Abstract
Protection of the endothelium is provided by circulating sphingosine-1-phosphate (S1P), which maintains vascular integrity. We show that HDL-associated S1P is bound specifically to both human and murine apolipoprotein M (apoM). Thus, isolated human ApoM+ HDL contained S1P, whereas ApoM− HDL did not. Moreover, HDL in Apom−/− mice contains no S1P, whereas HDL in transgenic mice overexpressing human apoM has an increased S1P content. The 1.7-Å structure of the S1P–human apoM complex reveals that S1P interacts specifically with an amphiphilic pocket in the lipocalin fold of apoM. Human ApoM+ HDL induced S1P1 receptor internalization, downstream MAPK and Akt activation, endothelial cell migration, and formation of endothelial adherens junctions, whereas apoM− HDL did not. Importantly, lack of S1P in the HDL fraction of Apom−/− mice decreased basal endothelial barrier function in lung tissue. Our results demonstrate that apoM, by delivering S1P to the S1P1 receptor on endothelial cells, is a vasculoprotective constituent of HDL.
Keywords: endothelial function, crystal structure, sphingolipids, vascular permeability, atherosclerosis
Sphingosine-1-phosphate (S1P), the phosphorylated metabolite of d-sphingosine, binds to five G protein-coupled receptors (S1P1–S1P5) and regulates a plethora of biological actions (1–6). In particular, the prototypical S1P1 receptor is essential for vascular maturation during development and promotes endothelial cell migration, angiogenesis, and barrier functions (7–9). Thus, S1P is required for maintenance of the barrier property of the lung endothelium (10). Plasma S1P, which is derived from several cellular sources (11, 12), is associated with HDL (∼65%) and albumin (∼35%) (3, 5). HDL-induced vasorelaxation as well as barrier-promoting and prosurvival actions on the endothelium have been attributed to S1P signaling (2, 4, 13). Hence, much of the endothelium-protective actions of HDL may result from the actions of S1P on the endothelial S1P receptors. However, the molecular nature of the S1P binding to HDL and interaction with S1P receptors has not been characterized.
Apolipoprotein M (apoM) is a lipocalin that resides mainly in the plasma HDL fraction (14). The retained hydrophobic NH2-terminal signal peptide anchors apoM in the phospholipid layer of the lipoprotein and prevents filtration of the ∼22-kDa protein in the kidney (15). The biological functions of apoM are understood only partly. Studies in apoM gene-modified mice suggest that apoM has antiatherogenic effects, possibly related in part to apoM's ability to increase cholesterol efflux from macrophage foam cells, to increased preβ-HDL formation, and to antioxidative effects (16–18). The recent elucidation of the crystal structure of human recombinant apoM (r-apoM) demonstrated a typical lipocalin fold characterized by an eight-stranded antiparallel β-barrel enclosing an internal binding pocket that probably facilitates binding of small lipophilic ligands (19). Indeed, r-apoM expressed in Escherichia coli was found to cocrystallize with myristic acid (19), illustrating that apoM can bind lipid compounds with fatty acid side chains, and in vitro binding experiments demonstrated that S1P displaced the myristic acid with an IC50 of 0.90 μM (19). We demonstrate here that apoM is the carrier of S1P in HDL, mediating vasoprotective actions on the endothelium.
