Abstract
Endogenous and pharmacologic glucocorticoids (GCs) limit inflammatory cascades initiated by Toll-like receptor (TLR) activation. A long-standing clinical observation has been the delay between GC administration and the manifestation of GC's anti-inflammatory actions. We hypothesized that the GCs would have inhibitory effects that target late temporal pathways that propagate proinflammatory signals. Here we interrogated signal transducer and activator of transcription 1 (STAT1) regulation by GC and its consequences for cytokine production during activation of macrophages with TLR-specific ligands. We found that robust STAT1 activation does not occur until 2–3 h after TLR engagement, and that GC suppression of STAT1 phosphorylation first manifests at this time. GC attenuates TLR4-mediated STAT1 activation only through induction of suppressor of cytokine signaling 1 (SOCS1), which increases throughout the 6-h period after treatment. Inhibition of TLR3-mediated STAT1 activation occurs via two mechanisms, impairment of type I IFN secretion and induction of SOCS1. Our data show that SOCS1 and type I interferons are critical GC targets for regulating STAT1 activity and may account for overall GC effectiveness in inflammation suppression in the clinically relevant time frame.
Keywords: knockout mice, JAK
Whereas inflammation is generally a protective innate immune response, inappropriate activation or inefficient regulation of inflammatory reactions contributes to a plethora of common human diseases (1). Endogenous and pharmacologic glucocorticoids (GCs) provide an essential restraint to limit the magnitude and duration of inflammatory responses, and are the mainstay of clinical intervention in treating patients suffering from several of those diseases (2). GCs function via the glucocorticoid receptor (GR), a member of the nuclear hormone receptor superfamily, and inactivation of GR in murine models has demonstrated the essential roles for GCs in survival and therapeutic efficacy (3–5).
Inflammatory triggers engage multiple components of innate and adaptive immunity with distinct functions. Among these components, Toll-like receptors (TLRs) are the best-studied innate immune sensors that recognize the molecular patterns present on foreign or autoantigens to initiate stimulus-dependent inflammatory responses (6). Various mechanisms for GC-mediated inhibition of TLR-driven inflammatory events have been implicated, including interaction with multiple signaling components, such as MAPKs, NF-κB, and activated protein 1 (2). Suppressive actions of GCs on these signaling pathways modulate a wide range of inflammatory molecules, including many cytokines. Most of these cytokines transmit signals through cell surface receptors to the nucleus by engaging a family of latent transcription factors, signal transducer and activator of transcription (STAT), for further initiation, propagation, and resolution of inflammation (7). Defining the regulation of STAT proteins by GC is of particular interest, given that STAT's later temporal activation corresponds to the time when most GC clinical efficacy is displayed (8).
GR physically interacts with STAT3, STAT5a, STAT5b, and STAT6, and functionally synergizes with them to promote STAT-responsive gene transcription. Conversely, no physical interaction is observed between STAT1 and GR, and the functional interaction is more complex (9); for example, GR cooperates with STAT1 to transcribe FcγRI (10). In contrast, IFN-γ–induced MHC class II gene expression is impaired by GR engagement, and pretreatment with dexamethasone (Dex), a synthetic GR agonist, inhibits IFN-γ–induced expression and activation of STAT1 (11). Apparently, GCs may have both synergistic and antagonistic effects on STAT1 function, indicating that STAT1 is a promising GC target for tailoring immunosuppressive effects.
Suppressors of cytokine synthesis (SOCSs) function in a negative-feedback loop to restrain inflammatory responses, including STAT activation (12); however, their involvement in GC-mediated immunosuppression is unknown. TLR agonists, such as LPS or CpG DNA, have been shown to induce SOCS1 (13). Moreover, stimulation with TLR ligands results in excessive production of TNF-α, IL-12, and IFN-γ in macrophages, dendritic cells, and fibroblasts from SOCS1-null mice (14–16). SOCS1 mRNA is induced by GCs in some hematopoietic and immune cell lines (17); whether SOCS1 is GC-regulated as a distal signal in TLR activation cascades has not been reported.
Previously, we found differential regulation of NF-κB– and MAPK-mediated inflammatory reactions by GCs depending on the nature of TLR/adapter protein recruited (18). However, the robust inhibitory effect of GCs on TLR-mediated inflammatory gene induction was only partly explained by attenuation of NF-κB or MAPKs, which are activated early and transiently after TLR engagement. In disorders associated with inflammation, such as asthma, treatment with GCs has limited or no effect shortly after administration, whereas significant efficacy is seen at 4–24 h after administration (19–21); thus, other delayed mechanisms of action must be in effect. In present study, we tested the hypothesis that GCs differentially modulate STAT1 activation depending on the nature of TLR engagement. Using GR-, STAT1-, or SOCS1-deficient macrophages, we found selective impairment of TLR3- or TLR4-induced STAT1 activation by GCs, with no effect on TLR9-induced STAT1 activation. Moreover, we have identified SOCS1 as a critical GC target for regulation of STAT1-mediated inflammatory reactions.
