Abstract
Hedgehog (Hh) signaling is critical to the patterning and development of a variety of organ systems, and both ligand-dependent and ligand-independent Hh pathway activation are known to promote tumorigenesis. Recent studies have shown that in tumors promoted by Hh ligands, activation occurs within the stromal microenvironment. Testing whether ligand-driven Hh signaling promotes tumor angiogenesis, we found that Hh antagonism reduced the vascular density of Hh-producing LS180 and SW480 xenografts. In addition, ectopic expression of sonic hedgehog in low-Hh–expressing DLD-1 xenografts increased tumor vascular density, augmented angiogenesis, and was associated with canonical Hh signaling within perivascular tumor stromal cells. To better understand the molecular mechanisms underlying Hh-mediated tumor angiogenesis, we established an Hh-sensitive angiogenesis coculture assay and found that fibroblast cell lines derived from a variety of human tissues were Hh responsive and promoted angiogenesis in vitro through a secreted paracrine signal(s). Affymetrix array analyses of cultured fibroblasts identified VEGF-A, hepatocyte growth factor, and PDGF-C as candidate secreted proangiogenic factors induced by Hh stimulation. Expression studies of xenografts and angiogenesis assays using combinations of Hh and VEGF-A inhibitors showed that it is primarily Hh-induced VEGF-A that promotes angiogenesis in vitro and augments tumor-derived VEGF to promote angiogenesis in vivo.
Through its regulation of epithelial-mesenchymal interactions and subsequent control of organ system patterning and growth, hedgehog (Hh) signaling is one of several conserved signaling pathways critical to embryogenesis. Key components of Hh signaling include the ligands sonic hedgehog (SHH), Indian hedgehog (IHH), and desert hedgehog (DHH); the negative and positive regulators of signaling, PTCH1 and SMO, respectively; and the GLI transcription factors controlling Hh target gene expression. Conserved Hh targets include GLI1 and PTCH1, and their expression is commonly used to measure Hh signaling activity.
Similar to other pathways active during development, Hh signaling has been shown to promote tumorigenesis. In several tumor types, tumorigenesis follows mutations in key Hh pathway regulators, resulting in hyperactive ligand-independent signaling within tumor cells. Alternatively, and in a broader range of tumors, Hh signaling promotes tumorigenesis through tumor cell production of Hh ligands (1). In tumors promoted by Hh ligands, recent studies have shown that canonical pathway activation is restricted to tumor stroma (2, 3).
To better understand how Hh ligands promote tumorigenesis, and because a role exists for Hh signaling during vascular development (4), we examined whether Hh signaling within tumors can influence angiogenesis, a critical determinant of tumor growth and viability. Though several recent studies have focused on defining a role for Hh in tumor angiogenesis, it remains unclear whether (i) Hh signaling promotes tumor angiogenesis (5), (ii) whether angiogenesis is influenced through canonical signaling, and (iii) which cells within tumors receive Hh's signal (6, 7).
Using Hh-producing and Hh-sensitive LS180 xenografts, we found that inhibiting Hh signaling decreased tumor angiogenesis. By overexpressing SHH in low Hh-expressing DLD-1 xenografts, we found that ectopic Hh signaling promoted tumor angiogenesis and that canonical Hh signaling was up-regulated in perivascular fibroblastic stromal cells surrounding small tumor vessels. Using an Hh-sensitive in vitro coculture assay, we found that angiogenesis correlated with Hh target gene induction in fibroblasts; parallel expression and functional assays identified VEGF-A as the predominant Hh-induced proangiogenic factor in vitro and in vivo. We also found VEGF-A expression was enriched in Hh-signaling active stoma relative to epithelium in metastatic human pancreatic adenocarcinomas (PDA) samples. Furthermore, SW480 xenografts given combinations of anti-VEGF and Hh pathway inhibitor (HPI) further supported a critical role for Hh-driven VEGF-A in driving Hh-induced angiogenesis in vivo.
Results
Hh Signaling Promotes Tumor Angiogenesis in LS180 Xenografts.
