (5.3 to 6.6) | 1.05 (0.98 to 1.13) | 5.4 | Ref | <0.001 |
| <150 min/weeke | 7.4 (5.9 to 9.3) | 7.1 (5.8 to 8.7) | 0.96 (0.81 to 1.13) | −4.4 | 0.679 | |
| 150–225 min/weeke | 5.6 (4.8 to 6.6) | 4.9 (4.2 to 5.7) | 0.87 (0.79 to 0.96) | −13.1 | 0.002 | |
| >225 min/weeke | 5.3 (4.4 to 6.4) | 4.7 (4.0 to 5.6) | 0.89 (0.79 to 0.99) | −11.2 | 0.010 | |
| HOMA-IR | ||||||
| Controls | 1.4 (1.2 to 1.5) | 1.4 (1.3 to 1.6) | 1.05 (0.97 to 1.13) | 4.8 | Ref | <0.001 |
| <150 min/week | 1.9 (1.4 to 2.4) | 1.8 (1.4 to 2.3) | 0.97 (0.81 to 1.16) | −3.4 | 0.860 | |
| 150–225 min/week | 1.3 (1.1 to 1.6) | 1.2 (1.0 to 1.4) | 0.87 (0.78 to 0.97) | −12.8 | 0.007 | |
| >225 min/week | 1.3 (1.0 to 1.6) | 1.1 (0.9 to 1.3) | 0.84 (0.75 to 0.95) | −16.0 | 0.002 | |
| Leptin (ng/ml) | ||||||
| Controls | 19.3 (17.5 to 21.2) | 19.1 (17.4 to 21.1) | 0.99 (0.94 to 1.05) | −0.7 | Ref | <0.001 |
| <150 min/week | 18.0 (15.4 to 20.9) | 17.3 (14.5 to 20.7) | 0.96 (0.87 to 1.07) | −3.6 | 0.513 | |
| 150–225 min/week | 19.9 (17.5 to 22.7) | 16.1 (13.8 to 18.7) | 0.81 (0.74 to 0.88) | −19.2 | <0.001 | |
| >225 min/week | 16.8 (14.4 to 19.6) | 11.8 (9.8 to 14.3) | 0.70 (0.61 to 0.81) | −29.6 | <0.001 | |
| Adiponectin/leptin | ||||||
| Controls | 0.64 (0.56 to 0.72) | 0.64 (0.56 to 0.72) | 1.00 (0.94 to 1.07) | 0.2 | Ref | <0.001 |
| <150 min/week | 0.66 (0.51 to 0.86) | 0.70 (0.52 to 0.94) | 1.06 (0.94 to 1.20) | 6.2 | 0.378 | |
| 150–225 min/week | 0.63 (0.54 to 0.75) | 0.77 (0.64 to 0.94) | 1.22 (1.11 to 1.34) | 21.9 | 0.001 | |
| >225 min/week | 0.68 (0.55 to 0.86) | 1.02 (0.78 to 1.33) | 1.48 (1.29 to 1.71) | 48.4 | <0.001 |
CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance.
Ratio of geometric means at 12 months to geometric means at baseline.
Percent change in proposed biomarker at 12 months from baseline for that level or group.
P values for change in proposed biomarker at 12 months from baseline between controls and that level of exercise adherence group adjusted for the baseline value.
Test for trend in change in proposed biomarker at 12 months from baseline cross controls and three adherence groups adjusted for the baseline value.
N=156, 40, 67, and 47 for controls and three exercise adherence levels ≤150, 150–225, >225 min/week, respectively.
The effects of the intervention on insulin, HOMA-IR, leptin, and the A/L ratio were attenuated after adjustment for adiposity change (Table 4). Adjustment for change in total or percent body fat had a greater impact on TER than changes in body weight or intra-abdominal fat, with effects becoming non-statistically significant for HOMA-IR (P=0.073 and P=0.065) and the A/L ratio (P=0.207 for both adiposity measures). Correlations between percent body fat change and metabolic changes in exercisers (data not shown) were strongest for leptin (rS=0.50, P<0.001) and the A/L ratio (rS=−0.55, P<0.001).
