Abstract
Background
Methadone clearance is highly variable and drug interactions are problematic. Both have been attributed to CYP3A, but actual mechanisms are unknown. Drug interactions can provide such mechanistic information. Ritonavir/indinavir, one of the earliest protease inhibitor combinations, may inhibit CYP3A. We assessed ritonavir/indinavir effects on methadone pharmacokinetics and pharmacodynamics, intestinal and hepatic CYP3A activity, and intestinal transporters (P-glycoprotein) activity. CYP3A and transporters were assessed with alfentanil and fexofenadine, respectively.
Methods
Twelve healthy human immunodeficiency virus-negative volunteers underwent a sequential 3-part crossover. On three consecutive days they received oral alfentanil/fexofenadine, intravenous alfentanil, and intravenous plus oral (deuterium-labeled) methadone, repeated after acute (3d) and steady-state (2 wk) ritonavir/indinavir. Plasma and urine analytes were measured by mass spectrometry. Opioid effects were assessed by miosis.
Results
Alfentanil apparent oral clearance was inhibited >97% by both acute and steady-state ritonavir/indinavir, and systemic clearance was inhibited >90%, due to diminished hepatic and intestinal extraction. Ritonavir/indinavir increased fexofenadine area under the plasma concentration-time curve 4-to 5-fold, suggesting significant inhibition of gastrointestinal P-glycoprotein. Ritonavir/indinavir slightly increased methadone N-demethylation, but had no significant effects on methadone plasma concentrations, or on systemic or apparent oral clearance, renal clearance, hepatic extraction or clearance, or bioavailability. Ritonavir/indinavir had no significant effects on methadone plasma concentration-effect relationships.
Conclusions
Inhibition of both hepatic and intestinal CYP3A activity is responsible for ritonavir/indinavir drug interactions. Methadone disposition was unchanged despite profound inhibition of CYP3A activity, suggesting little or no role for CYP3A in clinical methadone metabolism and clearance. Methadone bioavailability was unchanged despite inhibition of gastrointestinal P-glycoprotein activity, suggesting that this transporter does not limit methadone intestinal absorption.
Introduction
Methadone is a cost-effective opioid agonist which is highly efficacious in the treatment of acute, chronic, neuropathic, and cancer pain. It is increasingly being used as a first-line analgesic, as well as in opioid rotation strategies.1 Methadone maintenance is also a cornerstone therapy of opiate and opioid addiction, which prevents withdrawal and illicit drug use, and is a vital public health strategy for human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) risk reduction.2 Methadone prescribing rose 1300% between 1997 and 2006, attributable primarily to increased use for pain treatment.*,†
Methadone use is not, however, without limitations. An unfortunate, exponential, decade-long rise in methadone toxicity accompanied its growing clinical use, with adverse events increasing ~1800% between 1997–2004 and fatalities increasing 390% from 1999 to 2004.3‡,§ These problems have been attributed primarily to increased methadone use for pain treatment, rather than addiction, and have been the subject of two government-sponsored conferences.‡,§ There are considerable and (as yet) unpredictable inter- and intra-individual variabilities in methadone disposition and drug interactions, with consequent risks of withdrawal, inadequate analgesia, and/or toxicity.4–7 Both pharmacokinetic and pharmacodynamic variability are major impediments to optimal methadone use. Despite concerted research efforts, the causes remain poorly understood.8
Methadone is cleared mainly by cytochrome P450 (CYP)-catalyzed hepatic N-demethylation to the pharmacologically inactive primary metabolite 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), with some urinary excretion of unchanged drug. Clinical methadone metabolism and clearance have been attributed for over a decade to CYP3A4, based on extrapolation of in vitro drug metabolism studies, and numerous dosing guidelines and the methadone label** warn about the potential for CYP3A4-mediated methadone drug interactions and the need to adjust dosing accordingly.4,6,9–15 Methadone is also a substrate for the efflux transporter P-glycoprotein (P-gp), which influences methadone absorption, brain access, pharmacodynamics, and analgesia in animals.16,17 In humans, the role of P-gp in methadone disposition and clinical effects, is poorly understood.
Concomitant treatment with methadone and highly active antiretroviral therapy of HIV is common, due to the intertwined problems of opioid dependence and HIV/AIDS, and is increasing because antiretrovirals have essentially transformed AIDS from a terminal illness into a chronic disease.18 While some methadone-antiretroviral drug interactions have been observed, and typically ascribed to CYP3A4, the mechanism(s) in reality remain totally unknown.19,20 Ritonavir-indinavir was one of the first antiretroviral combinations. Like all protease inhibitors, indinavir and ritonavir are potent inhibitors of hepatic and intestinal CYP3A isoforms (3A4, 3A5, 3A7).20,21 Both drugs significantly inhibited human liver microsomal methadone metabolism in vitro.22 In contrast, limited, and somewhat inconsistent information is available about their effects on the disposition and clinical effects of methadone.14 Methadone elimination was unaffected by indinavir alone, and increased by ritonavir alone23,24 or in combination with saquinavir or lopinavir.25–27 Nonetheless, the apparent paradox of increased methadone elimination despite CYP3A4/5 inhibition was never explained, and this paradox challenges the basic tenet of CYP3A mediation of methadone elimination. Similarly unexplained was the absence of withdrawal symptoms in all these studies despite significantly reduced plasma methadone concentrations.
Therefore, due to increasing methadone use and adverse events, to provide fundamental insights into methadone metabolism and clearance, to better understand mechanisms of methadone drug interactions, and the absence of specific interactions information between methadone and the CYP3A4/5 inhibitors ritonavir/indinavir, this investigation assessed the influence of ritonavir/indinavir on methadone pharmacokinetics and pharmacodynamics. The purpose was to determine: 1) ritonavir/indinavir effects on hepatic CYP3A4/5, first-pass CYP3A4/5, and intestinal P-gp activities; 2) ritonavir/indinavir influence on methadone disposition and clinical effect; 3) the role of CYP3A4/5 and/or P-gp-mediated methadone bioavailability, first-pass metabolism, and systemic clearance in methadone clearance and its potential alteration by ritonavir/indinavir; 4) the influence of ritonavir/indinavir on methadone pharmacodynamics, 5) the ability of a clinical CYP3A4/5 probe to rapidly and noninvasively predict methadone disposition.
A comprehensive crossover investigation was conducted in healthy volunteers. Hepatic and first-pass CYP3A activities were evaluated using intravenous and oral alfentanil.28,29 Alfentanil is metabolized similarly by CYPs 3A4 and 3A5, while CYP3A7 has significantly less activity,30 and CYP3A5 polymorphisms have no effect on alfentanilclearance,29 hence alfentanil is considered a nonselective CYP3A4/5 (henceforth referred to as CYP3A) probe. Pupil diameter change (miosis) was used as a surrogate for alfentanil plasma concentrations to estimate alfentanil clearance and CYP3A activity noninvasively. Fexofenadine was used to assess P-gp activity.31 Intravenous and oral (deuterium-labeled) methadone were concurrently administered to assess IV and oral drug kinetics simultaneously, hepatic extraction and bioavailability, and, by avoiding a crossover design (for different routes of administration on different days) thereby diminish interday variability and increase protocol efficiency.32 Miosis was used to assess methadone clinical effects and pharmacodynamics.
Materials and Methods
Clinical Protocol
The investigation was approved by the University of Washington Institutional Review Board (Seattle, Washington) and subjects provided written informed consent. Eligibility criteria were 1) normal healthy volunteers 18–40 yr, 2) within 25% of ideal body weight (body mass index <30). Exclusion criteria were 1) major medical problems, 2) history of hepatic or renal disease, 3) family history of type 2 diabetes, 4) use of medications or nonprescription preparations known to alter CYP3A activity, 5) a known history of addiction to drugs or alcohol, or 6) access to and routine handling of addicting drugs in the regular course of employment. Females taking hormonal contraceptives were excluded. Both smokers and nonsmokers were enrolled. Subjects underwent a screening visit at which fasting blood glucose concentration and HIV serologic status were determined. Subjects were excluded if their glucose exceeded 110 mg/dl (because protease inhibitors can cause glucose intolerance) or they were HIV seropositive (because monotherapy can cause HIV resistance). The final study population was twelve subjects (six men, six women; 23 ± 5 yr, range 18–34; 68 ± 13 kg, range 50–95).
The protocol was a 3-period sequential crossover (control first, for logistical considerations) with each subject as their own control (fig. 1). Subjects were instructed to consume no food or beverages that contain grapefruit, apples or oranges for 7d before any study day, no alcohol or caffeine for 1d before each study session and on the study day, and no food or water after midnight before each study session. For each session, a catheter was placed in an arm vein for blood sampling and (if needed) a second catheter was placed for drug administration. Subjects (supine) were monitored with a pulse oximeter and automated blood pressure cuff, and received supplemental oxygen for saturations less than 94%.
Figure 1.
