Abstract
Objectives:
Central gray matter damage, the hallmark of term acute perinatal hypoxia-ischemia, frequently leads to severe cerebral palsy and sometimes death. The precision with which these outcomes can be determined from neonatal imaging has not been fully explored. We evaluated the accuracy of early brain MRI for predicting death, the presence and severity of motor impairment, and ability to walk at 2 years in term infants with hypoxic-ischemic encephalopathy (HIE) and basal ganglia–thalamic (BGT) lesions.
Methods:
From 1993 to 2007, 175 term infants with evidence of perinatal asphyxia, HIE, and BGT injury seen on early MRI scans were studied. BGT, white matter, posterior limb of the internal capsule (PLIC), and cortex and brainstem abnormality were classified by severity. Motor impairment was staged using the Gross Motor Function Classification System.
Results:
The severity of BGT lesions was strongly associated with the severity of motor impairment (Spearman rank correlation 0.77; p < 0.001). The association between white matter, cortical, and brainstem injury and motor impairment was less strong and only BGT injury correlated significantly in a logistic regression model. The predictive accuracy of severe BGT lesions for severe motor impairment was 0.89 (95% confidence interval 0.83–0.96). Abnormal PLIC signal intensity predicted the inability to walk independently by 2 years (sensitivity 0.92, specificity 0.77, positive predictive value 0.88, negative predictive value 0.85). Brainstem injury was the only factor with an independent association with death.
Conclusion:
We have shown that in term newborns with HIE and BGT injury, early MRI can be used to predict death and specific motor outcomes.
Central gray matter damage, the hallmark of acute perinatal hypoxia-ischemia in term infants,1 is an important cause of death and cerebral palsy (CP). CP is a lifelong condition affecting not only motor function, but the child's global development. Commonly associated impairments include learning, visual, feeding, and communication difficulties and epilepsy that all place a heavy burden on the children and their families.2
Coping with the birth of a severely asphyxiated baby is extremely distressing for parents. Apart from the initial concern that their infant may die, parents have to deal with uncertainty about their child's future. They usually want to know not only if their child will have a motor problem, but its severity and whether their child will be able to walk3; unfortunately, these questions can be difficult to answer.
MRI is the optimal modality for the early evaluation of the site and severity of brain injury and the prediction of outcomes in infants with hypoxic-ischemic encephalopathy (HIE).4,5 Lesions in the basal ganglia and thalami (BGT) and the posterior limb of the internal capsule (PLIC) are predictive of CP,1,6 but the precision with which this outcome and indeed survival can be determined from neonatal imaging has not been fully explored.
The aim of this study was to evaluate the accuracy of neonatal MRI for predicting death, the presence and severity of motor impairment, and ability to walk at 2 years in term newborn infants with HIE and BGT lesions.
METHODS
Standard protocol approvals, registrations, and patient consents.
Ethical permission for scanning the infants was obtained from the Hammersmith Hospital research ethics committee and individually from the parents.
Patients.
Between January 1, 1993, and December 31, 2007, all infants with neonatal encephalopathy (NE) born at or referred to Hammersmith/Queen Charlotte's Hospitals were included if they met all the following criteria: 1) ≥35 weeks gestational age; 2) signs of fetal compromise (abnormal cardiotocography, meconium stained liquor, a sentinel event immediately before delivery or during labor); 3) poor condition at birth (5-minute Apgar score <5, arterial cord blood pH < 7.1, need for major resuscitation); 4) NE (difficulty initiating and maintaining respiration, altered consciousness, abnormal tone and reflexes, with/without seizures7); 5) BGT injury on MRI scans obtained within 6 postnatal weeks; 6) outcome assessment at a minimal age of 12 months.
Exclusion criteria were identifiable metabolic disorder, severe congenital malformation/infection, genetic abnormality, and hypothermia treatment for NE.
Perinatal data.
Demographic data, family history, and antenatal and perinatal data were documented from detailed information proformas completed neonatally. An estimate of illness severity (perinatal index) at birth was derived (Apgar score <3 at 1 and <5 at 5 minutes, pH < 7, need for full resuscitation, including cardiac compressions and epinephrine).