Table 4.
Treatment effect ratios for changes in proposed biomarkers over 6 and 12 months from baseline, before and after adjustment for adiposity change
| Treatment effect ratio of exercise/control (95% CI)a | P value | |
|---|---|---|
| Insulin (μIU/ml) | ||
| No adjustment | 0.87 (0.81 to 0.93) | <0.001 |
| Adjustment for weight change | 0.90 (0.84 to 0.96) | 0.002 |
| Adjustment for % body fat change | 0.92 (0.86 to 0.99) | 0.021 |
| Adjustment for total body fat change | 0.92 (0.86 to 0.99) | 0.021 |
| Adjustment for intra-abdominal fat area change | 0.91 (0.85 to 0.98) | 0.011 |
| HOMA-IR | ||
| No adjustment | 0.86 (0.80 to 0.93) | <0.001 |
| Adjustment for weight change | 0.91 (0.84 to 0.98) | 0.009 |
| Adjustment for % body fat change | 0.93 (0.87 to 1.01) | 0.073 |
| Adjustment for total body fat change | 0.93 (0.87 to 1.00) | 0.065 |
| Adjustment for intra-abdominal fat area change | 0.92 (0.85 to 0.99) | 0.027 |
| Leptin (ng/ml) | ||
| No adjustment | 0.82 (0.78 to 0.87) | <0.001 |
| Adjustment for weight change | 0.91 (0.87 to 0.95) | <0.001 |
| Adjustment for % body fat change | 0.95 (0.90 to 0.99) | 0.023 |
| Adjustment for total body fat change | 0.94 (0.90 to 0.99) | 0.018 |
| Adjustment for intra-abdominal fat area change | 0.89 (0.85 to 0.95) | <0.001 |
| Adiponectin/leptin | ||
| No adjustment | 1.21 (1.13 to 1.28) | <0.001 |
| Adjustment for weight change | 1.08 (1.03 to 1.14) | 0.004 |
| Adjustment for % body fat change | 1.04 (0.98 to 1.09) | 0.207 |
| Adjustment for total body fat change | 1.03 (0.98 to 1.09) | 0.207 |
| Adjustment for intra-abdominal fat area change | 1.10 (1.03 to 1.17) | 0.003 |
CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance.
The geometric mean ratios were estimated from least square means for the difference in treatment effect between exercisers and controls averaged across the entire study period adjusted for the baseline values, and then back log-transformed.
Discussion
This year-long aerobic exercise intervention among postmenopausal inactive women resulted in reductions in insulin, HOMA-IR, and leptin concentrations and an increased A/L ratio, an emerging novel marker of IR. Changes in glucose, IGF1, IGFBP3, and adiponectin were not observed. This trial has provided new and strong evidence for the role of these biomarkers in the association between physical activity and breast cancer risk that supports preliminary findings from previous studies and expands that research to other biomarkers.
Only a few large RCTs have examined the effects of a long-term exercise-only intervention on these metabolic factors in older women. Of five previous noteworthy RCTs with similar study populations and/or outcomes of interest (Houmard et al. 2004, Frank et al. 2005, Giannopoulou et al. 2005, Pi-Sunyer et al. 2007, Arsenault et al. 2009), only one trial lead by McTiernan (Frank et al. 2005, McTiernan et al. 2005) was of comparable size, duration and scope to the ALPHA Trial. In contrast to the other trials, the ALPHA Trial included healthy, postmenopausal women with a BMI ranging from 22–40 kg/m2 rather than women with a BMI >25 kg/m2. Consequently, these results are more generalizable to the population at risk for postmenopausal breast cancer. The ALPHA Trial also had several other study design features that distinguish it from earlier RCTs, including the largest sample size of healthy women, the most tightly controlled exercise intervention that was supervised for the entire 12-month period, a minimal loss to follow-up, and the novel combination of biomarkers that were examined. In particular, no previous RCT has examined the effect of exercise on the ratio of adiponectin/leptin. Since this ratio appears to estimate IR (Finucane et al. 2009), it was only recently proposed as a biomarker of breast cancer risk (Cleary et al. 2009, Jarde et al. 2009). Thus, our study provides a new insight, using a potentially stronger indicator of breast cancer risk than leptin or adiponectin on its own, into how exercise impacts the causal pathway between exercise and postmenopausal breast cancer.
