Abstract
Recognition memory is thought to consist of two components: recollection and familiarity. Whereas it is widely agreed that the hippocampus supports recollection (remembering the episode in which an item was learned), there is uncertainty about whether it also supports familiarity (simply knowing that an item was encountered but without remembering the learning episode). We tested a counterintuitive prediction that follows from the idea that the hippocampus selectively supports recollection. Patients with hippocampal lesions should have strong experiences of familiarity as often as controls do; however, unlike controls, these experiences should not be accompanied by recollection. Accordingly, with methods that allow participants to report whether they remember an item as encountered previously or whether they simply know it is familiar, patients should express strong familiarity (in the absence of recollection) more often than controls. We indexed strong familiarity and recollection for previously studied words by obtaining confidence ratings together with Remember-Know judgments. The result was that patients provided fewer high-confidence Know responses than controls rather than more. Furthermore, the number of Know responses made by patients was substantially less than was predicted if recollection were impaired. This was true regardless of whether the prediction was based on the assumption that recollection and familiarity are independent or dependent processes. These results suggest that hippocampal lesions impair both recollection and familiarity. Unlike many previous studies of these constructs, the prediction (and the result) is independent of any particular theoretical model, and it holds even if Remember-Know judgments are not process-pure indicators of recollection and familiarity.
Keywords: amnesia, declarative memory, medial temporal lobe
Declarative memory depends on the integrity of anatomically related structures in the medial temporal lobe (the hippocampus, dentate gyrus, and subicular complex, together with the adjacent perirhinal, entorhinal, and parahippocampal cortices). Recognition memory is one of the most widely studied examples of declarative memory and refers to the ability to judge whether an item was previously encountered. Recognition memory is thought to consist of two components: recollection and familiarity (1, 2). Recollection involves remembering specific contextual details of a previous learning episode. Familiarity involves remembering only that an item was encountered previously but in the absence of any specific contextual details about the learning episode.
There has been considerable interest in whether recollection and familiarity might be distinguished on anatomical grounds. For example, it has been proposed that recollection depends on the hippocampus and that familiarity depends on the adjacent perirhinal cortex (3, 4). Alternatively, it has been proposed that medial temporal lobe structures work in a cooperative and complementary way and that these structures support both recollection and familiarity (5).
These ideas can potentially be tested in patients with circumscribed hippocampal lesions, and a number of methods have been used to assess the capacity for recollection and familiarity. In the Remember-Know procedure, individuals judge items to be old or new and then make either a Remember (R) response (if they can recollect details about their earlier encounter with the item) or a Know (K) response (if they judge the item to be familiar but cannot recollect anything about their encounter with the item).
We used this procedure to test a counterintuitive prediction that follows from the idea that the hippocampus selectively supports recollection. The prediction is that patients with bilateral lesions limited to the hippocampus should experience strong familiarity-based recognition in the absence of recollection (i.e., familiarity-based decisions made with high confidence and high accuracy) more often than controls do. The basis for this prediction is as follows. In controls, items that are learned sufficiently well to yield strong familiarity often yield recollection as well. That is, for controls, strong familiarity-based recognition in the absence of recollection is a relatively rare experience. For example, Know judgments made with high confidence (i.e., judgments indicating an experience of strong familiarity in the absence of recollection) tend to occur infrequently (6). In contrast, if recollection is selectively impaired in patients with hippocampal lesions, they should experience strong familiarity as often as controls do, but this experience should usually occur in the absence of recollection.
This prediction means that with the Remember-Know procedure, patients should provide many strong K responses but few strong R responses. Controls, by contrast, should provide many strong R responses but few strong K responses. Specifically, because the patients should have lost the recollection component of their recognition experience, thereby converting into a K response what for controls would be an R response, they should provide more high-confidence K responses than controls do.