Results and Discussion
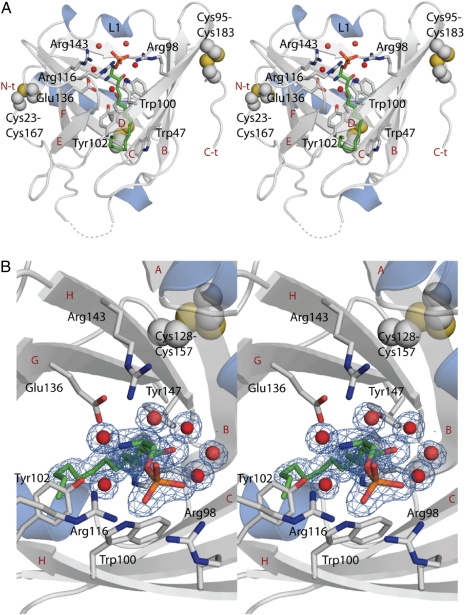
To study the molecular basis of apoM interaction with S1P, we determined the crystal structure of N-terminally truncated human apoM (residues 22–188) (r-apoM) in complex with S1P at 1.7-Å resolution. S1P is bound at the center of the calyx-like ligand-binding pocket (Fig. 1 and Fig. S1). It participates in numerous specific interactions with apoM (Fig. 1 and Fig. S2). The phosphate moiety interacts directly with the side chains of Arg98, Arg116, and Trp100 (Fig. 1 and Fig. S2). The S1P amino group is hydrogen-bonded to Glu136 and to Tyr102 and Arg143 via bridging water molecules. The hydroxyl group of S1P interacts via a bridging water molecule with the hydroxyl group of Tyr147 (Fig. 1 and Fig. S2). The hydrocarbon chain of S1P points toward the interior of the calyx. Hence, its interaction with apoM and its location coincide with that of myristic acid in a previous complex (Figs. S2 and S3) (19). When all these binding interactions are considered together, the lipid-binding site of apoM is highly complementary to the structure of S1P. In particular, the recognition of the phosphate group by several arginines hints that the S1P–apoM interaction is very specific.
Fig. 1.
The structure of the ApoM–S1P complex reveals the determinants of S1P-binding specificity. (A) Stereo view of the crystal structure of apoM with S1P at 1.7-Å resolution. S1P is shown as green sticks together with interacting residues. Strands B–F, the N terminus (N-t), and the C terminus (C-t) are labeled in red, and the interacting residues are labeled in black. (B) Top view of the S1P-binding site in close up. Electron density for S1P and surrounding water molecules is contoured at 1 σ and colored blue. Water molecules are shown as red spheres in both panels, and unmodeled loops toward the N terminus are shown as broken lines.
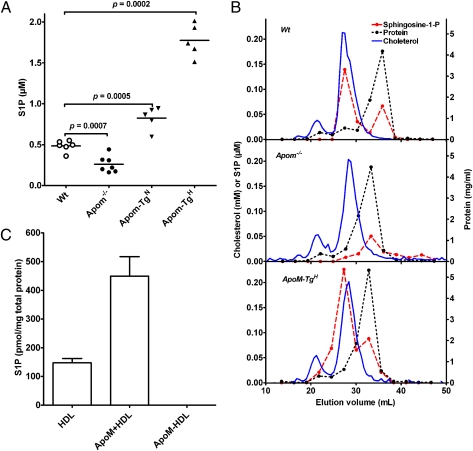
To elucidate whether apoM is the physiological carrier of HDL-associated S1P in vivo, plasma S1P was measured in apoM-knockout (Apom−/−) mice and in two lines of human apoM transgenic mice having either twofold (Apom-TgN) or 10-fold (Apom-TgH) increased plasma apoM concentrations (17). Compared with WT mice, plasma S1P was reduced by 46% in Apom−/− mice (P = 0.0007) and was increased by 71% (P = 0.0005) and by 267% (P = 0.0002) in the Apom-TgN and Apom-TgH mice, respectively (Fig. 2A). The plasma concentrations of HDL cholesterol, HDL total phospholipids, and apoA-I were affected only marginally in Apom−/− and Apom-TgH mice, demonstrating that the changes in S1P concentrations are related to apoM and not to variations in the amount of circulating HDL (17). When lipoproteins in WT mouse plasma were separated by gel filtration, the major peak of S1P coeluted with apoM in the HDL fractions, whereas a minor S1P peak coeluted with albumin (Fig. 2B). Apom−/− mice lacked S1P in the HDL fraction, but the S1P peak in the albumin fractions was present (Fig. 2B). Apom-TgH mice had increased S1P in HDL (Fig. 2B). This S1P was associated with apoM-containing HDL, as demonstrated by a parallel shift in S1P- and human apoM-elution profiles when a specific monoclonal antibody against human apoM (M58) was added to the plasma before gel filtration (Fig. S4 A and B).
Fig. 2.