As with the McTiernan trial (Frank et al. 2005), our study demonstrated decreased insulin concentrations and HOMA-IR in exercisers that differed significantly from changes in controls across 12 months of exercise; no significant change in glucose was found in either trial. Exercise also lowered circulating insulin significantly in the Dose–Response to Exercise in Postmenopausal Women (DREW) trial, a 6-month exercise RCT in 349 overweight/obese postmenopausal women with elevated blood pressure; glucose levels also decreased significantly (Arsenault et al. 2009). In general, exercise with moderate weight loss has been found to improve insulin sensitivity (Albright et al. 2000, Ryan 2000, Wareham et al. 2005) and prevent type 2 diabetes (Gill & Cooper 2008, Physical Activity Guidelines Advisory Committee 2008).
The impact of our intervention on breast cancer prevention has not been tested. However, varying insulin levels, comparable with the 12-month changes that we observed in exercisers (6.1 vs 5.3 μIU/ml), have significantly altered breast cancer risk in non-diabetic, postmenopausal women not using hormone therapy (Gunter et al. 2009). In a large etiologic study (Gunter et al. 2009), women with insulin levels 3.9 to <5.6 μIU/ml experienced a 41% lower risk of breast cancer than women with 5.6 to <8.8 μIU/ml insulin (midpoints of the ranges differed by 51%). Extrapolating those findings to our study, assuming a linear relation between insulin and breast cancer risk, we postulate that the 10% decrease in insulin experienced by our exercise group would lower breast cancer risk by ∼8%.
It remains unclear what exercise dose is optimal for improving insulin sensitivity. In secondary analyses, we observed significant trends between exercise adherence and insulin and HOMA-IR changes respectively. Another RCT, the Studies of Targeted Risk Reduction Interventions through Defined Exercise (STRRIDE), found improvements in insulin sensitivity in 154 overweight/obese adults that were greater with 170 min/week of exercise than with 115 min/week, supporting our finding of dose–response trends for insulin and HOMA-IR when exercise exceeded 150 min/week (Houmard et al. 2004). In another comparable trial (Frank et al. 2005), there was no benefit from exercising <130 min/week, but decreases in insulin and HOMA-IR were observed for exercise durations of 131 to 190 min/week; smaller decreases occurred when duration exceeded 190 min/week.
Our findings for an effect of aerobic exercise on insulin and HOMA-IR scores, which was attenuated but not wholly explained by changes in adiposity, are biologically plausible. Exercise may lower serum insulin levels by promoting fat loss and preserving lean body mass, and independently of fat loss by increasing the number and activity of glucose transporters in muscle and adipose tissue (Ryan 2000). Another trial of older women (Frank et al. 2005) found decreased insulin and HOMA-IR scores only in women whose total body fat was decreased by the intervention, which suggests these changes were driven by fat loss. We similarly showed total and percent body fat loss to be mediators of exercise-induced changes in HOMA-IR (Table 4). The same trial (Frank et al. 2005) showed no difference across changes in body weight, percent body fat or intra-abdominal adiposity, concordant with our findings for insulin.
Early epidemiological evidence (Orenstein & Friedenreich 2004) does not support an inverse association between chronic exercise and circulating levels of IGF1 and IGFBP3, particularly in older women. This evidence is consistent with our findings and other comparable RCTs (McTiernan et al. 2005, Seo et al. 2010) with one exception: that RCT (Orsatti et al. 2008) showed significantly increased IGF1 with exercise. A recent review also suggested that long-term exercise may actually increase circulating IGF1 (Nindl & Pierce 2010). Therefore, the evidence is weak that chronic exercise lowers IGF1 in postmenopausal women.