MRI.
Infants were imaged in a 1.0-, 1.5-, or 3-T MRI scanner, with conventional T1-weighted spin echo, inversion recovery, and T2-weighted spin echo sequences. Diffusion-weighted images were not evaluated as they were not always available and the age range at scan was wide.4
Images were assessed for anatomic development, evidence of more prolonged/subacute problems or longstanding established injury, and unusual patterns of injury. Abnormal signal intensities (SI) within the BGT, white matter (WM), PLIC, cortex, brainstem, and cerebellum were documented (table e-1 on the Neurology® Web site at www.neurology.org).
Outcomes.
Most infants attended our follow-up clinic and were assessed using a standarized neurologic examination,8 the Griffiths Mental Developmental scales,9 and head circumference measurement. For those not seen by us, this information was obtained from their local pediatric neurodevelopmental team.
The Surveillance of Cerebral Palsy in Europe (SCPE) definition and classification for CP were applied.10 Gross Motor Function Classification System (GMFCS)11 and classification of Bimanual Fine Motor Function (BFMF)2 were used to grade functional impairment. We classified gross motor impairment in children with CP as mild (level I of the GMFCS), moderate (GMFCS levels II and III), and severe (GMFCS levels IV and V). Age at and reason for death was documented. Head growth was analyzed using the Child Growth Foundation database and the LMSGrowth program.12
Statistical analyses.
Data were analyzed using SPSS version 11.5 (SPSS, Chicago, IL). The relation between the presence and severity of WM, cortex, BGT, PLIC, and brainstem injury with motor outcome and death was assessed by univariate analysis using the appropriate tests (χ2, Fisher exact test, Mann-Whitney) and the Spearman rank correlation coefficient. A logistic regression analysis was performed to identify brain structures independently associated with motor outcome and death. Prenatal and perinatal factors were included in the univariate analysis and where appropriate, in the logistic regression analysis. The predictive ability of the MRI abnormalities in each specific area for motor outcome and death was determined by calculation of the predictive accuracy, sensitivity, specificity, and positive and negative predictive values. Differences with p level < 0.05 were considered significant.
RESULTS
Of the 555 infants in our NE database (1993–2007), 175 fulfilled all entry criteria. Of the 380 infants not included were 186 with HIE without BGT lesions (normal MRI or WM/cortical injury only); 64 with metabolic diagnoses, congenital malformations/infections, or genetic abnormalities; 20 < 35 weeks GA; 41 treated with hypothermia; 59 scanned after 6 weeks; and 10 lost to follow-up.
The main antenatal and perinatal data are summarized in table e-2.
MRI analysis.
Median age at MRI scan was 10 days (range 2–42); 33% of infants were scanned between 2 and 7 days, 40% between 8 and 21 days, and 27% between 21 and 42 days.
BGT and PLIC abnormalities.
BGT lesions were mild in 28 infants (16%), moderate in 37 (21%), and severe in 110 (63%). The PLIC appeared normal in 24 infants (14%), equivocal in 18 infants (10%), and abnormal in 133 infants (76%).
WM and cortical abnormalities.
WM abnormality was mild in 26 infants (15%), moderate in 78 (45%), and severe in 65 (37%). Six infants had normal WM appearances. Cortical involvement was not seen in 17 infants (10%), was mild in 56 (32%), moderate in 55 (31%), and severe in 45 (26%).
Brainstem abnormality.
Brainstem lesions were seen in 119 infants (68%). Mesencephalic and pontine abnormalities were mild to moderate in 31 and 69 infants and severe in 85 and 38 infants, respectively. A total of 90% of infants with severe BGT lesions had some brainstem injury, which was severe in 67%. Only 2 infants with mild BGT injury had brainstem involvement.
Cerebellar abnormalities.
Cerebellar abnormalities were noted in 22 infants (13%) and were always associated with severe brainstem or BGT injury. In 12 infants scanned early (2–12 days) the abnormality was mainly mild vermis hypoplasia but also abnormal SI mostly in the dentate nuclei and a small unilateral hemorrhagic infarction in one infant. In 10 infants scanned later (13–42 days), the abnormality was mainly an increase in sulcal spaces suggesting atrophy but also abnormal SI in the cerebellar hemispheres.