The human colorectal carcinoma cell line LS180 expresses Hh ligands, and LS180 xenograft growth is inhibited by the HPI GDC-0449 (Fig. 1 A and B). To test whether Hh signaling promotes tumor angiogenesis during Hh-sensitive tumor growth, we compared the vascular densities of LS180 xenografts following treatment with either GDC-0449 or vehicle. Following 3 d of twice-daily dosing (75 mg/kg per dose), tumors treated with GDC-0449 showed an approximate 30% decrease in vascular density compared with control tumors (relative vessel area, 1.36% vs. 1.89%, P = 0.008; relative vessel perimeter, 0.37 vs. 0.47 μm−1, P = 0.03; Fig. 1 C and D). In addition, GDC-0449 treatment significantly reduced tumor vascular complexity as assessed by branch points normalized to viable area (relative branch point, 13.0 vs. 7.6 μm−2, P = 0.05; Fig. S1A). These data show that in Hh-sensitive tumor models, Hh signaling promotes tumor angiogenesis.
Fig. 1.
Hh signaling promotes tumor angiogenesis in LS180 xenografts. (A) FACS analysis of cell-surface ligand expression in LS180 cells using the Hh antibody 5E1. (B) LS180 tumor growth during treatment with vehicle or the HPI GDC-0449 (100 mg/kg, orally, twice daily). Data are mean ± SD (n = 10/group). (C and D) Tumor vascular density analysis of LS180 xenografts treated with GDC-0449 (75 mg/kg, orally, twice daily) vs. vehicle following 3 d of drug administration. GDC-0449 reduces tumor relative vessel area (C; *P = 0.01) and relative vessel perimeter (D; *P = 0.03) compared with vehicle treatment (n = 10/group). The middle line of each diamond represents the mean, and the upper and lower borders of the diamonds the 95% confidence interval.
Isolated Endothelial Cells Do Not Display Canonical Hh Signaling.
To begin to understand the cellular basis underlying Hh regulation of tumor angiogenesis, we asked if isolated endothelial cells (ECs) demonstrated Hh signaling. First, human umbilical vein endothelial cells (HUVECs) were cultured with 1 μg/mL SHH for up to 72 h; we found no induction of GLI1 or PTCH1 (Fig. S2A, a). In contrast, this same concentration of SHH stimulated the expression of GLI1 (five- to ninefold) and PTCH1 (2.5- to fivefold) in the myofibroblast cell line CCD-18Co (Fig. S2A, b). Second, culturing HUVECs in isolation with 1 μg/mL SHH had no effect on HUVEC growth (Fig. S2A, c). To test if tonic Hh signaling occurred in isolated ECs, we cultured HUVECs with 100 nM of GDC-0449 for 24–72 h and found no changes in GLI1 or PTCH1 expression (Fig. S2A, d). In addition, the growth rate of HUVEC cultures treated with GDC-0449 was not different from those treated with vehicle (Fig. S2A, e). We further asked if microvascular endothelial cells (HMVECs) derived from a variety of human tissues responded to canonical Hh signaling. We treated lung HMVEC (HMVEC-L), skin HMVEC (HMVEC-D), and skin lymphatic HMVEC (HMVEC-lyp) with 1 μg/mL SHH or vehicle in conditions favoring endothelial growth (supplemented with 10 ng/mL VEGF-A) for up to 72 h and found no induction of GLI1 or PTCH1 (Fig. S2B, a), and no difference in growth rate (Fig. S2B, c). However, under the same conditions, SHH induced both GLI1 (five- to 15-fold) and PTCH1 (two- to fivefold) in CCD-18Co cells (Fig. S2B, b). To test if tonic Hh signaling occurred within isolated HMVECs, we cultured HMVECs with 100 nM of GDC-0449 or vehicle in the presence of 10 ng/mL of VEGF-A for up to 72 h and found no changes in GLI1 or PTCH1 expression (Fig. S2B, d), and no difference in growth rate (Fig. S2B, e). The fact that isolated endothelial cells fail to respond to either SHH or a potent HPI suggests that endothelial cells are not directly responsive to Hh signals during Hh-mediated angiogenesis.