Study protocol for methadone-ritonavir/indinavir interaction. Shaded boxes show drug administration and follow-up blood and urine sampling. Numbers in the shaded box indicate alfentanil dose. Other doses are shown in the first column.
First-pass CYP3A activity and intestinal P-gp (and other transporters) activity were evaluated on day 1 using oral alfentanil and fexofenadine as in vivo probes.28,29,33,34 Subjects received ondansetron (4 mg IV) for antinausea prophylaxis followed 30 min later by oral alfentanil (43 and 23 μg/kg at control and ritonavir/indinavir sessions, respectively) with 100 cc water. Fexofenadine (60 mg) was administered with 100 cc water 1 hr after alfentanil. Subjects received a standard breakfast and lunch 3 and 6 hr, respectively, after alfentanil. Venous blood was sampled for 48 hr after alfentanil dosing and plasma stored at −20°C for later analysis. Coincident with blood sampling, dark-adapted pupil diameter was measured using a Pupilscan Model 12A infrared pupillometer with 0.1 mm resolution (Keeler Instruments, Broomall, PA).28,29 Each recorded value was the mean of triplicate measurements, which typically agreed to within 0.1–0.3 mm.
Hepatic CYP3A activity was evaluated on day 2 using intravenous alfentanil as an in vivo probe.28,29,34 Subjects received ondansetron followed 30 min later by alfentanil bolus (15 and 10 μg/kg at control and ritonavir/indinavir sessions, respectively). Subject received a standard breakfast 4 hr after alfentanil, and free access to food and water thereafter. Venous blood was sampled for 24 hr after alfentanil dosing, and dark-adapted pupil diameter was measured coincident with blood sampling.
Methadone metabolism and clearance were assessed on day 3 (with follow-up on days 4–7) by simultaneously administering IV and oral methadone.24,32 Subjects received IV ondansetron followed 30 min later by oral deuterated racemic (d5)-methadone HCl (11.0 mg, equivalent to 9.86 mg free base) with 100 ml water, and IV racemic unlabelled (d0)-methadone HCl (6.0 mg, equivalent to 5.4 mg free base, Roxane Laboratories, Columbus, OH). Deuterated methadone hydrochloride was synthesized and used under Investigational New Drug approval.32 Venous blood samples were obtained for 96 hr after methadone, plasma separated and stored at −20°C for later analysis, and dark-adapted pupil diameter was measured coincident with blood sampling. Subjects were fed a standard breakfast 4 hr after methadone and had free access to food and water thereafter. Continuous urine samples were collected at 24, 48, 72, and 96 hr. Nausea and/or vomiting were treated with ondansetron (4 mg IV or 8 mg orally) as needed.
Hepatic and first-pass CYP3A, intestinal transporters activity and methadone disposition were assessed at baseline. After 1–2 months, subjects then began taking ritonavir/indinavir 100/800 mg twice daily (approximately 7 am and 7 pm). Dosing was adjusted on study days (morning dose at lunch, and evening dose at 11 pm) to preclude an acute inhibitor effect from the morning dose. First-pass CYP3A and transporter activities were determined on day 2 of ritonavir/indinavir, and methadone disposition on day 3 (with follow-up on days 4–7). At steady-state ritonavir/indinavir, first-pass CYP3A and intestinal transporters activity were assessed on day 15, hepatic CYP3A assessed on day 17, and methadone disposition on day 17 (with follow-up on days 18–21). Because the duration of ritonavir administration may affect the degree of CYP3A alteration,23,24,34,35 both acute and steady-state ritonavir/indinavir were evaluated. A potential for CYP3A induction by steady-state ritonavir was reported.35 Since the time course of potential CYP3A induction was unknown, and time to evaluate hepatic CYP3A, first-pass CYP3A, and P-gp activities potentially not sufficient before commencement of induction, only first-pass CYP3A and P-gp activities were evaluated in the acute ritonavir/indinavir phase. First-pass is a greater determinant than hepatic metabolism of oral drug disposition, and hence the more important parameter to evaluate.
Sample size was determined using a simplified analysis (paired t-test) for comparing the outcome variable methadone systemic clearance. A previous study found 22 and 33% interday/intrasubject variability in IV and oral methadone clearances, respectively.32 To detect a 30% change in clearance, using a paired t-test, with 33% variability, 1-β =0.8, α =0.05, would require 12 subjects.
Analytical Methods
Plasma alfentanil and fexofenadine concentrations were simultaneously quantified using solid-phase extraction and electrospray liquid chromatography-mass spectrometry as described previously.33 Plasma and urine methadone and EDDP enantiomer concentrations were quantified using automated online extraction, stereoselective liquid chromatography, and electrospray mass spectrometry as described previously, except that standard curves for both d0- and d5-methadone were used.24,36
Data Analysis
Plasma methadone and EDDP data were analyzed using noncompartmental methods, assuming complete absorption, as described previously (WinNonlin 5.2, Pharsight Corp, Mountain View, CA).28 Systemic clearance of intravenous alfentanil and d0-methadone was (CLIV)=doseIV/AUCIV, apparent oral clearance of alfentanil and d5-methadone was (CL/F)=doseoral/AUCoral, bioavailability was (Foral)= (AUCoral/doseoral) × (doseIV/AUCIV), volume of distribution based on the terminal phase was (Vz)=Dose/(AUC × λ) where λ is the terminal elimination rate constant, and steady-state volume of distribution was (Vss)=CL × mean residence time. Hepatic extraction (EH) was CLH/Qp, where hepatic plasma flow (Qp) was estimated as 15.2 ml/kg/min. Renal clearance (Clr and CLr/F) was determined as: amount excreted in urine/AUC0-∞. EDDP formation clearance was determined from urine data as: Clf = fraction of dose recovered in urine × CLIV and Clf/F = fraction of dose recovered in urine × CL/Fl, for IV and oral dosing, respectively. Hepatic clearance (CLH) was CLiv-CLr. Alfentanil plasma data were similarly analyzed, as described previously.28,29 Gastrointestinal extraction was EG = 1-FG where FG was Foral/(Fabs(1-EH)); the oral dose was assumed to be entirely absorbed and thus Fabs was considered to be unity. Alfentanil and methadone effect (miosis) vs time curves were treated analogously to conventional plasma concentration curves, to determine the area under the effect curve (AUEC).28,29
Statistical Analysis
Differences between treatment groups for pharmacokinetic and effect parameters were analyzed using one-way repeated measures analysis of variance followed by the Student-Newman-Keuls test for multiple comparisons, or paired t-tests, as appropriate (SigmaStat 3.5, Systat Corp, Point Richmond, CA). Non-normal data were log transformed for analysis, but reported as the nontransformed results. Statistical significance was assigned at p< 0.05. Results are reported as the arithmetic mean ± standard deviation (SD). Plasma AUC and urine data were also assessed as ratios (treated/control) and the geometric mean and 90% confidence interval of the geometric mean. Confidence intervals excluding 1.0 were considered statistically significant. Relationships between methadone and alfentanil clearances were evaluated by linear regression analysis.
Results
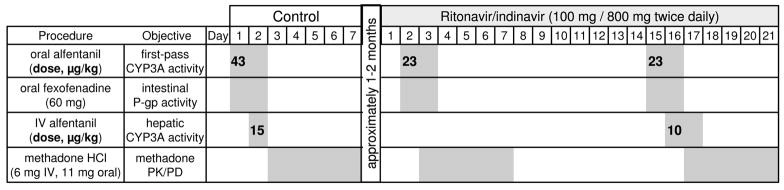
Ritonavir/indinavir profoundly inhibited both hepatic and intestinal CYP3A activities. Effects of ritonavir/indinavir on alfentanil plasma concentrations are shown in figure 2, and pharmacokinetic parameters are provided in table 1. The AUC0-∞/dose ratio (ritonavir-indinavir/control) for IV alfentanil was increased nearly 13-fold, half-life increased 11-fold, and systemic clearance and hepatic extraction were both decreased 92% by steady-state ritonavir/indinavir, indicating >90% inhibition of hepatic CYP3A activity. Acute and steady-state ritonavir/indinavir increased oral alfentanil AUC0-∞/dose ratio (ritonavir-indinavir/control) 40- and 30-fold and decreased apparent oral clearance 98% and 97%, respectively, and both protease inhibitor regimens increased the Cmax/dose ratio 4-fold, and the half-life 12 to 13-fold. These results indicate >97% inhibition of first-pass CYP3A activity. Ritonavir/indinavir increased alfentanil bioavailability from 34 to 81% and reduced alfentanil intestinal extraction to 38% of control (from 48 to 18%), indicating significant inhibition of intestinal CYP3A activity.
Figure 2.
Effect of ritonavir/indinavir on first-pass and hepatic CYP3A activity, assessed using alfentanil as a CYP3A probe. Shown are alfentanil concentrations after (A) oral and (B) IV administration. Subjects received (A) 43 and 23 μg/kg oral alfentanil at the control and ritonavir/indinavir (RTV/IND) sessions, respectively, and (B) 15 and 10 μg/kg IV alfentanil at the control and ritonavir/indinavir sessions, respectively. Concentrations are shown dose-normalized. Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
Table 1.