Results
TLR-Induced Proinflammatory Cytokine Secretion in Control and STAT1-Null Macrophages.
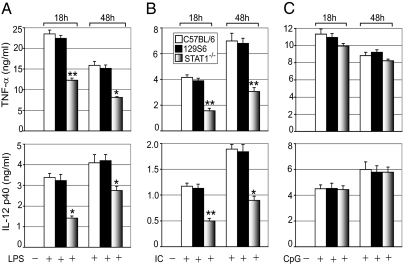
We first evaluated the relative contribution of STAT1 to TLR-mediated proinflammatory cytokine secretion. Macrophages isolated from control and STAT1-null (STAT1−/−) mice were treated with the TLR4 ligand LPS, the TLR3 ligand Poly (I:C), or the TLR9 ligand CpG. This study used primarily animals on a C57BL/6 background, whereas commercially available STAT1−/− mice are maintained on a 129S6 background. Secretion of TNF-α, IL-6, and IL-12(p40) was comparable in C57BL/6 and 129S6 control macrophages, indicating no significant difference in proinflammatory cytokine secretion between the two strains (Fig. 1). Both TNF-α and IL-12(p40) secretion after LPS or Poly (I:C) treatment were markedly reduced in STAT1−/− macrophages compared with control (STAT1+/+) macrophages. We found 48% (P < 0.01) and 49% (P < 0.05) reductions in TNF-α secretion after 18 h and 48 h of LPS treatment, respectively, along with 58% (P < 0.05) and 33% (P < 0.05) reductions of IL-12(p40) secretion after 18 h and 48 h of LPS treatment, respectively (Fig. 1A). We also found 49% (P < 0.01) and 51% (P < 0.01) reductions in TNF-α after 18 h and 48 h of Poly (I:C) treatment, respectively, as well as 62% (P < 0.01) and 56% (P < 0.05) reductions in IL-12(p40) after 18 h and 48 h of Poly (I:C) treatment, respectively (Fig. 1B). There was no significant difference in proinflammatory cytokine secretion between control and STAT1−/− macrophages when treated with CpG (Fig. 1C). Absence of STAT1 had a less pronounced effect on TLR-induced IL-6 secretion. We found 25% (P < 0.05) to 29% (P < 0.05) and 27% (P < 0.05) to 13% (P = 0.06) reductions in IL-6 levels after LPS and Poly (I:C) treatment, respectively (Fig. S1 A and B). Consistent with the foregoing results, we found no significant difference in secreted IL-6 levels in CpG-treated control and STAT1−/− macrophages (Fig. S1C).
Fig. 1.
TLR-induced cytokine secretion in control and STAT1-null macrophages. Effect of LPS (100 ng/mL) (A), Poly (I:C) (50 μg/mL) (B), and CpG (2 μM) (C) on proinflammatory cytokine secretion in C57BL/6 (□), 129S6 (■), and STAT1−/− ( ) macrophages. Cells were treated with TLR ligands for the indicated periods. Concentrations of TNF-α and IL-12p40 in the culture media were analyzed by ELISA. Data are mean ± SEM; n = 3. *P < 0.05; **P < 0.01 for STAT1−/− macrophages compared with control 129S6 macrophages.
) macrophages. Cells were treated with TLR ligands for the indicated periods. Concentrations of TNF-α and IL-12p40 in the culture media were analyzed by ELISA. Data are mean ± SEM; n = 3. *P < 0.05; **P < 0.01 for STAT1−/− macrophages compared with control 129S6 macrophages.
Effect of GCs on TLR-Induced STAT1 Phosphorylation.