Canonical Hh Signaling Within Perivascular Stromal Cells Is Associated with Augmented Tumor Angiogenesis in DLD-1 Xenografts.
To test the sufficiency of Hh signaling to drive tumor angiogenesis, we chose a human colorectal carcinoma cell line, DLD-1, expressing low levels of endogenous Hh ligands, and derived a stable cell line, DLD-1.SHH, expressing >100-fold higher levels of SHH (Fig. 2A). When grown as xenografts in nude mice, DLD-1.SHH cells formed tumors that induced the canonical mouse Hh targets Gli1 and Ptch1 (Fig. 2B). After 6–14 d of growth, DLD-1.SHH tumors were larger than control tumors (Fig. S3A) and often developed a dark red or bruised appearance, which control tumors lack (Fig. 2 C and D). Histologically, DLD-1.SHH tumors often displayed features associated with the “angiogenic switch” (8), which did not show in control tumors (Fig. 2 C and D), although without a significant difference in viable tumor area relative to control tumors (Fig. S3B). Notably, the most robust Hh signaling in DLD-1.SHH tumors was observed in fibroblastic cells juxtaposed to small vessels within the growing tumor mass (Fig. 2 E–J). After 8 d of growth in immunocompromised mice, histologic analysis and quantification of the vasculature in DLD-1.SHH xenografts showed a greater than twofold increase in relative tumor vessel area compared with low Hh-expressing DLD-1.Vec control tumors (2.11% vs. 1.03%, P = 0.0056), and a 1.9-fold increase in relative vessel perimeter (0.68 vs. 0.35 μm−1, P = 0.004; Fig. 2 K and L). In addition, the DLD-1.SHH tumors displayed a more complex vascular structure compared with control tumors, with more relative branchpoints 17.6 vs. 12.1 μm−2, P = 0.02 (Fig. S1B). These data demonstrate that canonical Hh signaling within perivascular tumor stroma coincides with increased tumor angiogenesis, consistent with the hypothesis that paracrine Hh signals received by perivascular stromal cells augment tumor angiogenesis in vivo.
Fig. 2.
Canonical Hh signaling in stromal perivascular cells is associated with augmented tumor angiogenesis. (A) FACS analysis of cell-surface ligand expression in parental DLD-1 (Left) and in DLD-1.SHH vs. DLD-1.Vec cells (Right) using Hh antibody 5E1. (B) RT-PCR transcript comparisons for day 8 (D8) DLD-1.Vec vs. DLD-1.SHH tumors. Mouse Gli1 and Ptch1 transcripts were quantified using species-specific primers, and the mouse Rpl19 gene was used as an internal control for each animal. Data are mean ± SD (n = 10/group). (C Upper) Representative gross images of DLD-1.SHH tumors. Left-sided hash marks are separated by 1 mm. Note the enlarged, dark red appearance of the tumors. (Lower) H&E-stained section of DLD-1.SHH tumor showing numerous red blood-cell lakes (hemorrhages) marked by an asterisk (16). (D) Representative gross images (Upper) and H&E-stained section images (Lower) of DLD-1.Vec control tumors. (E–H) Isotopic in situ hybridization (ISH) analysis of mouse Ptch1 gene expression in D8 DLD-1.SHH tumor sections. (F and H) Insets of E and G showing Ptch1 up-regulation in stromal perivascular cells. Arrows highlight silver grains that identify sites of probe hybridization. Note that silver grains overlie cells surrounding tumor blood vessels (BV). (I and J) ISH analysis of Ptch1 expression in a DLD-1.Vec control tumor section. Few Ptch1 transcripts were detected adjacent to tumor blood vessels. (K and L) Vascular density analysis of D8 DLD-1.SHH (n = 9) and DLD-1.Vec (n = 10) xenografts. SHH overexpression in DLD-1 tumors significantly increased relative vessel area (*P = 0.006) and relative vessel perimeter (*P = 0.004). (Magnification: D, 70×; E, G, and I, 200×.)
In Vitro Angiogenesis Correlates with Hh Target Gene Induction in Fibroblasts.