In the Remember-Know procedure, Know judgments typically reflect weaker memory than Remember judgments (6–8). Accordingly, one reason that K responses might be frequent among patients with hippocampal lesions is simply because their memory is weaker than the memory of controls. However, not all Know judgments reflect weak memory. Some are made with high confidence and high accuracy (9), just as most Remember judgments are. If hippocampal lesions selectively impair recollection, the counterintuitive prediction is that Know judgments made with high confidence and high accuracy should also occur more often in amnesic patients than in controls. Because our prediction does not require a precise quantification of recollection and familiarity, it is less subject to theoretical assumptions than other studies of these constructs. Thus, the prediction holds whether recollection and familiarity are independent (i.e., uncorrelated) or dependent (i.e., correlated) processes; it holds whether or not familiarity is governed by an equal-variance signal-detection process (as in ref. 10); and it holds even if Remember-Know judgments are not process-pure indicators of recollection and familiarity.
In our study, participants studied 100 words and then took a recognition memory test (1 = definitely new, 20 = definitely old). For words declared old (ratings of 11–20), participants then judged whether the word was recollected (Remember), was familiar (Know), or was a guess (Guess). Then, to examine the frequency of R and K responses in each group after removing the effect of memory strength, we compared performance for items endorsed with high confidence (ratings of 17–20).
Results
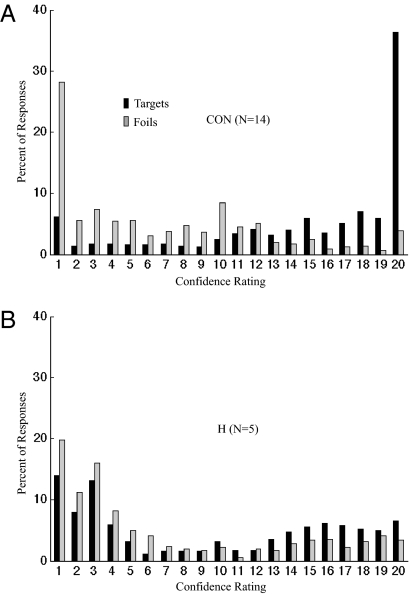
Fig. 1 shows the percent of responses for targets (studied words) and foils (new words) at each level of confidence on the Old/New recognition test. For both controls (Fig. 1A) and patients (Fig. 1B), responses were well distributed across all 20 levels of confidence. Confidence ratings for targets from 11 to 20 and for foils from 1 to 10 indicate correct responses. Fig. 2 shows that the overall performance of the patients was worse than that of the controls (59.6 ± 2.6% correct vs. 77.4 ± 2.1% correct; P < 0.001). The result was the same when performance was calculated as a discriminability score (d′ = 0.68 ± 0.18 vs. 1.75 ± 0.16; P < 0.001).
Fig. 1.
Percent of responses at each confidence rating for targets (black bars) and foils (gray bars). 1 = definitely new; 20 = definitely old. (A) CON, controls; (B) H, patients with hippocampal lesions.
Fig. 2.
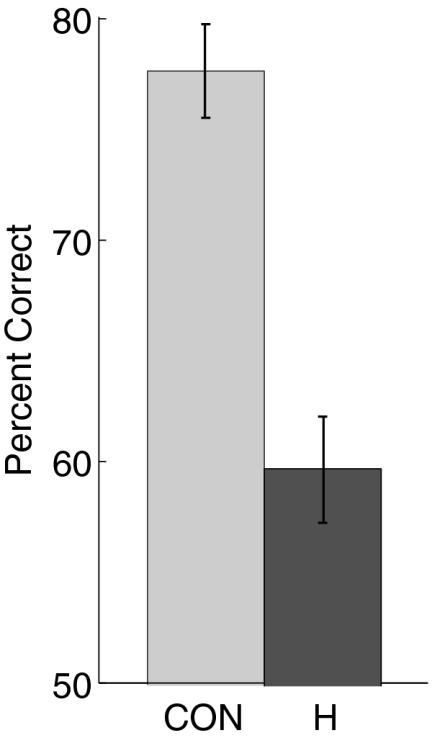
Percent of correct responses on the Old/New test of recognition memory for controls (n = 14) and patients with hippocampal lesions (n = 5). Error bars indicate SEM. CON, controls; H, patients with hippocampal lesions.