Intravenous and Oral Alfentanil Pharmacokinetic Parameters.
| Control | Acute Ritonavir/Indinavir | Steady-state Ritonavir/Indinavir | |
|---|---|---|---|
| IV alfentanil | |||
| Cmax, ng/ml | 85 ± 21 | 72 ± 16 | |
| Cmax/dose, ng · ml−1 · mg−1 | 84 ± 23 | 107± 21 * | |
| AUC0-∞, ng · h−1 · ml−1 | 51 ± 16 | 458± 229 * | |
| AUC0-∞/dose, ng · h−1 · ml−1 · mg−1 | 50 ± 12 | 680 ± 323 * | |
| AUC0-∞/dose ratio, ritonavir-indinavir/control | 12.7 (8.5–18.9) | ||
| CLIV, ml · kg−1 · min−1 | 5.30 ± 1.47 | 0.44 ± 0.19 * | |
| Elimination t1/2, h | 1.0 ± 0.2 | 10.8 ± 6.1 * | |
| EH | 0.35 ± 0.10 | 0.03 ± 0.01 * | |
| Oral alfentanil | |||
| Cmax, ng/ml | 25 ± 8 | 60 ± 21 * | 60 ± 21 * |
| Cmax/dose, ng · ml−1 · mg−1 | 9 ± 2 | 35 ± 5 * | 38 ± 14 * |
| AUC0-∞, ng · h−1 · ml−1 | 52 ± 25 | 1130 ± 420 * | 834 ± 358 *† |
| AUC0-∞/dose, ng · h · ml−1 · mg−1 | 18 ± 7 | 657 ± 133 * | 538 ± 253* |
| AUC0-∞/dose ratio, ritonavir-indinavir/control | 40 (24–66) | 30 (18–52) | |
| CL/F, ml · kg−1 · min−1 | 16.8 ± 7.5 | 0.4 ± 0.1 * | 0.5 ± 0.2 * |
| Elimination t1/2, h | 1.0 ± 0.1 | 12.2 ± 3.0 * | 13.3 ± 6.2 * |
| Foral | 0.34 ± 0.08 | 0.81 ± 0.08 a | |
| EG | 0.48 ± 0.10 | 0.18 ± 0.10 a | |
AUC = area under the plasma concentration-time curve; Cmax = peak plasma concentration; CLIV = systemic clearance of IV alfentanil; CL/F = apparent oral clearance of alfentanil; EH = hepatic extraction; EG = intestinal extraction; Foral = bioavailability.
Significantly different from control (P < 0.05).
Significantly different from acute ritonavir-indinavir (P < 0.05).
Subjects received 15 and 10 μg/kg intravenous (IV) alfentanil and 43 and 23 μg/kg oral alfentanil at the control and ritonavir/indinavir sessions, respectively. Peak plasma concentration (Cmax) and area under the concentration-time curve (AUC) are shown normalized to dose. All other variables are not dose adjusted. Results are the arithmetic mean ± SD (n=12), except the AUC0-∞/dose ratio (ritonavir-indinavir/control), which is the geometric mean (90% confidence interval).
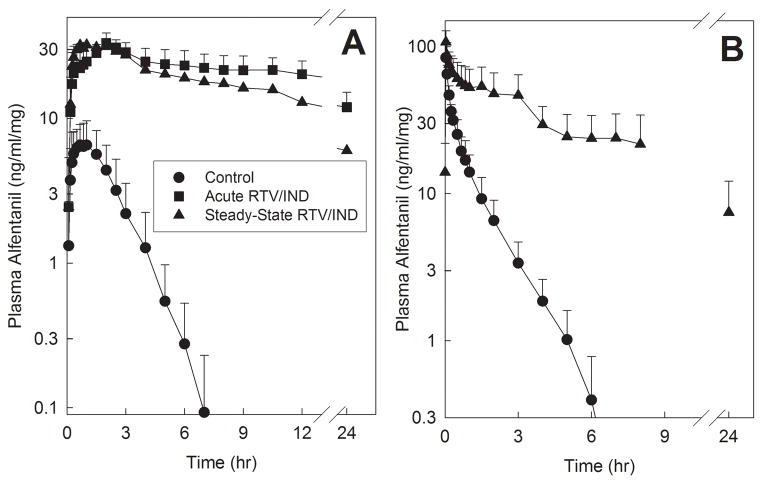
Pupil data were available before plasma alfentanil concentrations, and used as an early surrogate for alfentanil clearance to assess ritonavir-indinavir effects on CYP3A (fig. 3 and table 2). Ritonavir-indinavir increased and prolonged alfentanil miosis. The AUEC0-∞/dose ratio for IV alfentanil was significantly increased by steady-state ritonavir-indinavir, and the ratio for oral alfentanil was significantly increased by both acute and steady-state ritonavir/indinavir.
Figure 3.
Effect of ritonavir/indinavir on first-pass and hepatic CYP3A activity, assessed using alfentanil as a CYP3A probe. Pupil diameter change from baseline (miosis) was used as a surrogate for alfentanil plasma concentrations. Shown is dose-normalized miosis after (A) 43 and 23 μg/kg oral alfentanil at control and ritonavir/indinavir sessions, respectively, and (B) 15 and 10 μg/kg IV alfentanil at control and ritonavir/indinavir sessions, respectively. Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
Table 2.
Ritonavir–indinavir Effects on Methadone and Alfentanil Pupil Effect Parameters.
| Control | Acute Ritonavir/Indinavir | Steady-state Ritonavir/Indinavir | |
|---|---|---|---|
| IV alfentanil | |||
| Maximum miosis, mm | 4.5 ± 0.7 | 4.2 ± 0.8 | |
| Maximum miosis/dose, mm/mg | 4.5 ± 0.9 | 6.4 ± 1.3 * | |
| AUEC0-∞/dose, mm · h−1 · mg−1 | 3.4 ± 1.9 | 16.1 ± 10.5 * | |
| AUEC0-∞/dose ratio, ritonavir-indinavir/control | 4.6 (3.1, 6.8) | ||
| Oral alfentanil | |||
| Maximum miosis, mm | 1.7 ± 0.6 | 3.8 ± 1.0 * | 3.6± 1.0 * |
| Maximum miosis/dose, mm/mg | 0.6 ± 0.2 | 2.3 ± 0.9 * | 2.3 ± 0.6 |
| AUEC0-∞/dose, mm · h−1 · mg−1 | 0.9 ± 0.5 | 34 ± 30 * | 18± 16 *† |
| AUEC0-∞/dose ratio, ritonavir-indinavir/control | 41 (21–81) | 24 (14–23) | |
| Methadone | |||
| Maximum miosis, mm | 2.9 ± 1.0 | 3.5 ± 0.7 | 3.2 ± 0.8 |
| AUEC0-∞, mm/h | 52 ± 24 | 65 ± 26 | 48 ± 25 |
| AUEC0-∞ ratio, ritonavir-indinavir/control | 1.22 (0.94–1.57) | 0.90 (0.75–1.09) | |
Significantly different from control (P<0.05);
significantly different from acute ritonavir/indinavir (P < 0.05).
Subjects received 15 and 10 μg/kg intravenous (IV) alfentanil and 43 and 23 μg/kg oral alfentanil at the control and ritonavir/indinavir sessions, respectively. Maximum miosis and area under the effect-time curve (AUEC) for alfentanil are shown normalized to dose. All other variables are not dose adjusted. Subjects received 6.0 mg IV and 11.0 mg oral methadone HCl at all sessions. Results (n = 12) are the arithmetic mean ± SD, except the AUEC0-∞/dose and AUEC0-∞ ratios (ritonavir-indinavir/control), which are the geometric mean (90% confidence interval).
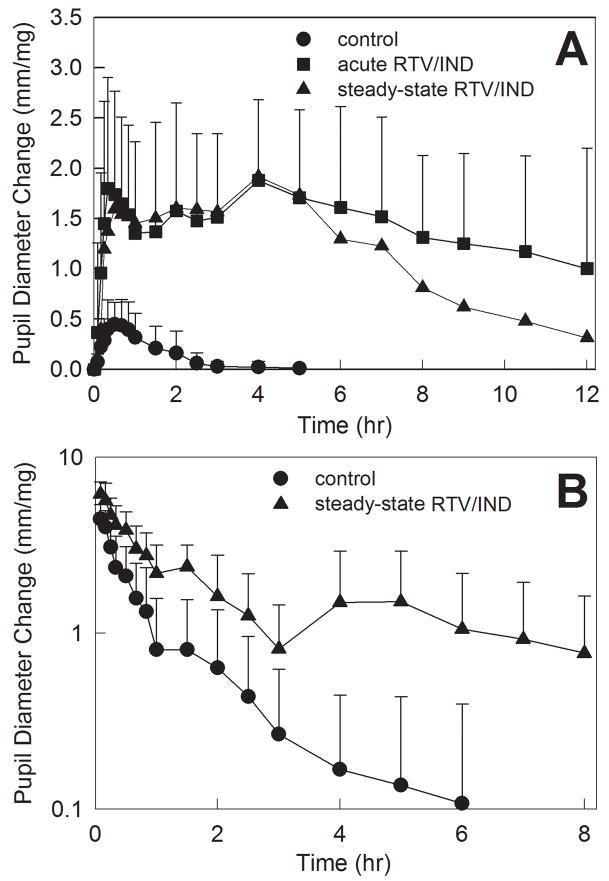
Disposition of oral fexofenadine was used to probe the activity of the intestinal efflux pump P-gp, and any other intestinal transporters for which fexofenadine is a substrate. Both acute and steady-state ritonavir/indinavir significantly increased fexofenadine peak plasma concentrations and AUC (fig. 4 and table 3). There were no differences between acute and steady-state ritonavir/indinavir effects on intestinal transporters.