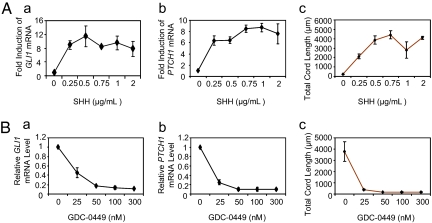
To better understand the molecular mechanisms by which perivascular fibroblastic cells drive tumor angiogenesis, we developed an in vitro, Hh-responsive angiogenesis assay by coculturing HUVECs with CCD-18Co human colon myofibroblasts (Fig. S4). Under coculture conditions, canonical Hh signaling within CCD-18Co cells was sensitive to varying doses of both SHH and the HPI GDC-0449; the magnitude of angiogenesis stimulated during coculture closely paralleled the induction of Hh targets GLI1 and PTCH1 in CCD-18Co cells (Fig. 3). To test whether Hh-driven angiogenesis could be generalized to fibroblasts and endothelial cells derived from other tissue types, we performed the same coculture angiogenesis assay by pairing HUVECs with fibroblast cell lines derived from breast, lung, and skin. Breast and lung fibroblast lines were Hh responsive, and the fold induction of GLI1 and PTCH1 in the fibroblasts paralleled the increase in angiogenesis seen in HUVEC cocultures. In contrast, and consistent with a requirement for canonical SHH signaling within fibroblasts, human normal skin dermal fibroblasts (HNDF) demonstrated neither canonical Hh signaling nor the ability to promote angiogenesis (Table S1). To test whether endothelial cell lines derived from a variety of human tissues were responsive to CCD-18Co proangiogenic signals, we paired CCD-18Co cells with HMVECs from lung and skin, and with lymphatic HMVECs derived from skin. Following coculture, all three HMVEC lines responded robustly to Hh-driven, CCD-18Co proangiogenic signals (Table S1). Taken together, these data indicate that Hh-responsive proangiogenic gene products, produced by fibroblasts from many tissue types, are capable of driving both blood vessel and lymphatic angiogenesis.
Fig. 3.
Angiogenesis correlates with Hh target gene induction in myofibroblasts. (A) GLI1 and PTCH1 target gene induction in CCD-18Co myofibroblasts and angiogenesis are dosage sensitive to SHH. (A, a and b) RT-PCR analysis of GLI1 and PTCH1 in cultured CCD-18Co cells treated with increasing doses of SHH for 48 h. (A, c) Hh-mediated angiogenesis in CCD-18Co/HUVEC cocultures following increasing doses of SHH. Note that HUVEC cord formation, reflected by the total cord length, is dosage sensitive to SHH and mirrors Hh target gene induction in CCD-18Co cells. (B) GLI1 and PTCH1 target gene induction in CCD-18Co cells and angiogenesis are dosage sensitive to Hh inhibition. (B, a and b) RT-PCR analysis of GLI1 and PTCH1 in cultured CCD-18Co cells treated with 1 μg/mL SHH and increasing doses of GDC-0449 for 48 h. (B, c) Hh-mediated angiogenesis in CCD-18Co/HUVEC cocultures following increasing doses of GDC-0449. Note that HUVEC cord formation is dosage sensitive to GDC-0449 and mirrors the change in Hh target gene expression in CCD-18Co cells. Data are mean ± SD (n = 3).
VEGF-A Is the Predominant Hh-induced Proangiogenic Factor Driving Tumor Angiogenesis in Vitro and in Vivo.
To test whether the proangiogenic signal(s) from fibroblasts was secreted, we allowed pure cultures of CCD-18Co cells grown in the presence or absence of SHH to condition media [SHH-containing conditioned media (SCM); vehicle-containing conditioned media (VCM)] (Fig. S5A). CCD-18Co cells cultured in the presence of SHH for 48 h showed a 20- to 30-fold induction of SHH-driven GLI1 and PTCH1 (Fig. S5B). Subsequently, SCM and VCM were added to fibroblast/HUVEC cocultures to which no exogenous SHH was added (“naïve” pairings). To prevent residual SHH from inducing Hh signaling in naïve CCD-18Co, the α-Hh antibody 5E1 (66.7 nM) was added to SCM before the start of angiogenesis cocultures (Fig. S5C). Consistent with the hypothesis that fibroblasts produce secreted, proangiogenic factors following Hh pathway activation, HUVECs paired with naïve CCD-18Co cells demonstrated more angiogenesis when cultured in SCM than in VCM (P = 0.018; Fig. 4A).