Neurodevelopmental outcomes.
Median age at follow-up was 24 (range 12–48) months; 82% of surviving children were ≥18 months and 61% were ≥24 months; 28% (49) of infants died from neurologic problems, 23 neonatally (18 after withdrawal of intensive care), 17 during year 1, and 9 later.
Of the 126 surviving infants, 89 (71%) had CP. In 54% the pattern was spastic, in 35% it was dystonic or spastic-dystonic, and in 11% it was athetoid. Only 2 children had a hemiplegia and none had a diplegia. The severity of gross motor impairment was mild (GMFCS level I) in 9%, moderate (levels II/III) in 14%, and severe (levels IV/V) in 77%. There was a close relationship between gross and fine motor function and 60% of children had BFMF levels 4/5. Only 9 children with CP were able to walk at 2 years, and only one before 18 months. Of the 37 infants (29%) without CP, 14 had minor neurologic abnormality (mild asymmetries of tone, tremor, or abnormal reflexes), but all walked independently by 2 years.
MRI and motor outcome.
The severity of BGT injury correlated significantly with the presence and severity of motor impairment (Spearman rank correlation 0.77; p < 0.001). These data are detailed in table 1 and figure 1. The association between WM, cortical and brainstem injury with motor impairment was significant on univariate analysis, but only BGT injury correlated significantly in a logistic regression model (LRM). The predictive accuracy of severe BGT lesions for severe motor impairment (CP GMFCS levels IV/V) was 0.89 (95% confidence interval [CI] 0.83–0.96). Severe BGT lesions could predict severe motor impairment with a sensitivity of 0.96, a specificity of 0.77, a positive predictive value (PPV) of 0.85, and a negative predictive value (NPV) of 0.94. In children with moderate BGT injury, where prediction is most difficult, there were no differences in perinatal factors, or different patterns of injury in tissues other than the BGT between those who had severe motor impairment and those who were mildly affected or did not have CP.
Table 1.
Relation between the severity of basal ganglia and thalamic (BGT) injury and the severity of motor impairment
Abbreviation: PLIC = posterior limb of the internal capsule.
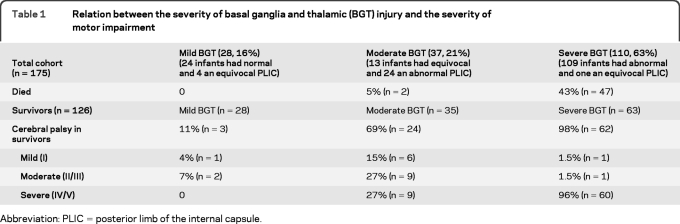
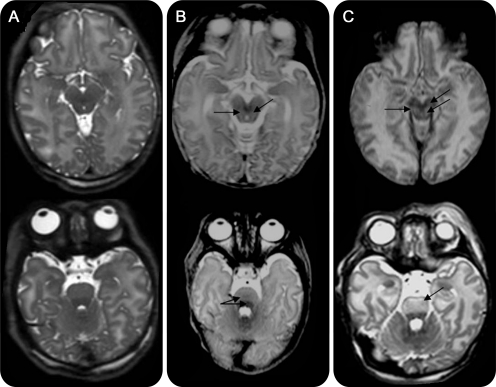
Figure 1. Basal ganglia and thalami (BGT) injury and motor outcome.
Axial T1-weighted images showing (A) mild, focal abnormal signal intensity (SI) in the BGT (arrow) with normal symmetric high SI from the myelinated posterior limb of the internal capsule (PLIC) (short arrows); (B) moderate, multifocal abnormal SI in the BGT (arrows); note the absence of high SI from myelin in the PLIC; and (C) severe, widespread abnormal SI in the BGT. There is no high SI from myelin in the PLIC. The relation between the severity of BGT injury and the severity of motor impairment is shown in table 1 (Spearman rank correlation 0.77; p < 0.001).