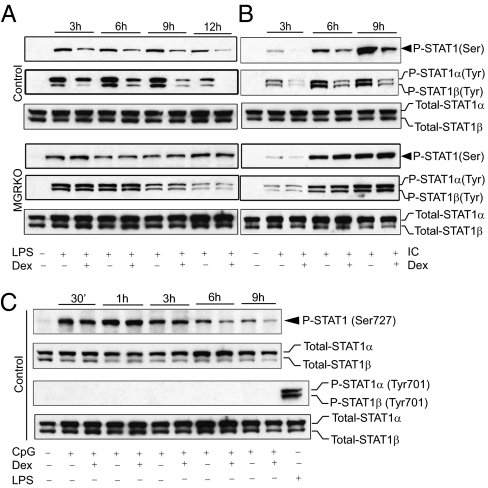
We next tested the hypothesis that engagement of GR with Dex regulates TLR ligand–induced STAT1 phosphorylation at Ser727 and Tyr701, essential prerequisites for STAT1 activation (22). We investigated both early and late effects of GC on TLR-induced STAT1 activation. LPS induced low levels of phosphorylation at Ser727 of STAT1 as early as 30 min after stimulation and more robust phosphorylation after 2 h of stimulation (Fig. S2A). Dex had no effect on STAT1 Ser727 phosphorylation until 2 h after LPS treatment. We found minimal phosphorylation at Tyr701 of STAT1 after 2 h of LPS treatment, but no effect of Dex at this time (Fig. S2A). Poly (I:C) did not induce phosphorylation at Ser727 and Tyr701 of STAT1 until 2 h after treatment, and Dex pretreatment had no detectable effect (Fig. S2B). LPS or Poly (I:C) treatment induced robust phosphorylation both at Ser727 and Tyr701 of STAT1 after 3 h of treatment (Fig. 2 A and B). Phosphorylation at Ser727 of STAT1 was found as early as 30 min after CpG treatment, but no detectable phosphorylation at Tyr701 was detected at this time point (Fig. 2C). Pretreatment with Dex markedly suppressed LPS- and Poly (I:C)-induced STAT1 phosphorylation throughout the later phase of the time course (2 h and thereafter). Densitometric analysis indicated that Dex caused 3.5- to 10.5-fold inhibition of LPS-induced STAT1 phosphorylation at Ser727 and 2- to 30-fold inhibition at Tyr701. GR-deficient macrophages displayed no Dex sensitivity for LPS-induced STAT1 phosphorylation (Fig. 2A). Similar suppression by Dex of Poly (I:C)-induced STAT1 phosphorylation was observed in control macrophages, but not in macrophages with conditional deletion of GR (MGRKO). We found a 2- to 3.5-fold inhibitory effect of Dex on Poly (I:C)-induced STAT1 phosphorylation at Ser727 and a 3.5- to 6-fold inhibitory effect at Tyr701 (Fig. 2B). In contrast, we detected no detectable effect of Dex on early-phase CpG-induced phosphorylation at Ser727 of STAT1, and only modest suppression at late phases (Fig. 2C).
Fig. 2.
Effect of GCs on TLR-induced STAT1 phosphorylation. Control or MGRKO macrophages were treated with or without Dex (100 nM) for 3 h, followed by LPS (A), Poly (I:C) (B), and CpG (C) treatment for the indicated periods. Cell lysates were analyzed by Western blot analysis using anti-phospho STAT1 Ser727 (P-STAT1 Ser), phospho STAT1 Tyr701 (P-STAT1 Tyr), and total STAT1 (Total-STAT1) antibodies. Representative of between three and five independent experiments.
Interferon α/β Receptor 1 Activation and TLR-Induced STAT1 Phosphorylation.
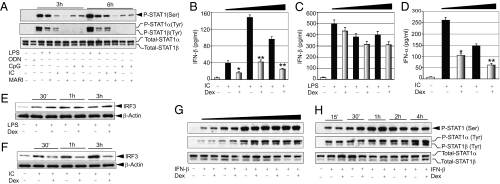
Type I interferons function via their cognate receptors, interferon α/β receptors (IFNARs), and are known to play a critical role in STAT1 induction (23). We examined the contribution of type I interferons in TLR-induced STAT1 activation using anti-IFNAR1 neutralizing antibody (MARI). Cells were pretreated with MARI and evaluated for TLR ligand–dependent STAT1 phosphorylation at early (3 h) and later (6 h) phases of STAT1 induction. We found a strong inhibitory effect of MARI on LPS-induced STAT1 phosphorylation (Fig. S3). MARI had little effect on LPS-induced STAT1 phosphorylation at Ser727, but a considerable inhibitory effect at Tyr701 (Fig. 3A). A pronounced inhibitory effect of MARI also was observed on Poly (I:C)-induced STAT1 phosphorylation. Densitometric analysis revealed that MARI caused a 2.8- to 5.5-fold suppression of Poly (I:C)-induced Ser727 phosphorylation and a 5- to 8-fold suppression of Tyr701 phosphorylation (Fig. 3A). MARI pretreatment had no effect on CpG-induced STAT1 phosphorylation at Ser727.
Fig. 3.
(A) IFNAR1 activation and TLR-induced STAT1 phosphorylation. Macrophages were pretreated with anti-IFNAR1 antibody (MARI) for 1 h, followed by treatment with LPS, Poly (I:C), CpG, or control oligonucleotide (ODN) for the indicated periods. Cell lysates were analyzed by Western blot analysis as described in Fig. 2. Representative of three independent experiments. (B–F) GC modulation of TLR-induced type I IFN secretion and nuclear IRF3. Macrophages were treated with Dex, followed by treatment with Poly (I:C) (B and D) or LPS (C) for 3, 6, or 9 h for IFN-β and for 12 or 18 h for IFN-α. Concentrations of IFN-α and IFN-β in the culture media were analyzed by ELISA. Data are mean ± SEM; n = 3. *P < 0.05; **P < 0.01 for Dex-treated cells compared with the group treated with Poly (I:C) only. (E and F) Cells were treated as described previously, and nuclear extracts were analyzed by Western blot analysis using anti-IRF3 or anti–β-actin antibodies. (G and H) Effect of GCs on IFN-β–induced STAT1 phosphorylation. Cells were pretreated with Dex, followed by treatment with varying doses of IFN-β (31.25–500 U/mL) for 1 h (G) or 125 U/mL of IFN-β for the indicated periods (H). Cell lysates were analyzed as described in Fig. 2. Representative of three independent experiments.