Fig. 4.
VEGF-A is the predominant Hh-induced proangiogenic factor driving tumor angiogenesis in vitro and in vivo. (A) Conditioned media from SHH-stimulated CCD-18Co cells promotes HUVEC cord formation. Data are mean ± SD (n = 3). (B) U133P Affymetrix array analysis comparing gene expression of CCD-18Co cells cultured with vehicle vs. 1 μg/mL SHH for 72 h. GLI1 and PTCH1 were induced, as expected, as well as the genes of several secreted proangiogenic factors. Values represent the fold change in expression in SHH- vs. vehicle-treated CCD-18Co cells. The P values were obtained from all probes measuring a gene's transcripts. An asterisk signifies that similar fold changes were seen with RT-PCR analyses following 48 h of SHH exposure. (C and D) ISH analysis of Vegf-A expression in DLD-1.SHH tumors. Similar to mouse Ptch1 (Fig. 2 E–H), Vegf-A is up-regulated in perivascular stromal cells in DLD-1.SHH tumors (D, arrows) Inset of C. BV locates blood vessels that are identified by their pink-red extracellular matrix and lumens. (E and F) ISH analysis in DLD-1.Vec control tumors shows little perivascular Vegf-A expression. (G) SHH-induced (1 μg/mL) in vitro angiogenesis was dosage sensitive to increasing concentrations of the α-VEGF-A antibody, G6-31. Data are mean ± SD (n = 3). (H) SHH-induced angiogenesis was abolished by 66.7 nM G6-31, indicating that SHH-induced angiogenesis in this model system is VEGF dependent. Data are mean ± SD (n = 3). (I) Vascular density analysis of Hh-producing SW480 xenograft tumors following 6 d of treatment with either HPI Hh-Antag (100 mg/kg, orally, twice daily), G6-31 (5 mg/kg, i.p., 2×/week), or a combination of both (n = 10/group). *P = 0.026, **P = 0.0001, ***P = 0.0002. No significant difference was seen between the G6-31 and combination group (P = 0.38). (Magnification: C and E, 280×.)
To identify the Hh response genes responsible for paracrine, Hh-driven tumor angiogenesis, we performed U133P Affymetrix array analyses of CCD-18Co cells cultured for 72 h with vehicle vs. 1 μg/mL SHH. Interestingly, several genes encoding known, secreted, proangiogenic factors were induced by SHH, including VEGF-A, HGF, and PDGF-C, and RT-PCR confirmed the up-regulation of these genes (Fig. 4B). To verify that each of these proangiogenic factors was induced by Hh in tumors, we compared VEGF-A, PDGF-C, and HGF transcript levels in day 8 xenografts of DLD-1.Vec and DLD-1.SHH tumors using RT-PCR with species-specific primers. Of the three proangiogenic factors identified by array analyses of CCD-18Co, only Vegf-A was found to also be up-regulated in SHH overexpressing tumors (Fig. S6 A and B). Consistent with VEGF-A behaving as a canonical Hh target in tumors, Vegf-A transcripts, like Ptch1 transcripts, were particularly enriched within perivascular stromal cells adjacent to small tumor blood vessels (cf. Fig. 4D to Fig. 2 F and H).
Because Hh-induced VEGF-A and angiogenesis correlate in vitro and in vivo, we tested VEGF-A's functional relevance to Hh-driven angiogenesis. First, we quantified SHH-induced angiogenesis in cocultures while inhibiting VEGF-A. Using the potent VEGF-A antibody G6-31, we found that Hh-mediated angiogenesis was dose dependent on VEGF-A (Fig. 4G). Conversely, with high G6-31 concentrations (1–100 nM), the vast majority of Hh-mediated angiogenesis was eliminated (Fig. 4H), demonstrating that Hh-induced VEGF-A is the predominant proangiogenic factor in this simplified system.