We next examined performance separately for R and K responses when the two kinds of responses were matched for memory strength at a high-confidence level (ratings of 17–20). As one might expect, when high-confidence ratings were given, both groups tended to perform rather well. For R responses, patients scored 71.5 ± 7.5% correct and controls scored 89.6 ± 3.2% correct (P < 0.05). For K responses, the corresponding values were 68.7 ± 7.5% correct and 77.8 ± 5.5% correct (P = 0.4). The fact that patients scored somewhat poorer than controls, even when confidence ratings were similar, suggests that the patients used a comparatively relaxed criterion when making their judgments. As would be expected, there were very few Guess responses associated with high-confidence ratings (patients did not indicate Guess for any of their correct responses, and controls indicated Guess for only 3.4 ± 1.7% of their correct responses).
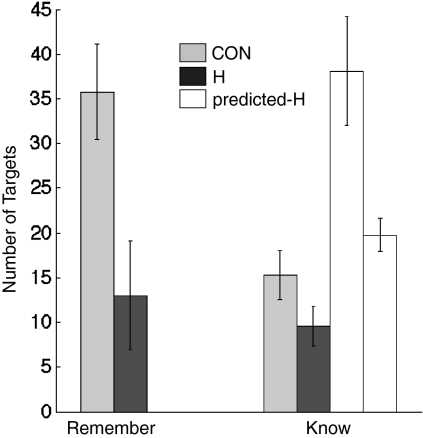
The question of interest concerned the frequency of correct R and K responses to study words that were given with high confidence. If recollection (and remembering) were impaired in patients with hippocampal lesions, there should be a dramatic reduction in high-confidence R responses in the patient group. Fig. 3 shows that, indeed, the patients did have fewer high-confidence R responses than controls (13.0 ± 6.1 vs. 35.8 ± 5.4; P < 0.05). In addition, if recollection (and remembering) were impaired in the patients, many items that would have been given R responses by the patients (if recollection had been available to them) should now receive K responses. In that case, the number of high-confidence K responses in patients should be even higher than in controls. However, contrary to this expectation, the patients gave numerically fewer high-confidence K responses than controls (9.6 ± 2.2 vs. 15.3 ± 2.8; P > 0.10). (The same was true for the frequency of lower confidence K responses, which ranged from 11 to 16; for patients and controls: 7.0 ± 1.9 vs. 8.7 ± 2.6).
Fig. 3.
Number of targets out of 100 that were identified with high confidence (confidence ratings from 17 to 20) for controls (n = 14) and patients with hippocampal lesions (n = 5). CON, controls; H, patients with hippocampal lesions. The data are shown separately for items given Remember judgments (Remember), and for items given Know judgments (Know). Also shown are two different predictions (predicted-H) for what the number of K responses should be for the patients if hippocampal damage selectively impaired recollection (discussed in section on data analysis in Materials and Methods). One predicted value was calculated under the assumption that all R responses are potentially K responses (the assumption of complete dependence, tall white bar). The other predicted value was calculated under the assumption that Remember and Know judgments are independent (shorter white bar).
We also calculated how many high-confidence K responses would, in fact, be predicted for the patients, given their reduced capacity for Remembering (Fig. 3 and section on data analysis in Materials and Methods). Under the assumption that recollection and high-confidence familiarity are fully dependent, the predicted number of high-confidence K responses for the patients was 38.1 ± 6.1. Under the assumption that recollection and familiarity are fully independent, the predicted number of high-confidence K responses for the patients was 19.8 ± 1.9. Contrary to these two predictions, the observed number of high-confidence K responses in the patient group was markedly lower than the predicted values (for the dependent method, P < 0.005; for the independent method, P < 0.01).