All the children with normal PLIC SI could walk independently by 2 years (mean age 14 ± 2.8 months, table 2 and figure 2). Of the children with an abnormal PLIC, only 12% could walk at 2 years, and most of them started walking after 18 months. An abnormal PLIC predicted the inability to walk independently by 2 years with a sensitivity of 0.92, a specificity of 0.77, a PPV of 0.88, and a NPV of 0.85. SI in the PLIC was equivocal in 18 infants, 12 of whom were walking by 2 years (6 by 18 months and 6 between 18 and 24 months), and 6, all of whom had CP, were not walking.
Table 2.
Relation between the signal intensity in the posterior limb of the internal capsule (PLIC) and the ability to walk at 2 years
Abbreviation: BGT = basal ganglia and thalami.
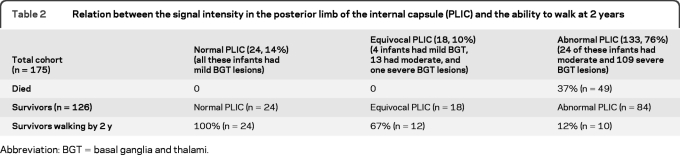
Figure 2. Signal intensity (SI) in the posterior limb of the internal capsule (PLIC) and ability to walk at 2 years.
Axial T1-weighted images showing (A) normal SI from myelin in the PLIC (arrow); (B) equivocal (reduced) SI in the PLIC (arrow); and (C) abnormal (absent) SI from myelin in the PLIC. The relation between SI in the PLIC and ability to walk at 2 years is shown in table 2 (p < 0.001).
MRI and death.
The presence and severity of brainstem injury had the strongest association with death neonatally and later (figure 3). The type and severity of brainstem lesions were similar between infants who died neonatally after withdrawal of care and those who died later. Mesencephalic and pontine lesions were independently and together related to death. BGT injury was associated with death on univariate analysis, but only brainstem injury correlated significantly in a LRM. The predictive accuracy of brainstem injury for death was 0.77 (95% CI 0.69–0.86). The LRM probability of death in the first 3 years with severe injury in mesencephalon and pons together was 61%.
Figure 3. Brainstem injury and death.
Axial T2-weighted images at the level of the mesencephalon (top row) and the pons (bottom row) showing (A) normal brainstem (56 infants, none died); (B) moderate brainstem injury (40 infants, 25% died), loss of anatomic details and focal high signal intensity (SI) in mesencephalon (arrows), and excessive differentiation between anterior and posterior pons (double arrow); and (C) severe injury (79 infants, 49% died); widespread abnormal SI in the mesencephalon and the pons (arrows). The difference among the 3 groups was statistically significant (p < 0.001).
The outcome in the 22 infants with cerebellar abnormality was extremely poor: 14 (63%) died and all 8 survivors developed severe CP (GMFCS levels IV/V).
Infants with a severe perinatal index were more likely to die neonatally (36% vs 9%; p < 0.001); this factor was also independently associated with death in the LRM. However, the perinatal index did not reduce the strength of the association between brainstem injury and death. When severe brainstem injury coexisted with severe perinatal index, the probability of death increased to 80%.
No other antenatal or perinatal factors were associated with death or motor outcome.
DISCUSSION
One of the main problems clinicians face when caring for an asphyxiated infant is how to give honest and reliable prognostic information to parents. Our data show that neonatal MRI in term infants with HIE and BGT injury can be used to predict death and specific motor outcomes. BGT injury severity was the best predictor of the presence and severity of motor impairment and the PLIC SI was the best predictor of the ability to walk, a significant issue in the daily lives of children with CP and their families. Brainstem injury was the best predictor of death.