To test whether VEGF-A is also the predominant factor involved in mediating Hh-induced tumor angiogenesis in the DLD-1.SHH xenograft model, we treated tumors with the α-VEGF-A antibody B20-4.1.1. We found that α-VEGF treatment of DLD-1.SHH decreases the vascular density significantly compared with IgG treatment (relative vessel area: 1.13% vs. 4.47%, P = 0.0019; Fig. S3C), suggesting that the amount of VEGF-A induced by SHH in DLD-1.SHH tumors is sufficient to mediate tumor angiogenesis. Interestingly, the vascular density in α-VEGF–treated DLD-1.SHH tumors is also lower than IgG-treated DLD-1.Vec tumors (relative vessel area: 1.13% vs. 1.61%, P = 0.0019; Fig. S3C). These data suggest that VEGF-A produced independently of Hh signaling also contributes to vessel formation in DLD-1.vec tumors.
If Hh in vivo induces both VEGF-A and VEGF-A–independent proangiogenic factors, we would predict that Hh-expressing tumors that are only partially growth inhibited by α-VEGF would show a greater antiangiogenic response when both Hh signaling and VEGF-A are inhibited. To test this, we used human SW480 colorectal carcinoma cells, which express SHH, grow in immunocompetent mice, and are only partially growth inhibited by VEGF-A antibody G6-31 (9). We compared the vascular densities of SW480 xenografts following 6 d of treatment (before significant tumor size differences developed) with G6-31 (9, 10), the HPI Hh-Antag (2) or a combination of both G6-31 and Hh-Antag. Tumors treated with either G6-31 or Hh-Antag alone had diminished angiogenesis vs. vehicle control tumors, as determined by vascular density (relative vessel area: 1.02% vs. 1.78%, P < 0.001 for G6-31, and relative vessel perimeter: 1.48 vs. 1.78 μm−1, P = 0.03 for Hh-Antag; Fig. 4I). In addition, tumors treated with a combination of both G6-31 and Hh-Antag also showed reduced relative vessel area vs. vehicle-treated tumors (1.14% vs. 1.78%, P < 0.001). However, adding Hh-Antag in combination with α-VEGF did not result in a significant change in vascular density vs. G6-31 treatment alone (1.14% vs. 1.02%, P = 0.38). Compared with vehicle treatment, tumors treated either with G6-31 or G6-31 combined with Hh-Antag had decreased vascular branching (relative branch point, 3.47 vs. 2.21 μm−2, P = 0.0002, for G6-31; and 3.47 vs. 2.54 μm−2, P = 0.005, for G6-31 + Hh-Antag). However, adding Hh-Antag to G6-31 treatment did not change vascular branching compared with G6-31 alone (relative branch point: 2.20 μm−2 for Hh-Antag vs. 2.54 μm−2 for combination, P = 0.31; Fig. S1C). These results indicate that similar to SHH-producing LS180 xenografts, SW480 xenografts demonstrate lower vascular densities following Hh inhibition; however, in contrast to LS180 xenografts, SW480 tumor vascular complexity was insensitive to Hh inhibition. In addition, these in vivo data reinforce the importance of VEGF-A induction in Hh-driven tumor angiogenesis.
To test whether these findings in Hh-induced tumor angiogenesis in xenograft models extend to human disease, we examined VEGF-A and GLI1 transcripts in three human PDAs that have been reported previously to show a high stromal activation of the Hh pathway (3). Consistent with xenograft models, we found both significantly higher stromal GLI1 (160- to 953-fold higher than epithelial GLI1) and 1.5-fold higher tumor stromal VEGF-A, relative to tumor epithelial VEGF-A (Fig. S7).