The results presented thus far provide no indication that the patients experienced an increased frequency of high-confidence familiarity-based decisions (relative to controls), as would be expected if recollection were selectively impaired in the patients. One possible way to explain this result without rejecting the idea that recollection is selectively impaired by hippocampal lesions would be to suppose that the patients (but not the controls) misunderstood the distinction between Remembering and Knowing and provided high-confidence R responses for decisions that, in fact, were based on a strong sense of familiarity; that is, many of their R responses should actually have been Know judgments. To consider this possibility, we repeated the analyses just described but first added all high-confidence R responses made by the patients to their high-confidence Know judgments (the same change was not made to the control data). When this was done, the number of (corrected) high-confidence K responses made by the patients (22.6 ± 6.5) now exceeded the number made by the controls (15.3 ± 3.0), but the difference was not significant. This corrected value for high-confidence K responses for the patients (22.6 ± 6.5) was then compared with the predicted number based on the assumptions of dependence and independence and using the formulas presented in the section on data analysis in Materials and Methods (except that the predictions were now based on the assumption that the patients provided no R responses). The predicted numbers of high-confidence K responses were now 24.8 (assuming complete independence) and 51.1 (assuming complete dependence). (Note that there is no error term for these predictions because the R response frequency for patients was set to zero; formulas are presented in section on data analysis in Materials and Methods). The recalculated number of K responses made by patients (22.6 ± 6.5) fell just below the lower of these two values. Thus, even after making the strong (and rather unrealistic) assumption that all high-confidence R responses made by the patients should be recast as high-confidence familiarity-based (Know) decisions, there was no evidence that the patients experienced high-confidence familiarity as much as they should have if familiarity had been spared and only recollection affected.
Finally, it might be supposed that the patients did not exhibit a clear increase in high-confidence familiarity-based decisions because they were reluctant to express high confidence in any of their memory decisions (because they were aware of their memory impairment); that is, the patients may have had a stricter criterion for expressing high confidence than the controls. In that case, one could suppose that recollection was, in fact, selectively impaired and that the predicted increase in high-confidence familiarity-based decisions did not occur because the patients used an unusually strict criterion for making their decisions. However, as noted previously, the patients appeared to use a more relaxed criterion than controls, rather than a stricter criterion, because their high-confidence responses were less accurate than those of controls. To examine this issue further, we also looked at the relative frequency of high-confidence decisions made to the foils. A stricter criterion would be indicated if the patients had a low false-alarm rate for high-confidence responses. However, the patients actually had a somewhat higher false-alarm rate than the controls (13.0 ± 6.8% vs. 7.3 ± 2.4%), indicating, again, that the patients had a less strict criterion, rather than a more strict criterion, than the controls. Thus, the low frequency of high-confidence familiarity-based decisions exhibited by the patients was not an artifact of a strict response criterion. In short, if recollection were selectively impaired in the patients, they should have experienced an increase in high-confidence familiarity-based responses. This increase did not occur, and the fact that it did not occur cannot be attributed to a difference in response criterion between patients and controls.
Discussion
The present study tested a counterintuitive prediction about recognition memory performance after hippocampal lesions, which follows from the idea that the hippocampus supports recollection but not familiarity. We used the Remember-Know procedure to index recollection and familiarity. If hippocampal lesions selectively impair recollection, patients should provide fewer R responses than controls. In addition, and counterintuitively, patients should provide more strong K responses than controls [because a strong experience of familiarity without recollection is not a common phenomenon for controls (6)].
Contrary to this counterintuitive prediction, the patients provided numerically fewer, rather than more, high-confidence K responses than controls (Fig. 3). We also calculated how many high-confidence K responses would have been expected for the patients if recollection had been selectively impaired. These calculations were made in two different ways: first, under the assumption that R and K responses are dependent and, second, under the assumption that the responses are independent (Fig. 3). The number of K responses actually produced by patients was significantly below both of these predicted values. Note that we computed the expected number of high-confidence K responses based on two extreme assumptions (complete dependence or complete independence of R and K responses). However, the actual relationship between R and K responses probably lies intermediate to these extremes.
The results were the same when it was supposed that all the R responses made by patients should have been K responses (because the patients misunderstood the distinction between Remember and Know). In addition, the low number of high-confidence K responses made by patients could not be attributed to a stricter response criterion for patients than controls (i.e., a reluctance to endorse items as old with high confidence, either with an R or K response). In fact, patients had a more relaxed criterion for giving high-confidence responses than controls did. First, their high-confidence responses were less accurate than control responses. Second, their false-alarm rate for high-confidence responses was higher than that of controls. The point is that a more relaxed criterion for the high-confidence decision criterion would increase (not decrease) the number of high-confidence responses made by patients.