GC Modulation of TLR-Induced Type I IFN Secretion and Nuclear Interferon Regulatory Factor 3.
We explored the effect of Dex on TLR-induced type I IFN secretion and found a strong inhibitory effect of Dex on Poly (I:C)-induced type I IFN secretion. Dex caused 59% (P < 0.05), 72% (P < 0.01), and 74% (P < 0.01) suppression of IFN-β secretion after 3, 6, and 9 h of Poly (I:C) treatment, respectively, in control macrophages compared with non–Dex-treated control macrophages (Fig. 3B). Dex treatment also inhibited IFN-α secretion by 60% after 12 h and by 58% after 18 h of Poly (I:C) treatment compared with non–Dex- treated macrophages (P < 0.01) (Fig. 3D). Surprisingly, Dex had no significant effect on LPS-induced IFN-β secretion (Fig. 3C). No secretion of IFN-β or IFN-α was detected after stimulation with CpG.
Nuclear translocation of interferon regulatory factor 3 (IRF3) is an integral step in TLR-dependent induction of type I IFN synthesis (24). We found that the nuclear abundance of IRF3 was increased after treatment with LPS (Fig. 3E) or Poly (I:C) (Fig. 3F). LPS-induced elevation of nuclear IRF3 was essentially unaltered in the presence of Dex; however, Dex markedly suppressed Poly (I:C)-induced increases in nuclear IRF3 (Fig. 3F). Densitometric analysis indicated 2- to 3.5-fold suppression of Poly (I:C)-induced increases in nuclear IRF3. This data are in accord with our previous results showing inhibitory effects of Dex on Poly (I:C)-induced, but not LPS-induced, type I IFN synthesis.
IFN-β–Induced STAT1 Phosphorylation Is GC-Resistant.
Engagement of IFNARs by type I interferons potently induces phosphorylation and activation of STATs (23). To establish whether Dex impairs type I IFN function beyond modulation of secretion, we measured the direct effects of Dex on IFN-mediated STAT1 phosphorylation. Cells were pretreated with Dex, followed by stimulation with varying doses of IFN-β (31.25–500 U/mL) for 1 h. We found no inhibitory effect of Dex on IFN-β–induced STAT1 phosphorylation at Ser727 or Tyr701 (Fig. 3G). To evaluate the temporal effect of Dex pretreatment on IFN-β–induced STAT1 phosphorylation, we treated cells with IFN-β for different times, as shown in Fig. 3H. Treatment with IFN-β induced STAT1 phosphorylation in a time-dependent manner with no inhibitory effect of Dex (Fig. 3H). Taken together, these findings indicate that IFN-β– induced STAT1 activation is Dex-resistant.
SOCS1 Mediates GCl Inhibition of STAT1.
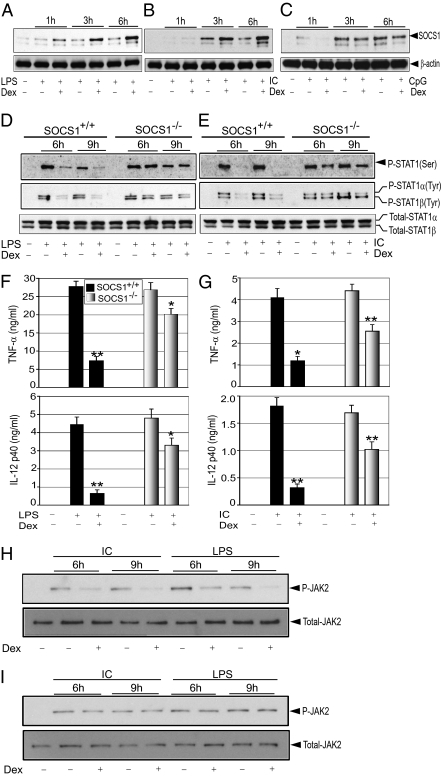
Impairment of type I IFN secretion confers Dex-dependent inhibition of Poly (I:C)-induced STAT1 phosphorylation, at least in part. However, changes in type I IFN secretion do not explain the inhibitory effect of Dex on LPS-induced STAT1 phosphorylation. Several lines of evidence suggest induction of SOCS1 as a potential regulatory mechanism for STAT activation (12). To establish the role of SOCS1 in mediating Dex-induced suppression of STAT1 activity, we evaluated TLR-induced SOCS1 expression in the presence or absence of Dex. The basal abundance of SOCS1 was low, and Dex alone had little effect on increasing it (Fig. S4). In contrast, SOCS1 expression was robustly induced after treatment with Dex in combination with LPS or Poly (I:C). Densitometric analysis indicated 2.6-, 4-, and 5-fold induction of SOCS1 expression by Dex treatment after 1, 3, and 6 h of LPS treatment, respectively (Fig. 4A). Dex also induced SOCS1 in Poly (I:C)-treated macrophages; 1.5- to 2-fold elevation of SOCS1 expression was seen in Poly (I:C)-treated macrophages when also treated with Dex (Fig. 4B). Dex treatment did not alter CpG-mediated induction of SOCS1 (Fig. 4C).