Given that leptin and adiponectin derive almost exclusively from adipose tissue, their levels should change with exercise-induced body fat loss and decreased central adiposity (Ritland et al. 2008), which was experienced in this trial (Friedenreich et al. 2010b). We observed reductions in leptin for exercisers, which increased in a dose-dependent manner with exercise adherence. Only a few studies have addressed the effects of exercise on leptin in postmenopausal women (Kohrt et al. 1996, Ryan et al. 2000, Hayase et al. 2002, Frank et al. 2005, Giannopoulou et al. 2005) and only two of them were RCTs (Frank et al. 2005, Giannopoulou et al. 2005). One trial (Frank et al. 2005) showed decreased leptin concentrations with exercise; however, in our most adherent subgroup, we observed more than twice the decrease in leptin (12% with >190 min/week (Frank et al. 2005) versus 30% with >225 min/week). In another RCT (Giannopoulou et al. 2005), leptin decreased in exercisers, but the change was of borderline statistical significance (P=0.06); this difference might be attributable to the higher volume of exercise undertaken in our trial or the minimal fat loss in the earlier study. Adiponectin levels have an inverse association with adiposity. Exercise may lower the levels of the obesity-related inflammatory markers, tumor necrosis factor-α, and interleukin 6, thereby increasing adiponectin gene expression and secretion (Bruun et al. 2003). However, we and others found no changes in adiponectin levels with exercise (Giannopoulou et al. 2005, Arsenault et al. 2009, Coker et al. 2009, Ligibel et al. 2009). Although RCT evidence relating chronic exercise to circulating adiponectin has been inconclusive (Simpson & Singh 2008), it is hypothesized that levels increase with sufficient weight loss (Kraemer & Castracane 2007, Christiansen et al. 2010), perhaps more than the 2.3 kg weight loss in our trial (Friedenreich et al. 2010b) and the others (Giannopoulou et al. 2005, Arsenault et al. 2009). We observed significantly greater increases in the A/L ratio in exercisers than in controls, driven mainly by decreased leptin. This finding indicates improved insulin sensitivity, as did the changes we observed for insulin and HOMA-IR; all three indices have been strongly associated with the insulin sensitivity index from hyperinsulinaemic–euglycaemic clamp studies (Finucane et al. 2009). Our secondary analysis suggests that total and percent body fat loss influenced the effect of exercise on the A/L ratio.
Our study's strengths include its large size, long duration, low drop-out rate, excellent adherence, reliable assays, and quantitative assessments of total and central adiposity. We studied the isolated effects of exercise by not intervening on diet and this RCT was the first to investigate sustained exercise-induced changes in the A/L ratio in older women. Limitations include some lack of compliance among the control group: 10% of controls increased recreational activity by 20 MET-hours/week. We used insulin and HOMA-IR as IR indicators, which are practical alternatives to the gold standard euglycaemic hyperinsulinemic clamp method (DeFronzo et al. 1979, Bergman et al. 1985). Furthermore, given the number of secondary analyses of these data, type I error is a possibility. In terms of generalizability, our findings describe non-diabetic, primarily Caucasian, normal weight to overweight and obese postmenopausal women who were inactive at baseline and, on average, had elevated leptin levels relative to normal weight women.
Conclusion
This RCT demonstrated that significant improvements in IR indicators, leptin, and A/L profiles can be achieved with sustained, moderate-to-vigorous aerobic exercise in previously inactive, postmenopausal women. Secondary analyses suggested that a decrease in total or percent body fat may be required to alter HOMA-IR and the A/L ratio. Similar to others, we observed no effect of long-term exercise on IGF1 or IGFBP3 levels. The benefits of improved insulin sensitivity include not only cancer risk reduction but also lower risk of metabolic syndrome, cardiovascular disease, and type 2 diabetes. Our finding of improved hormone levels with exercise ≥150 min/week is consistent with public health guidelines for prevention of all-cause mortality, cardiovascular disease, and type 2 diabetes (Physical Activity Guidelines Advisory Committee 2008) and lower than cancer prevention recommendations (World Cancer Research Fund and the American Institute for Cancer Research 2007), but because the ALPHA Trial was not designed to answer the question of exercise dose, further RCT evidence is needed to confirm our findings.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was funded by the Canadian Breast Cancer Research Alliance (#017468) and the Alberta Cancer Foundation (#22170). Dr C M F was funded by career awards from Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. Dr Courneya is supported by the Canada Research Chairs Program. The funders were not involved in any aspects of the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Acknowledgements
The study set-up was done by Kim van der Hoek and Marla Orenstein. The ALPHA Trial Study Coordinators were Rosemary Crosby and Ame-Lia Tamburrini. The Fitness Centre Managers were Ben Wilson, Lisa Workman, and Diane Cook. The Exercise Trainers were Shannon Hutchins, Kathy Traptow; Shannon Brown, Susan Daniel, Parissa Gillani, Stephanie Sanden, Karen Mackay, and Sandra Olsen. Data preparation and analysis were done by Sandra Blitz and Sony Brar.