A possible alternative explanation for the patients’ lower accuracy and higher false-alarm rate for high-confidence decisions is that the high-confidence criterion was not more relaxed but was, instead, more variable from item to item compared with controls. However, such variability would also result in an increased number of high-confidence responses (not a decreased number). The reason for this is that the criterion for making a decision with high confidence is usually placed high on the memory strength scale, such that only a small proportion of the Gaussian lure distribution falls to the right of it. Thus, an occasional shift of the criterion in the liberal direction (i.e., to the left) would result in a substantial increase in the proportion of the target and lure distribution falling to right of it (and a correspondingly large increase in the number of high-confidence hits and false alarms). However, an occasional shift of the criterion in the conservative direction (i.e., to the right) would result in a relatively small decrease in the proportion of the target and lure distribution falling to the right of it (and a correspondingly small decrease in the number of high-confidence hits and false alarms). Averaged across trials, the net change as a result of criterion variability would be an increase in the high-confidence false-alarm rate.
The point is that a more liberal or more variable high-confidence criterion would be expected to increase (not decrease) the number of high-confidence hits compared with what would otherwise occur. However, K response frequency in the patients was considerably lower than it should have been if hippocampal damage impaired only recollection. Accordingly, insofar as the Remember-Know method indexes recollection and familiarity, it appears that hippocampal damage in our patients impaired both recollection and familiarity.
Other studies have also used the Remember-Know procedure to assess the capacity of recollection and familiarity in memory-impaired patients with lesions that include the hippocampus (11–17). Patients typically exhibit a markedly reduced frequency of R responses but only a modest reduction (or no reduction) in the frequency of K responses. For example, in the present report, the overall frequency of R responses in patients was reduced by 47%, whereas the overall frequency of K responses in patients was reduced by only 31%. Similarly, in our earlier studies, overall R and K response frequencies were reduced by 62% and 24%, respectively (11), or by 20% and 12%, respectively (13).
However, the finding that K responses are less reduced in patients than R responses is not strong evidence that processes indexed by K responses are relatively preserved after hippocampal damage. A simpler explanation is that memory is weakened after hippocampal damage. As a result, the number of items that had been given K responses is reduced, but the reduction in K responses is largely compensated for by the conversion of R responses to K responses (7, 8, 11, 18). Thus, whenever memory is weaker, one should expect to find a sharp decrease in R responses associated with only a modest decrease in K responses.
Model-based estimates of recollection and familiarity go beyond raw Remember and Know scores by using formulas to compute quantitative estimates of recollection and familiarity. The most common approach uses the Independent Remember-Know (IRK) method (6), which assumes that recollection and familiarity are independent. The assumption of independence holds that recollected items are no more familiar, on average, than items that are not recollected. This assumption means that items given a Remember judgment would not necessarily be given a Know judgment if subjects were simply asked whether an item was familiar. Specifically, remembered items would be no more likely to yield a Know judgment than the other (non-Remember) items. In the IRK method, recollection is estimated by subtracting the Remember false-alarm rate from the Remember hit rate. Familiarity is estimated by first computing corrected Know hit rates and false-alarm rates [corrected Know hit rate equals Know hit rate/(1 − Remember hit rate); corrected Know false-alarm rate equals Know false-alarm rate/(1 − Remember false-alarm rate)]. Familiarity is then estimated either by subtracting the corrected Know false-alarm rate from the corrected Know hit rate or by computing a d′ score from the corrected Know hit rate and false-alarm rate.
Using the IRK method, several studies have suggested that familiarity is relatively spared after lesions that include the hippocampus (e.g., refs. 12 and 15–17). However, this conclusion is valid only if recollection and familiarity are, in fact, independent processes (because the IRK formulas are valid only if that assumption is true). To illustrate how assumptions about independence or dependence can dictate conclusions about the effect of hippocampal lesions, we computed familiarity estimates from patient K.N.’s data (15). Patient K.N. has bilateral lesions of the hippocampus that appear to spare adjacent cortex. Using the IRK method, Aggleton et al. (15) reported that K.N.’s recollection was impaired but his familiarity was spared. Indeed, we confirmed this conclusion by computing d′ familiarity scores from the values reported in their table 3 (15). The average IRK estimate of familiarity for controls (d′ = 1.28) was only slightly higher than the estimate for K.N. (d′ = 1.07).