Many studies have shown that MRI and other techniques are useful in predicting adverse outcome after neonatal HIE.4,5,13 However, “adverse outcome” is a broad, imprecisely used term that may have quite different implications for health professionals and parents: it may refer to children who die or are very severely disabled and those who are more mildly impaired (just because they have CP) even though they are able to walk and participate fully in family and social life. We used the GMFCS to determine the level of functional impairment of the children with CP. This is a widely used 5-level system designed to reflect differences in gross motor function, with an emphasis on sitting and walking.14 At 2 years, children at level I walk without limitations although with some impairment, children at levels II and III walk with limitations or need assistive devices, and children at levels IV and V are unable to walk. Several studies have reported that the common comorbidities of CP, such as learning disability, epilepsy, and visual impairment, and quality of life and social participation are directly related to GMFCS.15–17 Therefore, predicting the severity of CP is vital not only for anticipating future needs for physical problems and mobility but also for planning effective intervention programs and providing support and guidance to families regarding other aspects of development.
Almost 40% of our cohort were 12–24 months of age when they were assessed, though more than 80% were ≥18 months. We acknowledge that using the GMFCS for predicting later abilities in children between 1 and 2 years is less precise than in older children18 and that some 2-year-old nonambulatory children may walk later. The opposite scenario of deteriorating motor abilities is also possible.14 However, from a study of the predictive validity of the GMFCS in children younger than 2 years,18 it is reasonably certain that children who are functioning at GMFCS levels I–III before 2 years (mild and moderate CP) will walk as their preferred method of mobility, whereas children at levels IV and V (severe CP) will probably need a wheelchair to move around. All children in our study who were at level I were by definition walking at the time of our assessment and to our knowledge they continued to do so. Eleven children with GMFCS levels II–III were not walking at 2 years; all of them were seen again between 2 and 5 years and 4 of them are now walking.
Prediction of motor outcome was most difficult in infants with moderate BGT lesions. Almost 70% of these children had CP, but the grade of functional impairment was wide. Moderate lesions are not so well-defined as the mild or the severe ones and may reflect different levels of injury depending on several factors including the timing of the scan or biological susceptibility. Interestingly, we did not find any significant difference between scans done early and those done later in terms of prognostic accuracy. However, the timing of scans, imaging acquisition, and interpretation are very important in order to obtain the most accurate information and have been previously described in detail.19
Brainstem lesions have been described in neonatal HIE,20,21 but their relation to outcome has not been explored before. In a recent study of preterm infants with HIE, severe BGT and brainstem lesions together were associated with a severe outcome or death in 95% of cases.22 In our term cohort, brainstem injury was the only imaging factor with an independent association with death. No infant without brainstem injury died, regardless of the severity of the supratentorial lesions. The same effect has been described in children and adults with traumatic head injury,23,24 but not in adults with hypoxic encephalopathy, in whom cortical injury appears the most important factor related to death or permanent vegetative state.25 In adults with traumatic head injury, bilateral pontine lesions always predicted death.24 In our study, severe mesencephalic and pontine injury together predicted death in the first 3 years with a probability of 61%. This increased to 80% when the perinatal index was included in the analysis, suggesting that those infants who were most depressed at birth had had the most severe hypoxic insults. These infants did not necessarily have the most severe brain injury on MRI but many died early, perhaps before severe established lesions were apparent on their scans. Cardiac arrest and longer periods of resuscitation have been associated with early death in children and neonates.26,27
Most neonatal deaths occurred after withdrawal of intensive care. In these cases, the decision was made based on the history, clinical state, cranial ultrasound scan findings, and electrophysiologic data and not only on the lesions seen on the MRI scan. The patterns of injury seen in infants who died after withdrawal of care and in infants who died later were very similar. We found that brainstem injury had a similar predictive value for death whether infants died early or later so it is unlikely that the relation between brainstem lesions and outcome is affected by the decision for withdrawal of care.