ApoM gene dosage determines plasma S1P in genetically modified mice. (A) Plasma S1P in WT, Apom−/−, and apoM-transgenic female mice with ∼twofold (Apom-TgN) and ∼10-fold (Apom-TgH) increased plasma apoM. Each point represents data from an individual mouse; horizontal lines indicate means. (B) Lipoproteins in pools of plasma from WT (Top), Apom−/− (Middle), or Apom-TgH (Bottom) mice were separated by gel filtration on serially connected Superose 6 and 12 columns. The flow rate was 0.4 mL/min. Fractions of 275 μL were collected. Aliquots of 10 consecutive fractions were pooled before measuring S1P (red filled symbols) and protein (dotted black line). Cholesterol concentration (solid blue line) was determined in each fraction. The scale bar for cholesterol (mmol/L) and S1P (μmol/L) is shown on the left y axis. Protein (mg/mL) is shown on the right y axis. (C) S1P was measured with HPLC in purified preparations of human total HDL, apoM+ HDL, and apoM− HDL. Values are mean ± SEM (n = 3). S1P was not detectable in apoM− HDL. Results were confirmed by LC-MS/MS.
Importantly, the amount of apoM in HDL is sufficient to accommodate and account for all HDL-bound S1P. The average plasma apoM concentration is similar in mice and humans, i.e., ∼0.9 μmol/L (17). Hence, the apparent molar ratio between HDL-bound S1P and plasma apoM is ∼1:3 in WT and Apom-TgN mice and ∼1:6 in Apom-TgH mice. On gel filtration of human plasma, the majority of S1P coeluted with HDL, indicating that in humans, also, the main part of lipoprotein-bound S1P is associated with HDL (Fig. S4C). When human HDL was separated by affinity chromatography into apoM+ HDL and apoM− HDL fractions, S1P was found exclusively in apoM+ HDL (Fig. 2C). These data indicate that S1P in HDL is bound to apoM in both humans and mice. Intrinsic fluorescence binding studies of human r-apoM showed that apoM can bind S1P with an IC50 of 0.9 μM (19). With the same experimental setup but using murine r-apoM, an IC50 for S1P of 0.95 ± 0.05 μM (n = 3) was obtained (Fig. S5), further supporting the idea that apoM is the physiological carrier of HDL-associated S1P.
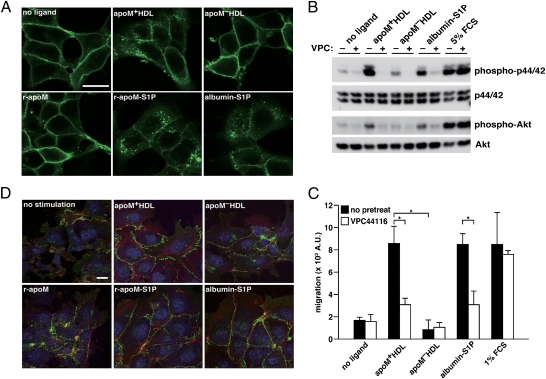
The biological effects of S1P are mediated by activation of the G protein-coupled S1P receptors, leading to activation of downstream effectors such as p44/42, MAPK, and Akt (20). To assess whether apoM+ HDL and apoM-bound S1P can activate the S1P1 receptor, we performed a ligand-induced receptor internalization assay in HEK293 cells stably expressing the GFP-tagged S1P1 receptor (21). Both ApoM+ HDL and r-apoM–bound S1P induced robust internalization of GFP-S1P1 receptor, similarly to albumin-bound S1P that was used as a positive control (Fig. 3A). Neither apoM− HDL nor r-apoM without S1P caused receptor internalization. These data indicate that the apoM–S1P complex can activate the S1P1 receptor, whether it is part of an HDL particle or not.
Fig. 3.