However, the assumption of independence minimizes the difference between these two scores. Indeed, the difference in familiarity estimates for controls and K.N. increases monotonically as the positive correlation between recollection and familiarity increases from zero (the independence assumption) to 1.0 (the dependence assumption). In the case of dependence, which assumes that any item given a Remember judgment would have received a Know judgment if subjects had simply been asked to judge familiarity, the estimated d′ familiarity scores for controls and K.N. are 2.12 and 1.45, respectively. Thus, using the same data but with the assumption of dependence, the conclusion would be that familiarity was substantially impaired by hippocampal lesions.
All previous studies that have used the IRK method to conclude that hippocampal lesions spare familiarity are similarly committed to the strong assumption that recollection and familiarity are independent. However, these same studies would instead conclude that hippocampal lesions impair familiarity if one adopted the reasonable assumption that recollection and familiarity are dependent. The assumption of dependence is that items given Remember judgments would have been familiar enough to be given Know judgments had subjects been asked to base their decision on familiarity alone.
Instead of relying on model-based estimates of familiarity to answer the question of whether or not hippocampal lesions affect familiarity, a better approach is to test predictions that hold regardless of whether one adopts the assumption of independence or dependence (or anything in between). We tested one such prediction here. We found that high-confidence K responses were not nearly as frequent in patients as they should have been if recollection were selectively impaired (and familiarity preserved).
This same finding has also been obtained in a different way using a source memory procedure (19). Participants studied 25 words; then, in a recognition test, they made confidence judgments ranging from 1 to 6 for 25 old words and 25 new words (1 = definitely new, 6 = definitely old). For words declared old (confidence ratings of 4–6), they also answered a source question (Was the word studied with instructions to form an indoor image or an outdoor image?). If recollection were selectively impaired, patients should have experienced high-confidence familiarity (old/new judgments made correctly with high confidence) as often as controls, but this experience of familiarity should usually not have been accompanied by successful recollection (correct source judgments). As a result, high-confidence familiarity judgments without source recollection should have occurred more frequently in patients than in controls. Contrary to this expectation, the frequency of high-confidence familiarity judgments in the absence of source recollection was less common in patients than it was in controls.
The present findings, together with these earlier results (19), provide evidence that hippocampal lesions impair familiarity as well as recollection. Recollection and familiarity are identifiable psychological constructs that are useful for understanding the nature of recognition memory. However, this distinction does not appear to illuminate the function of the hippocampus. Instead, as discussed elsewhere, findings from neuroanatomy and neurophysiology are more likely to inform the functional organization of structures within the medial temporal lobe (20).
Materials and Methods
Participants.
Five memory-impaired patients participated (Table 1). All the patients had moderately severe memory impairment. On immediate and delayed (12 min) recall of a short prose passage, they recalled 3.8 and 0.4 segments, respectively. Controls recalled 7.9 and 6.6 segments, respectively. K.E. became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. L.J. (the only female) became amnesic in 1988 during a 6-mo period with no known precipitating event. Her memory impairment has been stable since that time. Patients G.W. and R.S. became amnesic in 2001 and 1998, respectively, following a drug overdose and associated respiratory failure. J.R.W. became amnesic in 1990 after cardiac arrest. Estimates of medial temporal damage were based on quantitative analysis of MRI scans and either 19 controls (for the four male patients) or 11 controls (for L.J.) (21, 22). K.E., L.J., R.S., G.W., and J.R.W. have an average bilateral reduction in hippocampal volume of 49%, 46%, 33%, 48%, and 44%, respectively (all values >3 SDs from the control mean). On the basis of two patients (L.M. and W.H.) with similar bilateral volume loss in the hippocampus for whom detailed postmortem histological analysis was available (23), this degree of volume loss likely reflects nearly complete loss of hippocampal neurons (as also discussed in ref. 21). The volume of the parahippocampal gyrus is reduced by 17%, −8%, 1%, 12%, and 6%, respectively (all values within 2 SDs of the controls’ mean). Nine coronal MRI scans for each patient, together with detailed descriptions of the lesions, are available elsewhere (24).
Table 1.