Fig. 4.
(A–C) Effect of GCs on TLR-induced SOCS1 expression. Cells were treated with LPS (A), Poly (I:C) (B), and CpG (C) as described in Fig. 2. Cell lysates were analyzed by Western blot analysis using anti-SOCS1 or anti–β-actin antibodies. (D–G) SOCS1 mediates GC inhibition of STAT1. SOCS1+/+ or SOCS1−/− macrophages were treated with or without Dex for 3 h, followed by treatment with LPS (D) or Poly (I:C) (E) for the indicated periods. Cell lysates were analyzed by Western blot analysis using anti-phospho STAT1 Ser727 (P-STAT1 Ser), phospho STAT1 Tyr701 (P-STAT1 Tyr), and total STAT1 (Total-STAT1) antibodies. Representative of two or three independent experiments. Similar experiments were performed with LPS for 18 h (F) and Poly (I:C) for 48 h (G). Concentrations of TNF-α and IL-12p40 in the culture media were analyzed by ELISA. Data are mean ± SEM; n = 3. *P < 0.05; **P < 0.01 for Dex-treated cells compared with the group treated with TLR ligand only. (H and I) SOCS1 inhibits phospho-JAK2 abundance to inhibit STAT1 activation. SOCS1+/+ (H) or SOCS1−/− (I) macrophages were treated as described previously. Cell lysates were analyzed by Western blot analysis using anti-phospho JAK2 (P-JAK2), and total JAK2 (Total-JAK2) antibodies. Representative of two independent experiments.
To determine the importance of SOCS1 in the effects of GCs on STAT1 activation, we examined STAT1 activation in SOCS1-null (SOCS1−/−) macrophages. We found that Dex had markedly reduced efficacy in suppressing LPS- or Poly (I:C)-induced STAT1 phosphorylation at Ser727 and at Tyr701 in SOCS1−/− macrophages (Fig. 4D). Densitometric analysis indicated only 0–5% and 30–31% suppression for LPS-induced STAT1 phosphorylation at Ser727 and Tyr701, respectively, in SOCS1−/− macrophages, compared with 68–71% and 74–77% suppression in SOCS1+/+ macrophages. Moreover, densitometric analysis found only 27% and 11–43% suppression for Poly (I:C)-induced STAT1 phosphorylation at Ser727 and Tyr701, respectively, in SOCS1−/−macrophages compared with 80–87% and 70% suppression in SOCS1+/+ macrophages (Fig. 4E). We further explored the effect of Dex on TLR-induced proinflammatory cytokine secretion in SOCS1−/− macrophages. Pretreatment with Dex resulted in 73% and 85% suppression of TNF-α (P < 0.01) and IL-12(p40) (P < 0.01), respectively, in LPS-treated SOCS1+/+ cells, compared with only 25% (P < 0.05) and 31% (P < 0.05) suppression in SOCS1−/− cells (Fig. 4F). For Poly (I:C) treatment, Dex caused 71% and 83% inhibition of TNF-α (P < 0.05) and IL-12(p40) (P < 0.01), respectively, in SOCS1+/+ cells, compared with 42% (P < 0.01) and 40% (P < 0.01) inhibition in SOCS1−/− cells (Fig. 4G).
SOCS1 Inhibits Phosphorylated JAK2 Abundance to Impair STAT1 Activation.
One potential mechanism for SOCS1-mediated STAT1 suppression is the degradation of phosphorylated JAK2, the key upstream component for STAT1 phosphorylation and activation (12, 25, 26). We measured LPS- and Poly (I:C)-induced phosphorylated JAK2 in SOCS1+/+ and SOCS1−/− macrophages. Both LPS and Poly (I:C) enhanced the phosphorylation of JAK2 in SOCS1+/+ and SOCS1−/− cells (Fig. 4H). Pretreatment with Dex resulted in inhibition of TLR-induced phosphorylated JAK2 abundance (Fig. 4H). Densitometric analysis revealed 1.9- and 2.3-fold reductions in phosphorylated JAK2 by Dex after 6 and 9 h of Poly (I:C) treatment, respectively. Dex also inhibited phosphorylated JAK2 abundance in LPS-treated macrophages. A 2.2- to 2.5-fold reduction in phosphorylated JAK2 was seen in LPS-treated macrophages pretreated with Dex compared with non–Dex-pretreated macrophages. Strikingly, Dex had no detectable effect on LPS- or Poly (I:C)-induced phosphorylated JAK2 abundance in SOCS1−/− macrophages (Fig. 4I).