Figure 4.
Effect of ritonavir/indinavir on intestinal transporter activity, assessed using fexofenadine as a transporter probe. Each subject received 60 mg oral fexofenadine on all occasions. Each data point is the mean ± SD (n=12).
Table 3.
Fexofenadine Pharmacokinetic Parameters
| Control | Acute Ritonavir/Indinavir | Steady-state Ritonavir/Indinavir | |
|---|---|---|---|
| Cmax, ng/ml | 164 ± 125 | 404 ± 191* | 464 ± 228* |
| AUC0-∞, ng · h−1 · ml−1 | 859 ± 438 | 4160 ± 1510* | 3540 ± 1530* |
| AUC0-∞ ratio, ritonavir-indinavir/control | 5.0 (3.5–7..3) | 4.2 (2.9–5.9) | |
| CL/F, ml · kg−1 · min−1 | 20.8 ± 8.3 | 4.2 ± 2.0* | 5.3 ± 3.3* |
| Elimination t1/2, h | 12.4 ± 2.2 | 8.3 ± 3.2* | 7.5 ± 0.7* |
Significantly different from control (P < 0.05). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CL/F, apparent oral clearance of fexofenadine.
Results are the arithmetic mean ± SD (n=12), except the area under the concentration-time curve (AUC) ratio (ritonavir/control), which is the geometric mean (90% confidence interval).
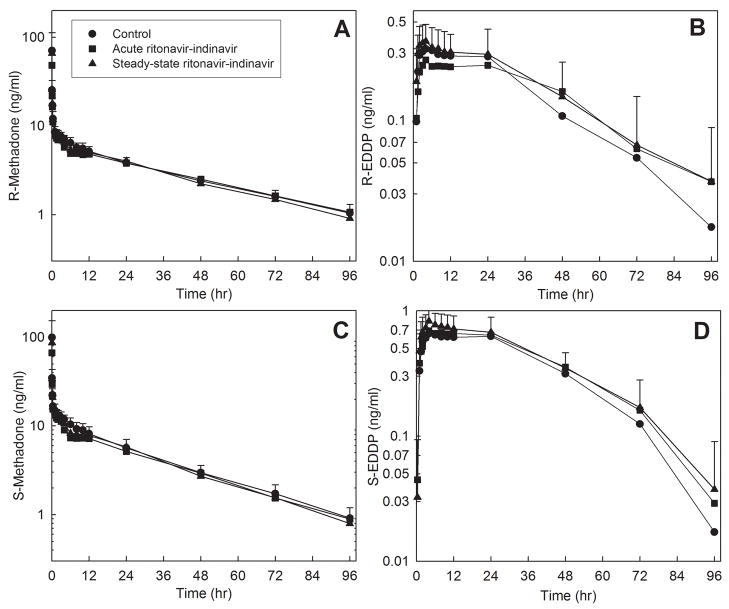
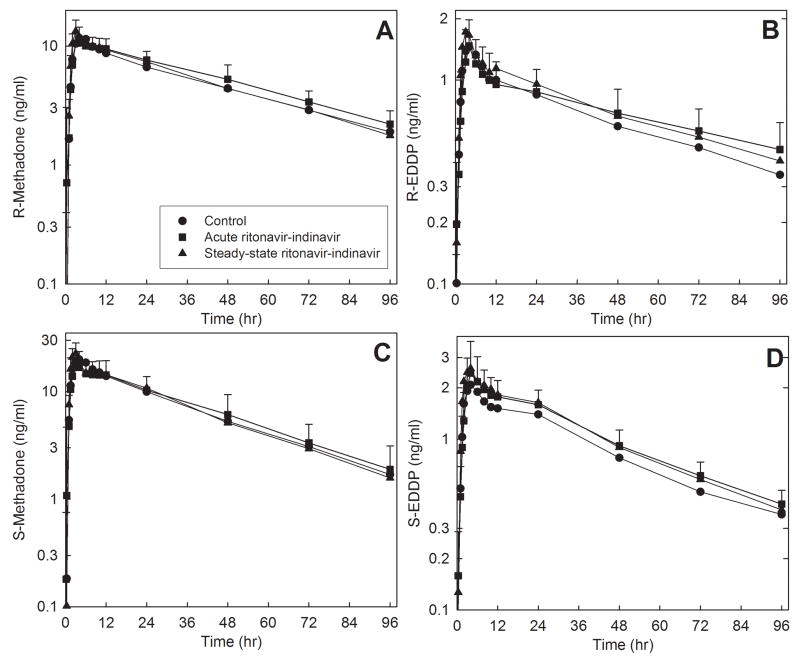
Disposition of methadone, after both IV and oral administration, was minimally affected by either acute or steady-state ritonavir/indinavir. Plasma R- and S-methadone concentrations in ritonavir/indinavir treated subjects were not different from those of untreated subjects, after IV methadone (fig. 5) and oral methadone (fig. 6). Acute and steady-state ritonavir/indinavir had no effect on the plasma AUC0-∞ ratio, systemic clearance, hepatic clearance, or hepatic extraction of R- and S- methadone after IV administration (table 4). Ritonavir/indinavir had no effect on the plasma AUC0-∞ ratio, apparent oral clearance, or bioavailability of R- and S- methadone after oral administration (table 5). Neither acute nor steady-state ritonavir/indinavir altered IV or oral methadone enantiomers renal clearance, which accounted for approximately 25% of both methadone enantiomers systemic clearance (table 6). Methadone N-demethylation was somewhat induced by ritonavir/indinavir, with greater effects on S- than R-methadone. Plasma R-EDDP but not S-EDDP concentrations were greater than controls in steady-state but not acute ritonavir/indinavir subjects for 10 hr after IV and oral methadone. S-EDDP/S-methadone AUC ratios were greater in acute and steady-state ritonavir/indinavir subjects than in controls for both IV and oral methadone. R-EDDP/R-methadone AUC ratios were not different from controls. EDDP enantiomer formation clearances after both IV and oral methadone were essentially unchanged.
Figure 5.
Ritonavir/indinavir effects on intravenous methadone disposition. Shown are plasma (A, B) R-methadone and R-EDDP (2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine) concentrations and (C, D) S-methadone and S-EDDP concentrations. Subjects received 6.0 mg IV methadone HCl (5.4 mg free base). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
Figure 6.
Ritonavir/indinavir effects on oral methadone disposition. Shown are plasma (A, B) R-methadone and R-EDDP (2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine) concentrations and (C, D) S-methadone and S-EDDP concentrations. Subjects received 11.0 mg oral methadone HCl (9.9 mg free base). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
Table 4.
Intravenous Methadone Pharmacokinetic Parameters.