Discussion
During the development of a variety of embryonic tissues, Hh signals originating from the epithelium drive canonical signaling in the adjacent mesenchyme, ultimately influencing the morphogenesis and growth of these structures (13). Recently, this Hh signaling relationship has been extended to tumor biology, where carcinoma cells have been shown to express Hh ligands and drive canonical signaling in tumor stroma, and more specifically in tumor-associated fibroblasts (3). A role for tumor stromal fibroblasts in promoting tumorigenesis is recognized and linked to these cells’ abilities to produce a variety of growth factors that influence carcinoma cell growth directly and/or indirectly (14). One indirect way that tumor fibroblasts promote tumorigenesis is by promoting tumor angiogenesis through secreted, paracrine signals (15).
The fact that tumor fibroblasts are Hh responsive and that Hh signaling promotes vascular development (4) supports the hypothesis tested here that in ligand-producing tumors, Hh signaling promotes tumorigenesis by stimulating tumor angiogenesis, supplementing the VEGF produced by tumor cells. We demonstrated a promotional role for Hh signaling in tumor angiogenesis by showing that inhibition of Hh signaling in LS180 and SW480 xenografts decreased tumor angiogenesis. Complementary to these loss-of-function studies, we found that overexpressing SHH in DLD-1 xenografts produced enlarged, hypervascularized tumors with histologic features of the angiogenic switch (16). The increased angiogenesis associated with ectopic SHH expression showed that canonical Hh signaling was up-regulated within fibroblastic stromal cells adjacent to small tumor blood vessels. To better understand the molecular and cellular underpinnings of Hh-mediated tumor angiogenesis, we showed, using an Hh-sensitive in vitro coculture assay, that canonical Hh signaling in fibroblasts from many tissues can drive angiogenesis through the production and secretion of proangiogenic factor(s). Though expression profiling, we identified several candidate proangiogenic factors unregulated in cultured fibroblasts, and through functional assays identified VEGF-A as the required Hh-induced proangiogenic factor in vitro. Furthermore, Hh-producing SW480 xenografts showed no additional antiangiogenic effect when given a hedgehog pathway inhibitor in combination with α-VEGF vs. α-VEGF alone, indicating that Hh-driven VEGF-A is also the critical determinant for Hh-induced angiogenesis in vivo.
Up-regulation of VEGF-A in diverse cell types may be induced by various signals (e.g., hypoxia, cytokines, hormones, lipids, and Hh ligands) (17, 18). Thus, Hh-driven VEGF in tumor stroma is in the context of this milieu of other proangiogenic contributions. In different tissues and tissue microenvironments, VEGF-A expression depends on the local balance of these signals. Our data extend these findings by showing that Hh-driven VEGF-A can modulate the balance of tumor angiogenesis.
Interestingly, recent studies have described noncanonical Hh signaling affecting cell proliferation, migration, and apoptosis pathways (19, 20). In endothelial cells in vitro, Hh is reported to affect tubulogenesis and survival by modulating RhoA GTPase and caspase activity (19, 20). We cannot rule out that in addition to modulating VEGF-A levels in stromal cells, SHH can directly affect angiogenesis via noncanonical signaling.
Our data show that for some tumor types, Hh's predominant tumor-promoting effect is through the induction of stromal VEGF-A. In tumors with an Hh-driven angiogenic component, we would expect little or no benefit in combining hedgehog pathway inhibitors with anti-VEGF therapy. In a recent phase II clinical trial, the HPI GDC-0449 failed to show added clinical benefit in previously untreated metastatic colorectal cancer patients when added to the standard care regime of Avastin (α-VEGF) and chemotherapy.† The data presented here support a role for Hh antagonists in antiangiogenic therapy. Though these data show that VEGF-A is the predominant proangiogenic factor mediating Hh-driven angiogenesis in several tumor models, this does not exclude other Hh proangiogenic targets from being relevant in subsets of human cancers. In addition to promoting angiogenesis, Hh has also been shown to influence other physiologic parameters within tumor stroma (21). The fact that Hh signaling can affect multiple parameters within tumor stroma simultaneously may, in some tumor types or models, result in a complex relationship between Hh signaling, angiogenesis, and blood flow (5). Future work will be required to better understand the full range of relevant Hh-driven, proangiogenic response genes expressed in human carcinomas and how best to target these with HPIs and/or other therapies.