Discussion
In this report, we define mechanisms through which GCs exert their anti-inflammatory effects. Our key findings are as follows: (i) STAT1 activation is an important downstream effector for TLR3 or TLR4 signaling, but not for TLR9 signaling; (ii) GCs have the ability to inhibit STAT1 activation resulting from TLR3 or TLR4 engagement; (iii) for TLR4 signaling, STAT1 suppression is entirely dependent on induction of SOCS1, whereas for TLR3 signaling, GC-suppressive effects result both from induction of SOCS1 and suppression of type I IFN secretion; and (iv) optimal SOCS1 induction requires coincident signals from TLRs and GR (Fig. S5). The increase in SOCS1 abundance is a critical anti-inflammatory action mediated by the gene expression–enhancing properties of ligand-bound GR, rather than a direct inhibitory interaction with proinflammatory transcription factors, such as NF-κB (2).
Whereas NF-κB and MAPKs are key signaling components that orchestrate TLR-initiated inflammatory actions, evidence from previous work suggests that GC-mediated suppression involves targets distinct from these intermediates (18). For example, pharmacologic inhibition of NF-κB or MAPK pathways produced only 50–60% suppression of proinflammatory cytokine secretion, compared with 80–90% suppression from Dex treatment. Activation of NF-κB and MAPKs is proximal to TLR ligation and accounts for the transcription and stabilization of several inflammatory signatures that function via secondary mediators, such as STATs, for the further progression or resolution of inflammation. We found that STAT1−/− macrophages secrete lower levels of IL-12(p40), TNF-α, and IL-6 compared with STAT1+/+ macrophages when treated with LPS or Poly (I:C) (Fig. 1 A and B). In contrast, CpG-mediated cytokine secretion is comparable in STAT1+/+ and STAT1−/− macrophages (Fig. 1C), indicating that TLR9-mediated inflammatory gene induction is largely STAT1-independent. This finding is in agreement with a previous report demonstrating that CpG DNA-induced IL-12 gene induction in bone marrow macrophages is independent of STAT1 activation (27). Taken together, our results indicate that STAT1 is an integral component of TLR3- and TLR4-inducible inflammatory reactions and a critical target for GC effects beyond NF-κB or MAPK pathways.
Consistent with the delayed onset of clinical efficacy, our data demonstrate late inhibitory effects of Dex on STAT1 activation compared with MAPKs or NF-κB suppression that occurs within minutes of TLR engagement. Engagement of TLR3 or TLR4 results in augmented phosphorylation of STAT1 at both Ser727 and Tyr701 residues, which are Dex- suppressible events, especially at the later phases after induction (Fig. 2 A and B). We found robust inhibitory effects of Dex on MAPK and NF-κB activation within 30–60 min of treatment with TLR ligands (3, 18). In contrast, we found no detectable effect of Dex treatment on TLR-induced STAT1 phosphorylation for similar periods with TLR ligands (Fig. S2). In cells pretreated with Dex for 3 h, the earliest inhibition of STAT1 phosphorylation was observed for Ser727 at 2 h after exposure to LPS. Our data suggest that 5–12 h of Dex treatment is required to inhibit STAT1 activation. This finding is consistent with clinical studies showing that corticosteroid treatment has little or no immediate effect, but demonstrates clinical utility after 4–24 h.
Given the wealth of data indicating type I IFNs as the major stimulatory signals for inducing STAT1, we asked whether GCs modulate STAT1 activation in macrophages by inhibiting IFN signaling. Our experiments with primary macrophages indicate that both IFN-α and IFN-β are Dex-suppressible in the context of Poly (I:C) treatment (Fig. 3 B and D). In addition, blockage of type I IFN signaling by pretreatment with IFNAR neutralizing antibody inhibits Poly (I:C)-induced STAT1 phosphorylation at Ser727 and Tyr701. Moreover, increased nuclear abundance of IRF3, a critical requirement for type I IFN transcription, is diminished by Dex treatment (Fig. 3F). Both dose–response and kinetic studies with IFN-β show Dex-resistance for direct IFN-β–mediated STAT1 phosphorylation at Ser727 or Tyr701 (Fig. 3 G and H). A similar observation was recently reported by Flammer et al. (28). Thus, GCs target the secretion of, but not the function of, type I IFNs to inhibit TLR3-mediated STAT1 induction. Surprisingly, increased nuclear IRF3 abundance, as well as secretion of IFN-β after LPS treatment, are Dex-resistant (Fig. 3A). The mechanism behind this difference in GC regulation of TLR3- and TLR4- mediated IFN-β and IRF3 nuclear abundance remains to be determined.