| R-methadone | S-methadone | |||||
|---|---|---|---|---|---|---|
| Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
|
| Cmax, ng/ml | 64 ± 40 | 50 ± 20 | 60 ± 26 | 90 ± 53 | 69 ± 26 | 80 ± 33 |
| AUC0-96, ng · h−1 · ml−1) | 290 ± 31 | 277 ± 83 | 279 ± 78 | 401 ± 81 | 353 ± 150 | 377 ± 170 |
| AUC0-∞, ng · h−1 · ml−1 | 347 ± 43 | 345 ± 105 | 326 ± 83 | 436 ± 91 | 390 ± 178 | 406 ± 185 |
| AUC0-∞ ratio, ritonavir-indinavir/control | 0.96 (0.81–1.13) | 0.92 (0.83–1.03) | 0.83 (0.69–1.00) | 0.88 (0.76–1.01) | ||
| CLIV, ml · kg−1 · min−1 | 1.98 ± 0.38 | 2.15 ± 0.82 | 2.15 ± 0.49 | 1.62 ± 0.44 | 2.04 ± 0.86 | 1.93 ± 0.78 |
| CLH, ml · kg−1 · min−1 | 1.42 ± 0.29 | 1.32 ± 0.35 | 1.57 ± 0.40 † | 1.26 ± 0.35 | 1.47 ± 0.64 | 1.55 ± 0.67 |
| Elimination t1/2, h | 37 ± 7 | 42 ± 14 | 36 ± 7 | 27 ± 4 | 27 ± 6 | 25 ± 5 |
| Vss, L/kg | 5.9 ± 1.4 | 7.2 ± 2.5 | 6.3 ± 1.8 | 3.4 ± 0.9 | 4.2 ± 1.6 | 3.8 ± 1.4 |
| EH | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.10 ± 0.03 † | 0.08 ± 0.02 | 0.10 ± 0.04 | 0.10 ± 0.04 |
| R-EDDP | S-EDDP | |||||
| Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
|
| Cmax, ng/ml | 0.35 ± 0.08 | 0.29 ± 0.11 | 0.37 ± 0.11 † | 0.73 ± 0.13 | 0.78 ± 0.27 | 0.86 ± 0.17 |
| AUC0-96, ng · h−1 · ml−1 | 14 ± 6 | 15 ± 7 | 16 ± 8 | 32 ± 8 | 35 ± 11 | 37 ± 10 |
| AUC∞, ng · h−1 · ml−1) | 19 ± 8 | 26 ± 10 | 21 ± 10 | 39 ± 10 | 42 ± 12 | 44 ± 10 |
| Elimination t1/2, h | 28 ± 12 | 50 ± 19 * | 32 ± 8 † | 29 ± 6 | 31 ± 10 | 29 ± 6 |
| AUC0-96, EDDP/methadone | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.06 ± 0.03 | 0.08 ± 0.03 | 0.11 ± 0.05 * | 0.11 ± 0.05 * |
| AUC∞, EDDP/methadone | 0.06 ± 0.03 | 0.07 ± 0.02 | 0.07 ± 0.03 | 0.10 ± 0.04 | 0.12 ± 0.06 * | 0.12 ± 0.05 * |
| AUC∞ (EDDP/methadone) ratio, ritonavir-indinavir/control) | 1.17 (0.88–1.55) | 1.23 (0.96–1.56) | 1.23 (1.07–1.41) | 1.27 (1.11–1.45) | ||
Significantly different from control (P < 0.05),
Significantly different from acute ritonavir-indinavir (P < 0.05). AUC = area under the plasma concentration-time curve; Cmax = peak plasma concentration; CLH = hepatic clearance; CLIV = systemic clearance; EDDP = 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine; EH = hepatic extraction; Vss = steady-state volume of distribution.
Subjects received 6.0 mg of IV methadone HCl at all sessions. Results are the arithmetic mean ± SD (n = 12), except area under the concentration-time curve (AUC) ratios (ritonavir-indinavir/control), which are the geometric mean (90% confidence interval).
Table 5.
Oral Methadone Pharmacokinetic Parameters.
| R-methadone | S-methadone | |||||
|---|---|---|---|---|---|---|
| Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
|
| Cmax, ng/ml | 13 ± 3 | 13 ± 4 | 14 ± 5 | 23 ± 6 | 21 ± 7 | 24 ± 10 |
| tmax, h | 4 (3–6) | 4 (3–10) | 3 (2–10) | 4 (2–6) | 4 (3–10) | 3 (2–10) |
| AUC0-96, ng · h ·ml−1) | 461 ± 72 | 512 ± 123 | 482 ± 148 | 639 ± 165 | 645 ± 280 | 638 ± 326 |
| AUC0-∞, ng · h−1 · ml−1) | 571 ± 115 | 650 ± 160 | 578± 162 | 710 ± 185 | 728 ± 341 | 698 ± 363 |
| AUC0-∞ ratio, ritonavir-indinavir/control | 1.13 (0.99–1.29) | 1.00 (0.89–1.12) | 0.96 (0.80–1.15) | 0.92 (0.78–1.08) | ||
| CL/F, ml · kg−1 · min−1 | 2.25 ± 0.54 | 2.00 ± 0.50 | 2.25 ± 0.53 | 1.87 ± 0.59 | 2.05 ± 0.94 | 2.14 ± 0.92 |
| Elimination t1/2, h | 39 ± 9 | 43 ± 11 | 37 ± 7 | 28 ± 5 | 29 ± 8 | 26 ± 5 |
| Vz/F, l/kg | 7.4 ± 1.6 | 7.3 ± 2.1 | 7.2 ± 1.8 | 4.4 ± 1.2 | 4.8 ± 1.9 | 4.6 ± 1.7 |
| Foral | 0.89 ± 0.12 | 0.97 ± 0.07 | 0.96 ± 0.07 | 0.88 ± 0.12 | 0.93 ± 0.06 | 0.92± 0.06 |
| R-EDDP | S-EDDP | |||||
| Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
|
| Cmax, ng/ml | 1.6 ± 0.4 | 1.7 ± 0.5 | 1.9 ± 0.4 | 2.4 ± 0.7 | 3.0 ± 1.3 * | 3.0 ± 0.9 * |
| AUC0-96, ng · h · ml−1 | 63 ± 11 | 69 ± 17 | 72 ± 18 | 87 ± 19 | 102± 18 * | 104± 21 * |
| AUC∞, ng · h−1 ·ml−1 | 88 ± 18 | 119± 43 | 119± 43 | 107 ± 24 | 126± 20 * | 129 ± 23 |
| Elimination t1/2, h | 50 ± 15 | 74 ± 28 | 66 ± 25 | 37 ± 6 | 41 ± 10 | 39 ± 9 |
| AUC0-96, EDDP/methadone | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.05 | 0.14 ± 0.04 | 0.19 ± 0.09 * | 0.19 ± 0.09 * |
| AUC∞, EDDP/methadone | 0.16 ± 0.03 | 0.19 ± 0.06 | 0.22 ± 0.11 | 0.16 ± 0.05 | 0.21 ± 0.10 * | 0.22 ± 0.10 * |
| AUC∞ (EDDP/methadone) ratio, ritonavir-indinavir/control | 1.15 (0.99–1.33) | 1.30 (1.07–1.59) | 1.24 (1.05–1.46) | 1.33 (1.12–1.57) | ||
Significantly different from control (P<0.05). AUC = area under the plasma concentration-time curve; Cmax = peak plasma concentration; CL/F = apparent oral clearance; EDDP = 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine; Foral = bioavailability; Vz/F = apparent volume of distribution.
Subjects received 11.0 mg oral methadone HCl at all sessions. Results are the arithmetic mean ± SD (n = 12), except the time to peak concentration (tmax), which is the median (and range), and the area under the concentration–time curve (AUC) ratio (ritonavir-indinavir/control), which is the geometric mean (90% CI).
Table 6.
Methadone and Metabolite Renal Excretion and Clearance
| R-methadone | S-methadone | |||||
|---|---|---|---|---|---|---|
| Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/indiNavir |
Control | Acute Ritonavir/Indinavir |
Steady-state Ritonavir/Indinavir |
|
| Percent of dose recovered 0–96 h | ||||||
| IV d0-methadone | 28 ± 7 | 31 ± 7 | 27 ± 7 | 22 ± 6 | 23 ± 5 | 21 ± 6 |
| Oral d5-methadone | 23 ± 6 | 29 ± 4 | 23 ± 6 | 18 ± 6 | 21 ± 5 | 18 ± 5 |
| d0-EDDP | 20 ± 2 | 17 ± 5 | 19 ± 4 | 35 ± 4 | 35 ± 9 | 36 ± 6 |
| d5-EDDP | 21 ± 2 | 21 ± 4 | 21 ± 4 | 35 ± 3 | 39 ± 7 | 37 ± 5 |
| Clearance, ml · kg−1 · min−1 | ||||||
| IV d0-methadone Clr | 0.56 ± 0.16 | 0.64 ± 0.18 | 0.58 ± 0.17 | 0.36 ± 0.13 | 0.45 ± 0.18 | 0.38 ± 0.14 |
| Oral d5-methadone Clr | 0.45 ± 0.14 | 0.61 ± 0.25 | 0.49 ± 0.13 | 0.28 ± 0.10 | 0.43 ± 0.20 | 0.33 ± 0.11 |
| d0-EDDP Clf | 0.40 ± 0.10 | 0.34 ± 0.08 | 0.39 ± 0.09 | 0.58 ± 0.19 | 0.69 ± 0.31 | 0.68 ± 0.28 |
| d5-EDDP Clf | 0.42 ± 0.09 | 0.45 ± 0.17 | 0.45 ± 0.09 | 0.57 ± 0.17 | 0.80 ± 0.37* | 0.70 ± 0.28 |
Significantly different from control (P < 0.05). CLr = renal clearance; CLf = formation clearance; EDDP = 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine.
Results are the mean ± SD (n=12).