Materials and Methods
In Situ Hybridization.
Isotopic in situ hybridizations were performed as described (22).
Ptch1.
A 771-bp fragment, spanning from nucleotides (nt) 3,518–4,288 of mouse Ptch1 (reference sequence NM_008957) was amplified by using PCR primers (upper) 5-CCAATGGCCTAAACCGACTGC-3 and (lower) 5-CCCACGGCCTCTCCTCACA-3.
Vegf-A.
A 450-bp fragment from rat Vegf spanning from nt 1,311–1,760 (reference sequence NM_031836) was PCR amplified by using the primers (upper) 5-CAACGTCACTATGCAGATCATGCG-3 and (lower) 5-GGTCTAGTTCCCGAAACCCTGAG-3.
Statistical Analysis.
Two-tailed Student's t test was used to generate P value in this study. P ≤ 0.05 was set as significant.
Additional details can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Lowe, N. Ferrara, F. Peale, H. Koeppen, C. Siebel, and W. Ye for helpful discussions and suggestions, and L. Komuves and M. Gonzalez Edick for microscopy support and advice. L. Berry, D. Swinarski, S. Spencer, and E. Wu coordinated animal studies performed at Piedmont Research and provided dosing advice for Hh-Antag and G6-31. We thank R. Hart and K. West for quality testing of Hh-Antag; S. Scales for α-Hh 5E1 and advice for its use; M. Friesenhahn and N. Lewin-Koh for biostatistical support and advice; and P. Channavajhala for providing technical support with cell culture assays. The Genentech animal facility provided animal housing support; C. Espiritu, R. Taylor, C. Jones, F. Puth, M. Baca, and the Genentech Pathology Core laboratory provided tissue processing and stains; and J. Ernst and Genentech Protein Chemistry provided SHH protein.
Footnotes
Conflict of interest statement: All authors are employees of Genentech, Inc., a wholly owned subsidiary of F. Hoffmann-La Roche Ltd., and may own equity in Roche.
*This Direct Submission article had a prearranged editor.
†Berlin JD, Bendell J, Hart LL, Firdaus I, Gore I, et al., Thirty-Fifth European Society for Medical Oncology Congress, October 8–12, 2010, Milan, abstr LBA21.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE29316).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017945108/-/DCSupplemental.
References
- 1.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 3.Tian H, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc Med. 2004;14:308–313. doi: 10.1016/j.tcm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu JR, et al. Sonic hedgehog protein promotes bone marrow-derived endothelial progenitor cell proliferation, migration and VEGF production via PI 3-kinase/Akt signaling pathways. Acta Pharmacol Sin. 2006;27:685–693. doi: 10.1111/j.1745-7254.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, et al. Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived pro-angiogenic cells. PLoS ONE. 2010;5:e8824. doi: 10.1371/journal.pone.0008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 9.Bagri A, et al. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin Cancer Res. 2010;16:3887–3900. doi: 10.1158/1078-0432.CCR-09-3100. [DOI] [PubMed] [Google Scholar]
- 10.Liang WC, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 11.Williams JA. Hedgehog signaling pathway as a target for therapeutic intervention in basal cell carcinoma. Drug News Perspect. 2003;16:657–662. doi: 10.1358/dnp.2003.16.10.829296. [DOI] [PubMed] [Google Scholar]
- 12.Williams JA, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: Effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong CM, Patel N, Lin J, Jung HS, Widelitz RB. Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: Perspectives in development and evolution. Cell Mol Life Sci. 2000;57:1672–1681. doi: 10.1007/PL00000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 16.Ryschich E, Schmidt J, Hämmerling GJ, Klar E, Ganss R. Transformation of the microvascular system during multistage tumorigenesis. Int J Cancer. 2002;97:719–725. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 18.Pola R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 19.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–579. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins D. Hedgehog signalling: Emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Bailey JM, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jubb AM, Pham TQ, Frantz GD, Peale FV, Jr, Hillan KJ. Quantitative in situ hybridization of tissue microarrays. Methods Mol Biol. 2006;326:255–264. doi: 10.1385/1-59745-007-3:255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.