A growing body of evidence indicates that expression of SOCS1 modulates both the kinetics and magnitude of STAT1 activation (12). Our data show that Dex optimally induces SOCS1 expression when costimulated with LPS or Poly (I:C) (Fig. 4 A and B). To determine the functional importance of SOCS1 in this context, we analyzed the effects of GCs on STAT1 phosphorylation and cytokine secretion in SOCS1−/− macrophages. For LPS treatment, the suppressive effect of Dex on both STAT1 phosphorylation and TNF-α or IL-12(p40) secretion was markedly diminished in SOCS1−/− macrophages (Fig. 4D). A similar (albeit less robust) suppression occurs with Poly (I:C)-induced STAT1 phosphorylation and cytokine secretion in the presence of Dex in SOCS1−/− cells (Fig. 4E). The relatively less-robust SOCS1 regulation by GCs for TLR3 engagement likely is compensated for by suppression of type I IFN signaling. SOCS1 uses several putative mechanisms to limit STAT1 activation, one of which is to target proteasomal degradation of phosphorylated JAK2 (12). Consistent with this mechanism, Dex markedly reduces phosphorylated JAK2 in SOCS1+/+ cells, but has no effect in SOCS1−/− cells. Thus, targeting phosphorylated JAK2 is a key mechanism through which SOCS1 impairs STAT1 activation in GC-treated cells.
For decades, despite their adverse side effects, GCs have been the mainstay of therapy for patients with inflammatory disorders (2). Modulating key GC-regulated targets by means other than GR engagement is an alternative strategy in immunosuppressive therapy and an active area for drug discovery (1, 29). The JAK/STAT pathway is a relatively new focus of pharmaceutical research, but so far has been limited to JAK3 (CP690550) or Pan-JAK (INCB18424) inhibition in rheumatoid arthritis and psoriasis (29). However, beyond their functions in inflammatory pathways, JAK proteins play critical roles in growth, differentiation, survival, and developmental processes (30). The effects of JAK inhibitors on these other cellular functions remain to be delineated. A recent study found that intracellular delivery of recombinant cell-penetrating SOCS1 potently impaired IFN-γ–induced STAT1 phosphorylation and proinflammatory signatures (31). One attractive strategy through which the anti-inflammatory effects of GCs could be accomplished without detrimental side effects is via similar pharmacologic augmentation of SOCS1 action.
Materials and Methods
Mice and Cell Culture.
The mice used in these experiments were of a C57BL/6 × 129/Sv background and were 6–10 wk old. Vanderbilt University's Animal Care and Use Committee approved the experimental protocols. MGRKO mice were generated using LysM promoter–driven, Cre recombinase–mediated excision of exons 1C and 2 of the GR gene (4, 32). Sex-matched LysM-Cre–negative homozygous floxed GR littermates were used as controls for the MGRKO mice. Mice with homozygous disruption of the Stat1 gene (129S6/SvEv-Stat1tm1Rds) or control mice with a 129S6 background (129S6/SvEvTac) were purchased from Taconic. Macrophage-specific SOCS1-deficient mice were generated using Cre/loxP system by breeding Socs1lox/lox mice, which carry a Socs1 allele flanked by loxP sites. Mice expressing Cre under the endogenous lysozyme M promoter were used to delete Socs1lox in macrophages (33). Thioglycollate-elicited macrophages were isolated by peritoneal lavage and cultured as described previously (3).
Reagents and Antibodies.
Poly (I:C) (Amersham Biosciences), CpG oligonucleotide ODN 1826 and control ODN 1826 (InvivoGen), Dex (Amtech), LPS (Escherichia coli 0111:B4) (Sigma-Aldrich), and recombinant IFN-β (PBL) were purchased and reconstituted in accordance with the manufacturers’ instructions. The following antibodies were used: anti-actin (A 5060; Sigma-Aldrich); anti–Phospho-Stat1 (Ser727) (9177), anti–Phospho-Stat1 (tyr701) (9171), anti-Stat1 (9172), anti-IRF3 (4962), anti-JAK2 (3230), and anti–phospho-JAK2 (3776), (Cell Signaling Technology); and anti-GR (sc-1004) and anti-SOCS1 (sc-9021) (Santa Cruz Biotechnology). Anti-mouse IFNAR (MARI-5A3) and isotype-matched control (GIR-208) antibodies were kindly provided by Robert Schreiber (Washington University, St. Louis, MO).
Cytokine Measurement and Western Blot Analysis.