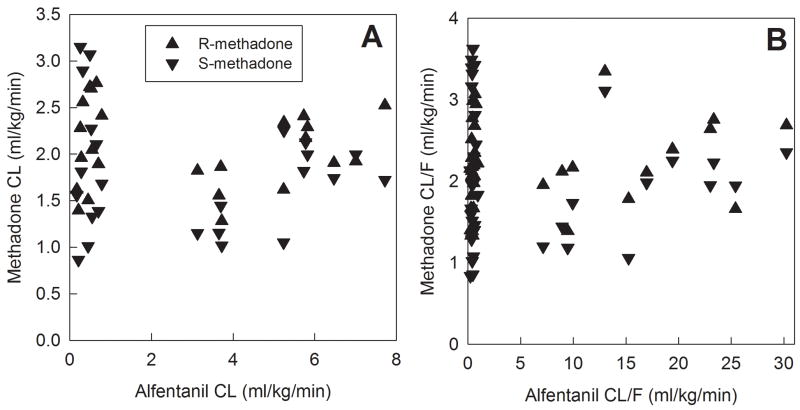
The relationship between methadone clearance and CYP3A activity, measured as alfentanil clearance, is shown in figure 7. For IV R- and S-methadone, there was no significant correlation between systemic methadone clearance and hepatic CYP3A activity (r2 ≤0.01 for both, p>0.05). For oral R- and S-methadone, there was similarly no significant correlation between methadone oral clearance and first-pass CYP3A activity (r2 = 0.04 and 0.01, both p>0.05).
Figure 7.
Relationship between methadone enantiomers clearance and CYP3A activity. (A) IV methadone clearance and hepatic CYP 3A activity (IV alfentanil clearance) (B) apparent oral methadone clearance and first-pass CYP 3A activity (oral alfentanil apparent clearance). Each data point is the result for a single subject. There were no significant correlations between methadone clearance and CYP3A activity.
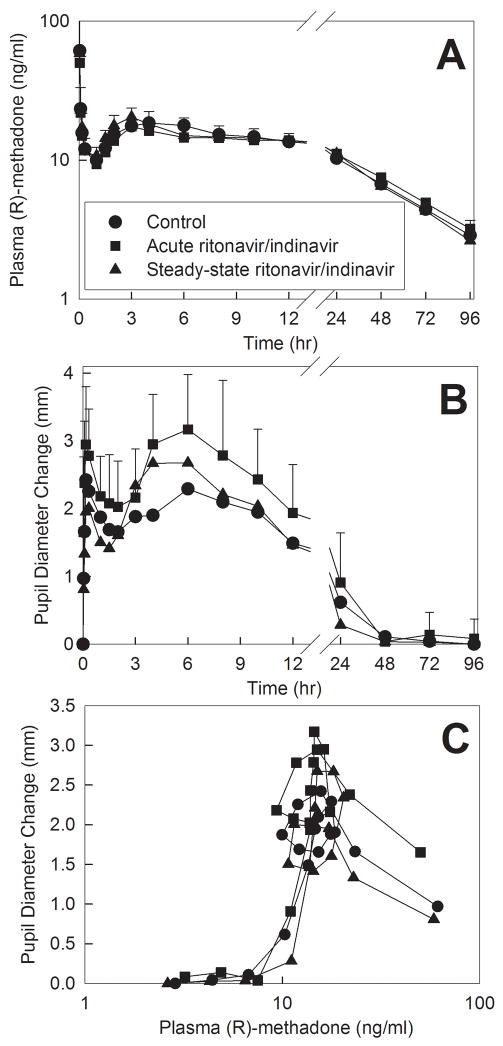
Methadone effects were assessed using changes in dark-adapted pupil diameter (miosis) (fig. 8). Plasma concentrations of total (sum of IV d0- and oral d5) R-methadone (the pharmacologically active enantiomer) are shown in figure 8A. There was a second plasma concentration peak at 3 hr, after the initial IV peak, reflecting the slow absorption of oral methadone. R-methadone concentrations were not different between treatments. Miosis was also not different in ritonavir-indinavir treated subjects and controls (fig. 8B), as was R-methadone pharmacodynamics, assessed by maximum miosis and AUEC (table 2) and the plasma concentration-effect curve (fig. 8C).
Figure 8.
Ritonavir/indinavir effects on methadone pharmacodynamics. Subjects simultaneously received 11.0 mg oral and 6.0 mg IV methadone HCl. Results are shown for (A) total plasma R-methadone concentrations, (B) dark-adapted pupil diameter change from baseline (miosis), and (C) plasma concentration-effect relationships (miosis vs R-methadone plasma concentration). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
Discussion
One major finding of this investigation was ritonavir/indinavir profoundly inhibited CYP3A activity. Steady-state ritonavir/indinavir inhibited more than 90% of hepatic CYP3A activity, as assessed using alfentanil clearance. Acute and steady-state ritonavir/indinavir both inhibited more than 97% of first-pass CYP3A activity, attributable to inhibition of both intestinal and hepatic CYP3A, as alfentanil hepatic extraction was reduced from 0.35 to 0.03 and intestinal extraction from 0.48 to 0.18. Changes in alfentanil miosis were qualitatively similar to the alterations in alfentanil plasma AUC ratios and hepatic and first-pass CYP3A activities, indicating that alfentanil miosis qualitatively reflected ritonavir/indinavir effects on CYP3A activity. There are considered to be relatively few well-characterized protease inhibitor interactions with model CYP3A substrates,37 and ritonavir/indinavir drug interactions in general are among the least well-studied. This is the first investigation to evaluate ritonavir/indinavir effects on both hepatic and first-pass CYP3A, and to show that this protease inhibitor combination diminishes both hepatic and intestinal CYP3A activities, and thus both liver and intestine are the site(s) of ritonavir/indinavir-CYP3A drug interactions.
CYP3A inhibition by ritonavir/indinavir was not unexpected. Indinavir and ritonavir are each potent inhibitors of hepatic and/or intestinal CYP3A4 and CYP3A5,20,21,37 both of which metabolize alfentanil.30 Whereas indinavir alone only increased the AUC of IV and oral alfentanil 2- and 3-fold, respectively (Kharasch, unpublished results, 2004), and ritonavir alone only increased the AUC of IV and oral alfentanil 4- and 10-fold,34 ritonavir/indinavir in the present investigation increased the AUC of IV and oral alfentanil 13- and 30-fold. Thus, ritonavir/indinavir effects likely reflect CYP3A inhibition by both protease inhibitors.
A second major finding of this investigation, which is the first to evaluate ritonavir/indinavir effects on apparent gastrointestinal P-gp activity, was that this protease inhibitor combination inhibited P-gp activity. Both short-term and steady-state ritonavir/indinavir significantly increased fexofenadine maximum plasma concentrations and AUC, which was not due to diminished elimination, suggesting impaired intestinal efflux. Fexofenadine is a well-characterized human P-gp substrate, is frequently used to assess P-gp pharmacogenetics and drug interactions,38–40 and well-known P-gp inhibitors such as ketoconazole, verapamil and erythromycin40–42 increase plasma fexofenadine concentrations. It is unlikely that ritonavir/indinavir effects on fexofenadine AUC are attributable to changes in CYP3A or renal P-gp activity, since these comprise <1% and only 5–10%, respectively, of fexofenadine elimination.38,40 Rather, they suggest ritonavir/indinavir inhibition of intestinal and/or hepatic P-gp. A previous investigation showed that acute and steady-state ritonavir alone increased fexofenadine AUC.34 Similarly, single-dose ritonavir and ritonavir/lopinavir and steady-state ritonavir/lopinavir also increased fexofenadine AUC, similarly ascribed to hepatic P-gp inhibition.43 Increases in fexofenadine AUC caused by acute and steady-state ritonavir/indinavir were greater than in these previous reports. An explanation is not apparent, since indinavir is a comparatively weak inhibitor of P-gp44,45 and other transporters.46 Since fexofenadine is not transported exclusively by P-gp, but is also a substrate for organic anion uptake transporters, exact mechanisms by which ritonavir/indinavir alters drug transport remain unknown.
The primary purpose of this investigation was to determine the effect of ritonavir/indinavir on methadone disposition, metabolism and clearance. This is the first investigation to evaluate ritonavir/indinavir effects on IV or oral methadone concentrations, concurrent effects on both IV and oral methadone, effects on methadone metabolism and on renal excretion, and compare short-term and steady-state ritonavir/indinavir effects on methadone disposition. There was some evidence for very minor induction of methadone N-demethylation. Ritonavir/indinavir (steady-state>acute) mildly increased (S-more then R-) EDDP/methadone plasma AUC ratios, although EDDP enantiomer formation clearances were essentially unchanged. Any minor induction of methadone metabolism was not at all reflected by changes in methadone plasma concentrations, systemic or apparent oral clearance, hepatic clearance, hepatic extraction, or bioavailability. Ritonavir/indinavir had no effect on methadone renal clearance.