Overt cerebellar injury is uncommon in neonates with HIE28 and we deliberately excluded infants with a clear developmental abnormality. The cerebellar abnormality we found appeared mild and we could not show statistically an effect on outcome independent of the associated BGT and brainstem injury. However, outcome was very severe in this group, and the appearance of the cerebellum may be an important sign when assessing scans in this context. It may represent a mild developmental variant that could be a marker for susceptibility to severer hypoxic-ischemic injury or represent atrophy when seen on later scans as an early consequence of severe central gray matter injury.29
All the infants included in this study had some degree of BGT injury. We did not include infants with WM damage only as it is less likely that this pattern results from acute perinatal hypoxia-ischemia alone.1,28,30 We know that in children with a history of HIE and WM damage in the absence of BGT injury the rate of CP and death is very low; in a study of 46 such infants none died, only 6 developed CP, and all but 2 were walking by 2 years.31 We also excluded infants treated with hypothermia as at the start of this study it was not known whether this therapy might alter the relationship between imaging and outcome.32,33 However, the recent results of the nested substudy of the Total Body Hypothermia for Neonatal Encephalopathy trial have shown that the accuracy of neonatal MRI for the prediction of outcome is not altered by therapeutic hypothermia.34
Our study has shown that in term infants with HIE and BGT injury, MRI can be used for the more accurate prediction of death and motor outcomes than has hitherto been generally accepted. This has important implications for parents as well as neonatologists and other health professionals involved in the immediate, short-term, and long-term care of newborn infants with hypoxic-ischemic encephalopathy.
Supplementary Material
Editorial, page 2048
Supplemental data at www.neurology.org
- BFMF
- Bimanual Fine Motor Function
- BGT
- basal ganglia and thalami
- CI
- confidence interval
- CP
- cerebral palsy
- GMFCS
- Gross Motor Function Classification System
- HIE
- hypoxic-ischemic encephalopathy
- LRM
- logistic regression model
- NE
- neonatal encephalopathy
- NPV
- negative predictive value
- PLIC
- posterior limb of the internal capsule
- PPV
- positive predictive value
- SCPE
- Surveillance of Cerebral Palsy in Europe
- SI
- signal intensity
- WM
- white matter
AUTHOR CONTRIBUTIONS
The original concept for the study came from Dr. Frances Cowan and Prof. Mary Rutherford; the data were collected by Ms. Joanna Allsop, Dr. Miriam Martinez-Biarge, and Dr. Olga Kapellou; the images were analyzed by Dr. Martinez-Biarge and Prof. Rutherford; and the outcome data were collected mainly by Dr. Cowan and Dr. Diane Gindner. Statistical analysis was conducted by Dr. Martinez-Biarge, Dr. Jesus Diez-Sebastian, and Dr. Cowan.
DISCLOSURE
Dr. Martinez-Biarge was funded by the Spanish Instituto de Salud Carlos III (grant number: FIS CM 06/00219). Dr. Diez-Sebastian, Dr. Kapellou, Dr. Gindner, and Dr. Allsop report no disclosures. Dr. Rutherford receives research support from the Medical Research Council UK. Dr. Cowan reports no disclosures.
REFERENCES
- 1. Okereafor A, Allsop J, Counsell SJ, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008;121:906–914 [DOI] [PubMed] [Google Scholar]
- 2. Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol 2002;44:309–316 [DOI] [PubMed] [Google Scholar]
- 3. Rosenbaum P. Cerebral palsy: what parents and doctors want to know. BMJ 2003;326:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rutherford M, Pennock J, Schwieso J, et al. Hypoxic-ischemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed 1996;75:F145–F151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143–149 [PMC free article] [PubMed] [Google Scholar]
- 6. Rutherford MA, Pennock JM, Counsell SJ, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998;102:323–328 [DOI] [PubMed] [Google Scholar]
- 7. Leviton A, Nelson KB. Problems with definitions and classifications of newborn encephalopathy. Pediatr Neurol 1992;8:85–90 [DOI] [PubMed] [Google Scholar]