Characteristics of memory-impaired patients
| WMS-R |
||||||||
| Patient | Age, y | Education, y | WAIS-III IQ | Attention | Verbal | Visual | General | Delay |
| K.E. | 68 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | 72 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| R.S. | 53 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| G.W. | 51 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| J.R.W | 47 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
The Wechsler Adult Intelligence Scale-III (WAIS-III) and the Wechsler Memory Scale-Revised (WMS-R) yield mean scores of 100 in the normal population, with an SD of 15. The WMS-R does not provide numerical scores for individuals who score below 50. Intelligence quotient (IQ) scores for J.R.W. and R.S. are from the WAIS-R.
The control group consisted of 14 adults (10 male) averaging 62.8 ± 2.7 y of age and 14.4 ± 0.5 y of education.
Materials and Procedure.
The stimuli were 200 nouns from the Toronto Word Pool (25), with a mean frequency of 61 (range: 1–712), a mean imagery rating of 5.1 (range: 1–7), and a mean concreteness rating of 5.3 (range: 1–7). Half of the words were assigned to the study list, and half of the words served as foils for the recognition test. Words were presented on a computer screen at both the study and test. The assignment of words as targets or foils was counterbalanced across participants.
During study, participants made a pleasant/unpleasant judgment on the keyboard for each of the 100 study words (2.5-s presentation time). A central cross was presented during each intertrial interval of 0.25 s (total study session = 5 min). The study session was divided into two equal blocks of 50 trials with a short break between blocks. Participants were asked to remember the target words for a subsequent memory test.
Following the study session (about 3−5 min), participants saw a mixed list of the 100 target words and 100 foil words. For each word, participants made a self-paced old/new recognition judgment using a 20-point scale, where 1 = definitely new and 20 = definitely old (total test session was about 20 min). Participants were instructed to use the entire scale. For words declared old (ratings of 11–20), participants also judged whether the word was recollected, was familiar, or was a guess, following a Remember-Know-Guess procedure (9, 26). The modified instructions emphasized that participants should use the R response only if they could actually describe specific details about the experience of studying the word. They were told that they should use the K response if they thought the word was familiar but could not recollect any details of their encounter with the word.
Data Analysis.
The frequency of correct high-confidence K responses (ratings of 17–20) given by patients was compared with predicted values based on the proposal that hippocampal damage selectively impairs recollection and spares familiarity. Two different predicted values were calculated. At one extreme, one assumes that all R responses are potentially high-confidence K responses (the assumption of complete dependence). Using this assumption, we first estimated how many high-confidence K responses the controls would have given if their capacity for recollection were reduced to the same extent as it was in the patients. Thus, we subtracted each patient's R response frequency from the mean R response frequency in controls. This subtraction yielded the number of R responses that would be lost by controls if their capacity for recollection were the same as in the patients. All these R responses are potentially high-confidence K responses under the assumption of complete dependence. Accordingly, these R responses were added to the mean number of high-confidence K responses actually observed in controls. The resulting number then represents the number of high-confidence K responses predicted for a given patient (because the patients are, theoretically, exactly like controls who have had recollection selectively reduced). The formula is:
where the notation nK or nR indicates the number of high-confidence K or R responses and upper bars indicate mean values. For example, if recollection were completely eliminated in the patients, such that nRpatient = 0 for all patients, high-confidence Know judgments for the patients would be predicted to increase by an amount equal to the mean number of Remember judgments provided by the controls. Note that for our patients, R responses were reduced but they were not completely eliminated.
At the opposite extreme to the assumption of complete dependence, one assumes complete independence between recollection and familiarity (and between Remember and Know judgments). According to this idea, if recollection were absent, some (but not all) R responses are potentially high-confidence K responses. Specifically, the proportion of control R responses that are potential K responses is the same as the observed proportion of high-confidence K responses (as opposed to guesses or misses) that were made by controls for all target items that did not receive an R response. Again, we first calculated how many high-confidence K responses the controls would have given if their capacity for recollection were reduced to the same extent as in the patients. To make this determination, we subtracted each patient's R response frequency from the mean R response frequency in controls. The number of R responses made by controls that would become high-confidence K responses under the assumption of independence was then added to the mean number of high-confidence K responses actually observed in controls. The predicted frequency of high-confidence K responses in patients under the assumption of independence is as follows:
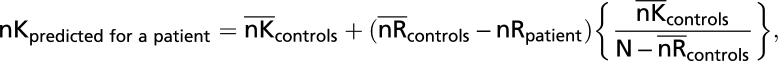 |
where the notation nK or nR indicates the number of K or R responses for patients or controls and the upper bars indicate mean values. The final term represents the proportion of K responses by controls for targets that are not Rs. N indicates the total number of targets.