References
- Albright A, Franz M, Hornsby G, Kriska A, Marrero D, Ullrich I, Verity LS. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Medicine and Science in Sports and Exercise. 2000;32:1345–1360. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Hormone and Metabolic Research. 2007;39:9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- Arsenault BJ, Cote M, Cartier A, Lemieux I, Despres JP, Ross R, Earnest CP, Blair SN, Church TS. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocrine Reviews. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. Journal of Clinical Endocrinology and Metabolism. 1989;68:374–378. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. PNAS. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. American Journal of Physiology. Endocrinology and Metabolism. 2003;285:E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Ando S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. Journal of Biological Chemistry. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiology, Biomarkers & Prevention. 2009;18:701–705. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- Chappell J, Leitner JW, Solomon S, Golovchenko I, Goalstone ML, Draznin B. Effect of insulin on cell cycle progression in MCF-7 breast cancer cells. Direct and potentiating influence. Journal of Biological Chemistry. 2001;276:38023–38028. doi: 10.1074/jbc.M106008200. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. American Journal of Physiology. Endocrinology and Metabolism. 2010;298:E824–E831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. Journal of Cellular Biochemistry. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Ray A, Rogozina OP, Dogan S, Grossmann ME. Targeting the adiponectin:leptin ratio for postmenopausal breast cancer prevention. Frontiers in Bioscience. 2009;1:329–357. doi: 10.2741/s30. [DOI] [PubMed] [Google Scholar]
- Coker RH, Williams RH, Kortebein PM, Sullivan DH, Evans WJ. Influence of exercise intensity on abdominal fat and adiponectin in elderly adults. Metabolic Syndrome and Related Disorders. 2009;7:363–368. doi: 10.1089/met.2008.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csizmadi I, Kahle L, Ullman R, Dawe U, Zimmerman T, Friedenreich CM, Bryant HE, Subar A. Adaptation and evaluation of the National Cancer Institute's Dietary History Questionnaire and nutrient database for use in Canadian Populations. Public Health Nutrition. 2007;10:88–96. doi: 10.1017/S1368980007184287. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. American Journal of Physiology. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Bussiere M, Dos SE, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochemical and Biophysical Research Communications. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiologica. 2006;186:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- Finucane FM, Luan J, Wareham NJ, Sharp SJ, O'Rahilly S, Balkau B, Flyvbjerg A, Walker M, Hojlund K, Nolan JJ, et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52:2345–2349. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher O, Gibson L, Johnson N, Altmann DR, Holly JM, Ashworth A, Peto J, Silva IS. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: a systematic review. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:2–19. [PubMed] [Google Scholar]
- Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, Irwin ML, Rudolph RE, Rajan KB, Stanczyk F, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obesity Research. 2005;13:615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:287–301. [PubMed] [Google Scholar]
- Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. British Journal of Sports Medicine. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Courneya KS, Neilson HK, Matthews CE, Willis G, Irwin M, Troiano R, Ballard-Barbash R. Reliability and validity of the Past Year Total Physical Activity Questionnaire. American Journal of Epidemiology. 2006;163:959–970. doi: 10.1093/aje/kwj112. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, Terry T, Boyd NF, Yaffe MJ, Irwin ML, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. Journal of Clinical Oncology. 2010a;28:1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenreich CM, Woolcott CG, McTiernan A, Terry T, Brant R, Ballard-Barbash R, Irwin ML, Jones CA, Boyd NF, Yaffe MJ, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. International Journal of Obesity. 2010b;35:427–435. doi: 10.1038/ijo.