- 8. Haataja L, Mercuri E, Regev R, et al. Optimality score for the neurological examination of the infant at 12 and 18 months of age. J Pediatrics 1999;135:153–161 [DOI] [PubMed] [Google Scholar]
- 9. Griffiths R. The Abilities of Young Children. London: Child Development Research Centre; 1970 [Google Scholar]
- 10. Cans C. Surveillance of Cerebral Palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000;42:816–824 [DOI] [PubMed] [Google Scholar]
- 11. Palisano RJ, Hanna SE, Rosenbaum PL, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther 2000;80:974–985 [PubMed] [Google Scholar]
- 12. Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407–429 [PubMed] [Google Scholar]
- 13. van Rooij LG, Toet MC, Osredkar D, et al. Recovery of amplitude integrated electroencephalographic background patterns within 24 hours of perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed 2005;90:F245–F251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palisano RJ, Cameron D, Rosenbaum PL, et al. Stability of the Gross Motor Function Classification System. Dev Med Child Neurol 2006;48:424–428 [DOI] [PubMed] [Google Scholar]
- 15. Himmelmann K, Beckung E, Hagberg G, Uvebrant P. Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol 2006;48:417–423 [DOI] [PubMed] [Google Scholar]
- 16. Vargus-Adams J. Health-related quality of life in childhood cerebral palsy. Arch Phys Med Rehabil 2005;86:940–945 [DOI] [PubMed] [Google Scholar]
- 17. Fauconnier J, Dickinson HO, Beckung E, et al. Participation in life situations of 8–12 year old children with cerebral palsy: cross sectional European study. BMJ 2009;338:b1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorter JW, Ketelaar M, Rosenbaum P, Helder PJ, Palisano R. Use of the GMFCS in infants with CP: the need for the reclassification at age 2 years and older. Dev Med Child Neurol 2009;51:46–52 [DOI] [PubMed] [Google Scholar]
- 19. Rutherford M, Biarge MM, Allsop J, Counsell S, Cowan F. MRI of perinatal brain injury. Pediatr Radiol 2010;40:819–833 [DOI] [PubMed] [Google Scholar]
- 20. Leech RW, Alvord EC., Jr Anoxic-ischemic encephalopathy in the human neonatal period. The significance of brain stem involvement Arch Neurol 1977;34:109–113 [DOI] [PubMed] [Google Scholar]
- 21. Pasternak JF, Gorey MT. The syndrome of acute near-total intrauterine asphyxia in the term infant. Pediatr Neurol 1998;18:391–398 [DOI] [PubMed] [Google Scholar]
- 22. Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res 2009;66:222–229 [DOI] [PubMed] [Google Scholar]
- 23. Woischneck D, Klein S, Reissberg S, et al. Prognosis of brain stem lesion in children with head injury. Child Nerv Syst 2003;19:174–178 [DOI] [PubMed] [Google Scholar]
- 24. Firsching R, Woischneck D, Klein S, Ludwig K, Döhring W. Brain stem lesions after head injury. Neurol Res 2002;24:145–146 [DOI] [PubMed] [Google Scholar]
- 25. Weiss N, Galanaud D, Carpentier A, Naccache L, Puybasset L. Clinical review: Prognostic value of magnetic resonance imaging in acute brain injury and coma. Crit Care 2007;11:230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saugstad OD, Ramji S, Rootwelt T, Vento M. Response to resuscitation of the newborn: early prognostic variables. Acta Paediatr 2005;94:890–895 [DOI] [PubMed] [Google Scholar]
- 27. Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med 2008;9:32–39 [DOI] [PubMed] [Google Scholar]
- 28. Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 2003;361:736–742 [DOI] [PubMed] [Google Scholar]
- 29. Le Strange E, Saeed N, Cowan FM, Edwards AD, Rutherford MA. MR imaging quantification of cerebellar growth following hypoxic-ischemic injury to the neonatal brain. AJNR Am J Neuroradiol 2004;25:463–468 [PMC free article] [PubMed] [Google Scholar]
- 30. Sie LT, van der Knaap MS, Oosting J, de Vries LS, Lafeber HN, Valk J. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics 2000;31:128–136 [DOI] [PubMed] [Google Scholar]
- 31. Bregant T, Rutherford M, Cowan F. White matter lesions in term infants with neonatal encephalopathy: Correlation with later scans and neurodevelopmental outcome. Early Hum Dev 2007;83:128 [Google Scholar]
- 32. Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics 1998;102:885–892 [DOI] [PubMed] [Google Scholar]
- 33. Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics 2000;106:684–694 [DOI] [PubMed] [Google Scholar]
- 34. Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.