ApoM-bound S1P activates S1P1-mediated intracellular signaling pathways. (A) Confocal microscopy of HEK293 cells stably expressing S1P1-GFP. Cells were serum starved, stimulated for 1 h with indicated ligands, fixed, and imaged. ApoM+ HDL (equivalent to ∼100 nM S1P as determined by LC/MS/MS) or ApoM− HDL was used at 100 μg/mL Fatty acid-free BSA and r-apoM were complexed with S1P and used at afinal concentration of 100 nM (equimolar for both protein and lipid). (Scale bar, 20 μm.) (B) HUVEC were serum starved and pretreated with 1 μM of the S1P1 antagonist VPC44116 for 30 min before stimulated with apoM+ HDL (20 μg/mL protein, 20 nM S1P), apoM−HDL (20 μg/mL protein), or albumin-S1P (100 nM S1P and equimolar protein) for 10 min. (Note that VPC44116 has no inhibitory effects on cells stimulated by FCS, because FCS can activate receptor systems other than S1P1.) Activation of p44/42 and Akt was examined by Western blot analysis using phospho-specific antibodies. (C) HUVEC were serum starved and pretreated with 1 μM VPC44116 for 30 min where indicated and subjected to migration assay with 10 μg/mL apoM+ HDL (10 nM S1P), 10 μg/mL apoM− HDL, or 10 nM albumin-S1P. Data are mean ± SD (n = 3). *P < 0.01. (D) Microscopy of HUVEC that were serum starved and stimulated with 100 μg/mL apoM+ HDL, apoM− HDL, 100 nM albumin-S1P, r-apoM–S1P, or 100 nM S1P-free r-apoM for 1 h. After fixation, VE-cadherin (green), nuclei (blue), and F-actin (red) were visualized with confocal microscopy. (Scale bar, 20 μm.)
To test activation of endogenous S1P1 receptors and the downstream signaling by apoM-bound S1P, human umbilical vein endothelial cells (HUVEC) were stimulated with various carriers complexed or not with S1P. Prominent phosphorylation of p44/42 and Akt was induced by apoM+ HDL but not by apoM− HDL (Fig. 3B). Moreover, blocking of S1P1 receptors with the S1P1-selective antagonist VPC44116 (22, 23) essentially abolished the effect of apoM+ HDL on p44/42 and Akt phosphorylation (Fig. 3B), indicating that the effects of apoM+ HDL were mediated by the S1P1 receptor. Albumin-bound S1P, apoM+ HDL and apoM-bound S1P showed similar time courses and dose responses in the activation of p44/42 and Akt (Fig. S6 A and B).
S1P is a potent chemoattractant for endothelial cells, which are essential for wound-healing response and angiogenesis (9, 24). ApoM+ HDL stimulated chemotaxis of HUVEC, and this effect was abolished by pretreatment with the S1P1 antagonist VPC44116 (Fig. 3C). Both albumin- and r-apoM–bound S1P worked as chemoattractants in a concentration-dependent manner, but r-apoM–bound S1P showed slightly higher activity, especially at lower S1P concentrations (Fig. S6C). S1P suppresses abnormal vascular permeability by inducing the assembly of vascular endothelial (VE)-cadherin–containing adherens junctions between endothelial cells (1, 9). As shown in Fig. 3D, HUVEC were well spread, contained F-actin,and formed adherens junctions when treated with apoM+ HDL and with albumin- and r-apoM–bound S1P. In contrast, adherens junctions and F-actin were not induced efficiently by apoM− HDL or by r-apoM without S1P.
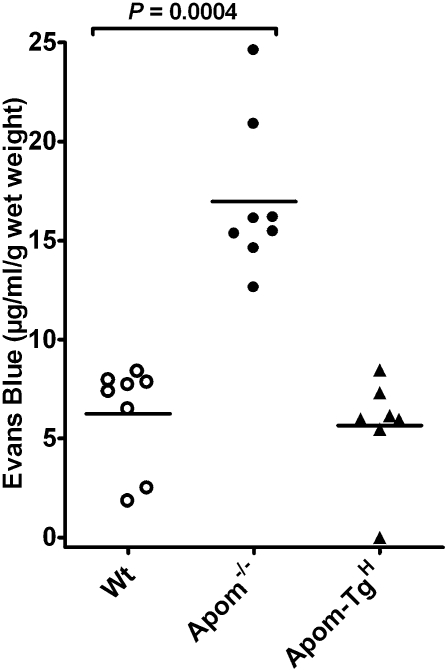
The importance of S1P in regulating vascular integrity in vivo has been shown in mice lacking plasma S1P (10). These mice extravasate albumin in their lungs, as demonstrated by i.v. injection of Evans Blue. After i.v. injection of Evans Blue, increased extravasation of Evans Blue in the lung was observed in the Apom−/− mice compared with WT and Apom-TgH mice (Fig. 4). This observation suggests that, even though Apom−/− mice have albumin-bound S1P in the circulation, this S1P cannot fully maintain the endothelial barrier function in the lung. However, the increased vascular leakage in the Apom−/− mice was not followed by general edema, because the weight of the lung tissue was unchanged in the different strains of mice (Fig. S7).