Cells were plated at 106 cells/mL in a 24-well plate (BD Labware) with 1.0 mL of complete medium. Cells were treated with Dex (Amtech) for 3 h at a concentration of 100 nM, followed by various TLR ligands, such as 100 ng/mL of LPS, 50 μg/mL of Poly (I:C), 2 μM CpG, and 2 μM control oligonucleotide (ODNc). Supernatants were collected, and cytokine concentrations were measured by ELISA for TNF-α, IL-6, IL-12 (BD Biosciences), IFN-α and IFN-β (PBL) in accordance with the manufacturer's instructions. Western blot analysis was performed as described previously (18).
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical significance was tested using the unpaired two-tailed Student t test. Differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (Grants AI50653 and MH79010) and Pfizer, Inc.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017296108/-/DCSupplemental.
References
- 1.O'Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 2.Rhen T, Cidlowski JA. Anti-inflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4–mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109:4313–4319. doi: 10.1182/blood-2006-10-048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer JA, et al. T-cell glucocorticoid receptor is required to suppress COX-2–mediated lethal immune activation. Nat Med. 2003;9:1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- 5.Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: Lessons from conditional knockout mice. Mol Cell Endocrinol. 2007;275:98–108. doi: 10.1016/j.mce.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 7.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH. An umbrella review: Corticosteroid therapy for adults with acute asthma. Am J Med. 2009;122:977–991. doi: 10.1016/j.amjmed.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens. 2006;68:1–12. doi: 10.1111/j.1399-0039.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 10.Aittomäki S, et al. Cooperation among Stat1, glucocorticoid receptor, and PU.1 in transcriptional activation of the high-affinity Fcγ receptor I in monocytes. J Immunol. 2000;164:5689–5697. doi: 10.4049/jimmunol.164.11.5689. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Li WP, Meng C, Ivashkiv LB. Inhibition of IFN-γ signaling by glucocorticoids. J Immunol. 2003;170:4833–4839. doi: 10.4049/jimmunol.170.9.4833. [DOI] [PubMed] [Google Scholar]
- 12.Piessevaux J, Lavens D, Peelman F, Tavernier J. The many faces of the SOCS box. Cytokine Growth Factor Rev. 2008;19:371–381. doi: 10.1016/j.cytogfr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 14.Chinen T, et al. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFN-γ and IL-4 are involved. Gastroenterology. 2006;130:373–388. doi: 10.1053/j.gastro.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 15.Hanada T, et al. Induction of hyper Th1 cell–type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. J Immunol. 2005;174:4325–4332. doi: 10.4049/jimmunol.174.7.4325. [DOI] [PubMed] [Google Scholar]
- 16.Kinjyo I, et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 17.Chinenov Y, Rogatsky I. Glucocorticoids and the innate immune system: Crosstalk with the Toll-like receptor signaling network. Mol Cell Endocrinol. 2007;275:30–42. doi: 10.1016/j.mce.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, et al. TAK1 targeting by glucocorticoids determines JNK and IkappaB regulation in Toll-like receptor–stimulated macrophages. Blood. 2010;115:1921–1931. doi: 10.1182/blood-2009-06-224782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson PG, Saltos N, Fakes K. Acute anti-inflammatory effects of inhaled budesonide in asthma: A randomized controlled trial. Am J Respir Crit Care Med. 2001;163:32–36. doi: 10.1164/ajrccm.163.1.9807061. [DOI] [PubMed] [Google Scholar]
- 20.Liu MC, et al. Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. J Allergy Clin Immunol. 2001;108:29–38. doi: 10.1067/mai.2001.116004. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo C, Rodrigo G. Early administration of hydrocortisone in the emergency room treatment of acute asthma: A controlled clinical trial. Respir Med. 1994;88:755–761. doi: 10.1016/s0954-6111(05)80198-2. [DOI] [PubMed] [Google Scholar]
- 22.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 23.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type–specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 25.Kamizono S, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 26.Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol Cell Biol. 2002;22:3316–3326. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford M, Schroeder AJ, Morse HC, 3rd, Vogel SN, Cowdery JS. CpG DNA-induced IL-12 p40 gene activation is independent of STAT1 activation or production of interferon consensus sequence binding protein. J Biomed Sci. 2002;9:688–696. doi: 10.1159/000067280. [DOI] [PubMed] [Google Scholar]
- 28.Flammer JR, et al. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol. 2010;30:4564–4574. doi: 10.1128/MCB.00146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 30.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiGiandomenico A, Wylezinski LS, Hawiger J. Intracellular delivery of a cell-penetrating SOCS1 that targets IFN-γ signaling. Sci Signal. 2009;2:ra37. doi: 10.1126/scisignal.1162191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 33.Chong MM, Metcalf D, Jamieson E, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 in T cells and macrophages is critical for preventing lethal inflammation. Blood. 2005;106:1668–1675. doi: 10.1182/blood-2004-08-3049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.