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler J, Haynes B, Ekse D, Dowsett M, Lonning PE. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. Journal of Steroid Biochemistry and Molecular Biology. 2007;104:27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, Kanaley JA. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Medicine. 2008;38:807–824. doi: 10.2165/00007256-200838100-00002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clinical Cancer Research. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. Journal of Clinical Oncology. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. Journal of National Cancer Institute. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase H, Nomura S, Abe T, Izawa T. Relation between fat distributions and several plasma adipocytokines after exercise training in premenopausal and postmenopausal women. Journal of Physiological Anthropology & Applied Human Science. 2002;21:105–113. doi: 10.2114/jpa.21.105. [DOI] [PubMed] [Google Scholar]
- Hosker JP, Matthews DR, Rudenski AS, Burnett MA, Darling P, Bown EG, Turner RC. Continuous infusion of glucose with model assessment: measurement of insulin resistance and beta-cell function in man. Diabetologia. 1985;28:401–411. doi: 10.1007/BF00280882. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. Journal of Applied Physiology. 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- IARC Working Group 2002 Weight Control and Physical Activity. In IARC Handbook of Cancer Prevention, vol 6. Eds H Vainio & F Bianchini. Lyon, France: IARC Press.
- Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, Yukawa M, Aiello E, Potter JD, McTiernan A. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. Journal of the American Medical Association. 2003;289:323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- Jarde T, Caldefie-Chezet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, Vasson MP. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocrine-Related Cancer. 2009;16:1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- Kabat GC, Kim M, Caan BJ, Chlebowski RT, Gunter MJ, Ho GY, Rodriguez BL, Shikany JM, Strickler HD, Vitolins MZ, et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. International Journal of Cancer. 2009;125:2704–2710. doi: 10.1002/ijc.24609. [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncology. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt WM, Landt M, Birge SJ., Jr Serum leptin levels are reduced in response to exercise training, but not hormone replacement therapy, in older women. Journal of Clinical Endocrinology and Metabolism. 1996;81:3980–3985. doi: 10.1210/jc.81.11.3980. [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Castracane VD. Exercise and humoral mediators of peripheral energy balance: ghrelin and adiponectin. Experimental Biology and Medicine. 2007;232:184–194. [PubMed] [Google Scholar]
- Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA: A Cancer Journal for Clinicians. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2008;13:371–379. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- Levy JC, Rudenski A, Burnett M, Knight R, Matthews DR, Turner RC. Simple empirical assessment of beta-cell function by a constant infusion of glucose test in normal and type 2 (non-insulin-dependent) diabetic subjects. Diabetologia. 1991;34:488–499. doi: 10.1007/BF00403285. [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Giobbie-Hurder A, Olenczuk D, Campbell N, Salinardi T, Winer EP, Mantzoros CS. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes & Control. 2009;20:1523–1528. doi: 10.1007/s10552-009-9358-3. [DOI] [PubMed] [Google Scholar]
- Lynch BM, Neilson HK & Friedenreich CM 2011 Physical activity and breast cancer prevention. In Recent Results in Cancer Research: Physical Activity and Cancer, vol 186, part 1, pp 13–42. Eds KS Courneya & CM Friedenreich. Berlin, Heidelberg, Germany: Springer-Verlag. ( 10.1007/978-3-642-04231-7_2) [DOI] [PubMed]
- Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Massa D, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. Journal of Molecular Medicine. 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- Mahabir S, Baer D, Johnson LL, Roth M, Campbell W, Clevidence B, Taylor PR. Body Mass Index, percent body fat, and regional body fat distribution in relation to leptin concentrations in healthy, non-smoking postmenopausal women in a feeding study. Nutrition Journal. 2007;6:3. doi: 10.1186/1475-2891-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Schwartz RS, Potter J, Bowen D. Exercise clinical trials in cancer prevention research: a call to action. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:201–207. [PubMed] [Google Scholar]
- McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Research. 2004;64:2923–2928. doi: 10.1158/0008-5472.CAN-03-3393. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Sorensen B, Yasui Y, Tworoger SS, Ulrich CM, Irwin ML, Rudolph RE, Stanczyk FZ, Schwartz RS, Potter JD. No effect of exercise on insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:1020–1021. doi: 10.1158/1055-9965.EPI-04-0834. [DOI] [PubMed] [Google Scholar]
- Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- Nindl BC, Pierce JR. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Medicine and Science in Sports and Exercise. 2010;42:39–49. doi: 10.1249/MSS.0b013e3181b07c4d. [DOI] [PubMed] [Google Scholar]
- Orenstein MR, Friedenreich CM. Review of physical activity and the IGF family. Journal of Physical Activity and Health. 2004;1:291–320. [Google Scholar]
- Orsatti FL, Nahas EA, Maesta N, Nahas-Neto J, Burini RC. Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas. 2008;59:394–404. doi: 10.1016/j.maturitas.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. PNAS. 1976;73:4536–4540. doi: 10.1073/pnas.73.12.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee 2008 Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC, USA: U.S. Department of Health and Human Services.
- Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocrine-Related Cancer. 2006;13:273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- Ritland LM, Alekel DL, Matvienko OA, Hanson KB, Stewart JW, Hanson LN, Reddy MB, Van L, Genschel U. Centrally located body fat is related to appetitive hormones in healthy postmenopausal women. European Journal of Endocrinology. 2008;158:889–897. doi: 10.1530/EJE-07-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B 1986 Fundamentals of Biostatistics. Edn 2. Boston, MA, USA: Duxbury Press.
- Rudenski AS, Matthews DR, Levy JC, Turner RC. Understanding "insulin resistance": both glucose resistance and insulin resistance are required to model human diabetes. Metabolism. 1991;40:908–917. doi: 10.1016/0026-0495(91)90065-5. [DOI] [PubMed] [Google Scholar]
- Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Medicine. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Pratley RE, Elahi D, Goldberg AP. Changes in plasma leptin and insulin action with resistive training in postmenopausal women. International Journal of Obesity and Related Metabolic Disorders. 2000;24:27–32. doi: 10.1038/sj.ijo.0801080. [DOI] [PubMed] [Google Scholar]
- Seo DI, Jun TW, Park KS, Chang H, So WY, Song W. 12 weeks of combined exercise is better than aerobic exercise for increasing growth hormone in middle-aged women. International Journal of Sport Nutrition and Exercise Metabolism. 2010;20:21–26. doi: 10.1123/ijsnem.20.1.21. [DOI] [PubMed] [Google Scholar]
- Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity. 2008;16:241–256. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- Turner RC, Levy JC, Rudenski AS, Hammersley M & Page R 1993 Measurement of insulin resistance and beta-cell function: the HOMA and CIGMA approach. In Current Topics in Diabetes Research, edn 12, pp 66–75. Eds F Belfiore, RN Bergman & GM Molinatti. Basel, Switzerland: Karger.
- Warburton DE, Katzmarzyk PT, Rhodes RE, Shephard RJ. Evidence-informed physical activity guidelines for Canadian adults. Canadian Journal of Public Health. 2007;98(Supplement 2):S16–S68. doi: 10.1139/H07-123. [DOI] [PubMed] [Google Scholar]
- Wareham NJ, Brage S, Franks PW, Abbott RA. Physical activity and insulin resistance. In: Kumar S, O'Rahilly S, editors. Insulin Resistance: Insulin Action and its Disturbances in Disease. Wiley; Chichester, UK: 2005. pp. 317–400. [Google Scholar]
- World Cancer Research Fund and the American Institute for Cancer Research 2007 Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC, USA: American Institute for Cancer Research.
- Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA. Circulating levels of leptin, adiposity and breast cancer risk. British Journal of Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. Journal of National Cancer Institute. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]