This formula can also be presented in a form that will be more familiar to some:
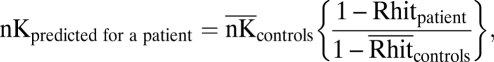 |
where Rhit patient represents the Remember hit rate for a patient and 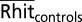 represents the mean Remember hit rate for controls. Note that when the Remember hit rate for patients is zero, this formula reduces to the standard formula for calculating K response frequency under independence.
represents the mean Remember hit rate for controls. Note that when the Remember hit rate for patients is zero, this formula reduces to the standard formula for calculating K response frequency under independence.
These two predicted values represent two extreme estimates based on the assumptions of complete dependence and complete independence. In reality, the relationship between recollection and familiarity is likely neither completely dependent nor completely independent, and the predicted value for the frequency of Know judgments in the patient group is probably intermediate between the two calculated values.
Acknowledgments
We thank Jennifer Frascino for assistance. This study was supported by the Medical Research Service of the Department of Veterans Affairs, National Institute of Mental Health Grant 24600, and National Institute on Aging Grant AG05131 to the Alzheimer's Disease Research Center at University of California at San Diego.
Footnotes
The authors declare no conflict of interest.
References
- 1.Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Suppes P, editors. Contemporary Developments in Mathematical Psychology. San Francisco: Freeman; 1974. pp. 243–290. [Google Scholar]
- 2.Mandler G. Recognizing: The judgment of previous occurrence. Psychol Rev. 1980;87:252–271. [Google Scholar]
- 3.Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonelinas AP. Consciousness, control, and confidence: The 3 Cs of recognition memory. J Exp Psychol Gen. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- 7.Dunn JC. Remember-know: A matter of confidence. Psychol Rev. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- 8.Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- 9.Wixted JT, Mickes L. A continuous dual-process model of remember/know judgments. Psychol Rev. 2010;117:1025–1054. doi: 10.1037/a0020874. [DOI] [PubMed] [Google Scholar]
- 10.Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- 11.Knowlton BJ, Squire LR. Remembering and knowing: Two different expressions of declarative memory. J Exp Psychol Learn Mem Cogn. 1995;21:699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- 12.Yonelinas AP, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 13.Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- 14.Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- 15.Aggleton JP, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Turriziani P, Serra L, Fadda L, Caltagirone C, Carlesimo GA. Recollection and familiarity in hippocampal amnesia. Hippocampus. 2008;18:469–480. doi: 10.1002/hipo.20412. [DOI] [PubMed] [Google Scholar]
- 17.Verfaellie M, Rajaram S, Fossum K, Williams L. Not all repetition is alike: Different benefits of repetition in amnesia and normal memory. J Int Neuropsychol Soc. 2008;14:365–372. doi: 10.1017/S1355617708080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn JC. The dimensionality of the remember-know task: A state-trace analysis. Psychol Rev. 2008;115:426–446. doi: 10.1037/0033-295X.115.2.426. [DOI] [PubMed] [Google Scholar]
- 19.Kirwan CB, Wixted JT, Squire LR. A demonstration that the hippocampus supports both recollection and familiarity. Proc Natl Acad Sci USA. 2010;107:344–348. doi: 10.1073/pnas.0912543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26:13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squire LR, et al. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friendly M, Franklin PE, Hoffman D, Rubin DC. The Toronto Word Pool: Norms for imagery, concreteness, orthographic variables, and grammatical usage for 1,080 words. Behav Res Methods Instrum Comput. 1982;14:375–399. [Google Scholar]
- 26.Rajaram S. Perceptual effects on remembering: Recollective processes in picture recognition memory. J Exp Psychol Learn Mem Cogn. 1996;22:365–377. doi: 10.1037//0278-7393.22.2.365. [DOI] [PubMed] [Google Scholar]