Another purpose of this investigation was to test the hypothesis that CYP3A mediates methadone disposition. For years, clinical methadone metabolism, clearance and disposition in general have been attributed to CYP3A4,4,9,10,15,19,47 and variability in CYP3A4 activity was considered the major factor responsible for interindividual differences in methadone bioavailability,6 based on extrapolation of early in vitro studies which identified methadone N-demethylation by human recombinant and hepatic and intestinal CYP3A4.11,32,48–50 Methadone interactions with highly active antiretroviral therapy and other drugs have been attributed to CYP3A4-mediated alterations in methadone disposition.12,19,26 Nevertheless, the present results provide robust and unequivocal evidence against a major role for CYP3A in IV and oral methadone clearance. Specifically, ritonavir/indinavir caused minor induction of methadone metabolism and no changes in hepatic, systemic, or oral clearance, despite more than 90% inhibition of hepatic CYP3A activity and more than 97% inhibition of first-pass CYP3A activity. There was no correlation between IV methadone clearance and hepatic CYP3A activity, nor between oral methadone apparent clearance and first-pass CYP3A activity. The hypothesis that CYP3A mediates methadone disposition is rejected. While the present investigation offers no insights into the CYP isoform which is responsible for clinical methadone metabolism and clearance, investigations stimulated in part by rejection of this hypothesis have, however, identified the prominent role of CYP2B6 in methadone metabolism in vitro,32,50–52 and clinical studies are consistent with this hypothesis.15,24,32,34,47,53
A fifth major finding of this investigation, which is the first to evaluate the relationship between ritonavir/indinavir effects on gastrointestinal P-gp activity and methadone bioavailability, was that bioavailability was unchanged despite significant inhibition of P-gp. While methadone appears to be a P-gp substrate,54 and an animal experiment suggested a role for intestinal P-gp in methadone absorption,55 the present investigation does not support a hypothesis that intestinal P-gp is significantly involved in methadone absorption and first-pass extraction. Clinically, P-gp polymorphisms had no effect on methadone enantiomer peak concentrations after oral administration.15 Thus, while methadone may be a P-gp substrate, passive methadone permeability may be sufficiently high to enable high absorption uninfluenced by P-gp activity.
The sixth major purpose of this investigation was to evaluate ritonavir/indinavir effects on methadone pharmacodynamics. Pupil diameter changes were used to assess plasma concentration-effect relationships. Methadone miosis, and concentration-effect relationships, were not significantly changed by acute or steady-state ritonavir/indinavir. This is in contrast to the effects of acute and steady-state ritonavir, and less so steady-state nelfinavir, which appeared to cause leftward and upward shifts of the R-methadone concentration-response curve, consistent with an increase in apparent potency and efficacy.24,53 Such effects were postulated to suggest methadone brain access by an active process, influenced by one or more blood brain barrier drug efflux and/or influx transporters, susceptible to a transport-mediated interaction with protease inhibitors. Consistent with this interpretation is the finding that indinavir was the least potent of any protease inhibitor in inhibiting activity of primary cultured bovine brain microvessel endothelial cell P-gp and other efflux transporters.56
The results of this investigation have significant clinical implications. Methadone is a cornerstone therapy for opiate dependence, but is being increasingly used to treat acute, chronic, perioperative, neuropathic, and cancer pain. Greater methadone use for pain therapy is primarily responsible for a 1300% increase in methadone prescriptions between 1997 and 2006.*, † Unfortunately, however, an exponential decade-long rise in methadone toxicity has accompanied this growing clinical use. Methadone-related adverse events increased ~1800% between 1997–2004 and fatalities increased 390% from 1999 to 2004, both of which have been attributed primarily to increased methadone use for pain treatment not addiction.3‡,§ The long elimination half-life and extreme variability in clearance (pharmacogenetic and/or drug interaction-related) are considered the greatest impediment to predictable methadone dosing and clinical effect.9 Concerted research efforts for over a decade have endeavored to identify the enzymes responsible for methadone elimination, pertinent drug interactions, and provide practitioner guidance. Based on extrapolation of in vitro studies, methadone metabolism and clearance in vivo have been ubiquitously attributed to CYP3A4,4,9,10,15,47 and methadone dosing guidelines warn about the potential for CYP3A4-mediated methadone drug interactions and the need to adjust dosing accordingly.4,6,9–11,13–15 The FDA-approved methadone label states “Since the metabolism of methadone is mediated primarily by CYP3A4, coadministration of drugs that inhibit CYP3A4 may cause decreased clearance of methadone. The expected clinical results would be increased or prolonged opioid effects.”†† Nevertheless, neither the role of CYP3A4 in methadone clearance nor effects of CYP3A4 inhibition were ever clinically tested. We performed the first ever assessment of CYP3A activity and methadone clearance, and found no influence of CYP3A inhibition (60%) on methadone plasma concentrations or clearances, and no correlation between methadone clearance and hepatic CYP3A activity.32 Subsequently, ritonavir (>90%),24 nelfinavir (>75%),53 and in this investigation ritonavir/indinavir (>90%) were found to inhibit hepatic and first-pass CYP3A profoundly without reducing methadone clearance, and lack of a correlation between methadone clearance and CYP3A activity has been a consistent finding.15,24,32,53 These results strengthen the original findings, and provide substantive evidence against a role for CYP3A in methadone drug interactions, and clinical methadone N-demethylation and clearance in general. Practitioner guidelines identifying methadone as a CYP3A substrate, and warning of CYP3A-mediated drug interactions, require thorough and thoughtful reevaluation. Indeed, the important message is that CYP3A-based guidelines used by clinicians to direct methadone therapy may be incorrect.
There are potential limitations with this investigation. First, a single methadone dose was evaluated. It is neither possible nor ethical to study healthy volunteers at “steady-state”, due to the risk of causing addiction. Nonetheless, the kinetics of a single low methadone dose appear similar to those of higher, steady-state methadone doses. Second, ritonavir/indinavir effects were evaluated in healthy volunteers, not HIV-infected patients. This was deliberate, because antiretroviral therapy involves several drugs, thereby precluding a mechanistic evaluation and attribution of results to any one specific agent (i.e., ritonavir-boosted indinavir). The present investigation was conducted when the standard ritonavir-boosted indinavir regimen was 100/800 mg twice daily, but that has now been changed to 100/400 mg twice daily.57 Nonetheless, a similar absent effect of lower dose ritonavir-indinavir would be expected, and the mechanistic results of the present investigation remain valid.
In summary, ritonavir/indinavir had no significant effects on methadone enantiomers systemic or apparent oral clearance, renal clearance, hepatic extraction or clearance, bioavailability, or plasma concentrations, despite significant (>90%) inhibition of hepatic and first-pass CYP3A activity. This suggests little or no role for CYP3A in clinical methadone metabolism and clearance.
Acknowledgments
Financial Disclosure: Supported by grants R01-DA14211, K24-DA00417 and R01-GM63674 (Dr. Kharasch), and M01-RR00037 (University of Washington General Clinical Research Center) from the National Institutes of Health, Bethesda, Maryland. Indinavir was the generous gift of Merck and Co., Inc. (Whitehouse Station, New Jersey)
We are grateful to Merck and Co, Inc., Whitehouse Station, New Jersey, for the generous gift of indinavir.
Footnotes
No author has any affiliation with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in the manuscript.
Governale L: Methadone utilization in the US 2002 - 2006, FDA Center for Drug Evaluation and Research, 2007, www.dpt.samhsa.gov/ppt/FINAL%20Methadone%20Governale%20SAMHSA%207-20-07.ppt, last accessed June 1, 2008.
Methadone mortality A reassessment, Drug Enforcement Administration, Office of Enforcement Operations, Pharmaceutical Investigations Section, Targeting and Analysis Unit, 2007, http://www.dpt.samhsa.gov/ppt/methadone%20ARCOS%2007-20-07.ppt, last accessed June 1, 2008.
Methadone mortality A reassessment. Summary report of the meeting. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration, Department of Health and Human Services, Rockville, MD, 2007, http://www.dpt.samhsa.gov/pdf/Methadone_Report_10%2018%2007_Brief%20w%20attch.pdf, last accessed June 1, 2008.
Methadone mortality - A reassessment. Background information. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration, Department of Health and Human Services, Rockville, MD, 2007, http://www.dpt.samhsa.gov/pdf/MethadoneBackgroundPaper_72007_2_.pdf, last accessed June 1, 2008.
www.fda.gov/CDER/Drug/infopage/methadone/default.htm, last accessed Dec 1, 2007
www.fda.gov/CDER/Drug/infopage/methadone/default.htm, last accessed Dec 1, 2007
References
- 1.Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev. 2007:CD003971. doi: 10.1002/14651858.CD003971.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–190. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 3.Webster LR. Methadone-related deaths. J Opioid Manag. 2005;1:211–7. doi: 10.5055/jom.2005.0045. [DOI] [PubMed] [Google Scholar]
- 4.Foster DJ, Somogyi AA, Dyer KR, White JM, Bochner F. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50:427–40. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster DJ, Somogyi AA, White JM, Bochner F. Population pharmacokinetics of (R)-, (S)- and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2004;57:742–55. doi: 10.1111/j.1365-2125.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari A, Coccia CP, Bertolini A, Sternieri E. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551–9. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Shah N, Lathrop SL, Landen MG. Unintentional methadone-related overdose death in New Mexico (USA) and implications for surveillance, 1998–2002. Addiction. 2005;100:176–88. doi: 10.1111/j.1360-0443.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark JD. Understanding methadone metabolism: a foundation for safer use. Anesthesiology. 2008;108:351–2. doi: 10.1097/ALN.0b013e318164937c. [DOI] [PubMed] [Google Scholar]
- 9.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 10.Shinderman M, Maxwell S, Brawand-Amey M, Golay KP, Baumann P, Eap CB. Cytochrome P4503A4 metabolic activity, methadone blood concentrations, and methadone doses. Drug Alcohol Depend. 2003;69:205–11. doi: 10.1016/s0376-8716(02)00320-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang J-S, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)-and (S)-methadone in vitro. Drug Metab Dispos. 2003;31:74–7. doi: 10.1124/dmd.31.6.742. [DOI] [PubMed] [Google Scholar]
- 12.McCance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: the importance of drug interactions between opioids and antiretroviral agents. Clin Infect Dis. 2005;41 (Suppl 1):S89–95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- 13.Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther. 2006;80:682–90. doi: 10.1016/j.clpt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41:563–72. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- 15.Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–81. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Thompson SJ, Koszdin KK, Bernards CM. Opiate-induced analgesia as increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–9. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–9. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 18.Muga R, Langohr K, Tor J, Sanvisens A, Serra I, Rey-Joly C, Munoz A. Survival of HIV-infected injection drug users (IDUs) in the highly active antiretroviral therapy era, relative to sex- and age-specific survival of HIV-uninfected IDUs. Clin Infect Dis. 2007;45:370–6. doi: 10.1086/519385. [DOI] [PubMed] [Google Scholar]
- 19.Maas B, Kerr T, Fairbairn N, Montaner J, Wood E. Pharmacokinetic interactions between HIV antiretroviral therapy and drugs used to treat opioid dependence. Expert Opin Drug Metab Toxicol. 2006;2:533–43. doi: 10.1517/17425255.2.4.533. [DOI] [PubMed] [Google Scholar]
- 20.Robertson SM, Penzak SR, Pau A. Drug interactions in the management of HIV infection: an update. Expert Opin Pharmacother. 2007;8:2947–63. doi: 10.1517/14656566.8.17.2947. [DOI] [PubMed] [Google Scholar]
- 21.Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3:583–98. doi: 10.1517/17425225.3.4.583. [DOI] [PubMed] [Google Scholar]
- 22.Iribarne C, Berthou F, Carlhant D, Dreano Y, Picart D, Lohezic F, Riche C. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug Metab Dispos. 1998;26:257–60. [PubMed] [Google Scholar]
- 23.Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–91. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber JG, Rosenkranz S, Segal Y, Aberg J, D’Amico R, Mildvan D, Gulick R, Hughes V, Flexner C, Aweeka F, Hsu A, Gal J. Effect of ritonavir/saquinavir on stereoselective pharmacokinetics of methadone: results of AIDS Clinical Trials Group (ACTG) 401. J Acquir Immune Defic Syndr. 2001;27:153–60. doi: 10.1097/00126334-200106010-00010. [DOI] [PubMed] [Google Scholar]
- 26.McCance-Katz EF, Rainey PM, Friedland G, Jatlow P. The protease inhibitor lopinavir-ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis. 2003;37:476–82. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- 27.Clarke S, Mulcahy F, Bergin C, Reynolds H, Boyle N, Barry M, Back DJ. Absence of opioid withdrawal symptoms in patients receiving methadone and the protease inhibitor lopinavir-ritonavir. Clin Infect Dis. 2002;34:1143–45. doi: 10.1086/339541. [DOI] [PubMed] [Google Scholar]
- 28.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass CYP3A activity. Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. 2007;82:410–26. doi: 10.1038/sj.clpt.6100237. [DOI] [PubMed] [Google Scholar]
- 30.Klees TM, Sheffels P, Dale O, Kharasch ED. Metabolism of alfentanil by cytochrome P4503A (CYP3A) enzymes. Drug Metab Dispos. 2005;33:303–11. doi: 10.1124/dmd.104.002709. [DOI] [PubMed] [Google Scholar]
- 31.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–71. [PubMed] [Google Scholar]
- 32.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Kharasch ED, Walker A, Hoffer C, Sheffels P. Evaluation of first-pass cytochrome P4503A (CYP3A) and P-glycoprotein activities using alfentanil and fexofenadine in combination. J Clin Pharmacol. 2005;45:79–88. doi: 10.1177/0091270004269705. [DOI] [PubMed] [Google Scholar]
- 34.Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–12. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenblatt DJ, von Moltke LL, Daily JP, Harmatz JS, Shader RI. Extensive impairment of triazolam and alprazolam clearance by short- term low-dose ritonavir: the clinical dilemma of concurrent inhibition and induction. J Clin Psychopharmacol. 1999;19:293–96. doi: 10.1097/00004714-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Whittington D, Sheffels P, Kharasch ED. Stereoselective determination of methadone and the primary metabolite EDDP in human plasma by automated on-line extraction and liquid chromatography mass spectrometry. J Chrom B. 2004;809:313–21. doi: 10.1016/j.jchromb.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 37.Ernest CS, II, Hall SD, Jones DR. Mechanism-based inactivation of cytochrome P450 3A (CYP3A) by HIV protease inhibitors. J Pharmacol Exp Ther. 2005;312:583–91. doi: 10.1124/jpet.104.075416. [DOI] [PubMed] [Google Scholar]
- 38.Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–21. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- 39.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 40.Lemma GL, Wang Z, Hamman MA, Zaheer NA, Gorski JC, Hall SD. The effect of short- and long-term administration of verapamil on the disposition of cytochrome P450 3A and P-glycoprotein substrates. Clin Pharmacol Ther. 2006;79:218–30. doi: 10.1016/j.clpt.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Petri N, Borga O, Nyberg L, Hedeland M, Bondesson U, Lennernas H. Effect of erythromycin on the absorption of fexofenadine in the jejunum, ileum and colon determined using local intubation in healthy volunteers. Int J Clin Pharmacol Ther. 2006;44:71–9. doi: 10.5414/cpp44071. [DOI] [PubMed] [Google Scholar]
- 42.Tannergren C, Knutson T, Knutson L, Lennernas H. The effect of ketoconazole on the in vivo intestinal permeability of fexofenadine using a regional perfusion technique. Br J Clin Pharmacol. 2003;55:182–90. doi: 10.1046/j.1365-2125.2003.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Heeswijk RP, Bourbeau M, Campbell P, Seguin I, Chauhan BM, Foster BC, Cameron DW. Time-dependent interaction between lopinavir/ritonavir and fexofenadine. J Clin Pharmacol. 2006;46:758–67. doi: 10.1177/0091270006288733. [DOI] [PubMed] [Google Scholar]
- 44.Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–70. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- 45.Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73:1573–81. doi: 10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 46.Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, Efferth T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother. 2007;59:238–45. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 47.Crettol S, Deglon JJ, Besson J, Croquette-Krokkar M, Gothuey I, Hammig R, Monnat M, Huttemann H, Baumann P, Eap CB. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Iribarne C, Berthou F, Baird S, Dreano Y, Picart D, Bail JP, Beaune P, Menez JF. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem. Res. Toxicol. 1996;9:365–73. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 49.Foster DJ, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: Lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–12. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 51.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–99. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 52.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2007;108:363–74. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 53.Kharasch ED, Whittington D, Walker A, Hoffer C, Sheffels P. Mechanism of nelfinavir influence on methadone pharmacokinetics, pharmacodynamics, and clinical effect (abstract) Clin Pharmacol Ther. 2008:PI–90. [Google Scholar]
- 54.Crettol S, Digon P, Golay KP, Brawand M, Eap CB. In vitro P-glycoprotein-mediated transport of (R)-, (S)-, (R,S)-methadone, LAAM and their main metabolites. Pharmacology. 2007;80:304–11. doi: 10.1159/000107104. [DOI] [PubMed] [Google Scholar]
- 55.Bouer R, Barthe L, Philibert C, Tournaire C, Woodley J, Houin G. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: In vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol. 1999;13:494–500. doi: 10.1111/j.1472-8206.1999.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 56.Bachmeier CJ, Trickler WJ, Miller DW. Comparison of drug efflux transport kinetics in various blood-brain barrier models. Drug Metab Dispos. 2006;34:998–1003. doi: 10.1124/dmd.105.006999. [DOI] [PubMed] [Google Scholar]
- 57.Cressey TR, Plipat N, Fregonese F, Chokephaibulkit K. Indinavir/ritonavir remains an important component of HAART for the treatment of HIV/AIDS, particularly in resource-limited settings. Expert Opin Drug Metab Toxicol. 2007;3:347–61. doi: 10.1517/17425255.3.3.347. [DOI] [PubMed] [Google Scholar]