Fig. 4.
The apoM–S1P complex maintains lung endothelial barrier function. WT, Apom−/−, and Apom-TgH mice were injected i.v. with Evans Blue (30 μg/g body weight). After 30 min the mice were perfused with saline; then the lungs were removed and used for extraction of Evans Blue. Each point represents the content of Evans Blue in the lungs of one mouse, and horizontal lines represent mean values.
Taken together, our data demonstrate that apoM is the physiological carrier protein of S1P in HDL and that apoM can deliver S1P to the S1P1 receptor on endothelial cells. Thus, apoM-bound S1P mediates S1P1 receptor activation, resulting in downstream (junction assembly) effects that are vasoprotective. These observations provide important information about the function of apoM and increase the understanding of the antiatherogenic effects of apoM demonstrated in several different mouse models (17, 18, 25). Atherosclerosis is a chronic inflammatory disease characterized by accumulation of oxidized lipoproteins and cholesterol-filled foam cells in the arterial intima. HDL can protect against atherosclerosis by pleiotropic mechanisms, e.g., by promoting cholesterol efflux from foam cells, by attenuating LDL oxidation, by anti-inflammatory or antiplatelet effects, and by protecting the endothelium. Although apoM enhances the antioxidant function of HDL and the ability of HDL to stimulate cholesterol efflux from foam cells, these antiatherogenic effects also are characteristics of apoM-free HDL (16, 17). In contrast, the S1P-mediated vasoprotective effects of HDL are unique, because apoM is the physiological carrier protein for S1P in HDL, and the subset of HDL that contains apoM mediates multiple S1P-dependent effects of HDL (1). However, in addition to the now-reported effects on HDL functionality, apoM also has putatively proatherogenic effects on the metabolism of very low-density lipoprotein (VLDL)/LDL. Hence, the concentrations of apoM and LDL cholesterol in human plasma correlate positively (26), and in mice the overexpression of human apoM delays the clearance of VLDL/LDL, resulting in increased plasma concentrations of VLDL/LDL (25). Thus, adverse effects on LDL metabolism may counteract the beneficial effects on vascular endothelium of HDL-associated apoM. These dual effects would agree with the lack of association between plasma apoM levels and the risk of coronary heart disease observed in human case-control studies (27).
Many effects on endothelial cells have been attributed to S1P in plasma. Recently, Camerer et al. (10) showed that plasma S1P plays an important role in maintaining vascular barrier function. Our in vivo data further support the conclusion that plasma S1P is essential in maintaining vascular integrity. However, the presence of S1P bound to albumin in plasma is not sufficient, because our results demonstrate that S1P carried by apoM in the HDL fraction has an important role in preserving vascular integrity. Thus, even though the estimated concentration of albumin-bound S1P is sufficient to activate the S1P receptors in the Apom−/− mice, these mice have decreased endothelial barrier function in the lung. Because apoM determines the S1P-binding capacity of HDL, we propose that elevation of apoM+ HDL would be vasoprotective by preventing endothelial injury, allowing endothelial regeneration, and maintaining vascular integrity.
Our results raise the interesting question of how S1P is delivered from apoM to S1P receptors. The specific anchoring of apoM to lipoproteins and in particular to HDL by its retained signal peptide suggests that association of apoM with HDL and lipids in general is an important component for its function. The structural elucidation of the apoM–S1P complex revealed several features that possibly promote S1P uptake or release upon membrane or even receptor associations. In the two complexes of apoM with S1P or myristic acid, the peptide segment preceding the first β-strand of the lipocalin fold adopts multiple conformations, thereby changing the exposure of several hydrophobic amino acids, partially uncovering the bottom of the binding calyx, and possibly facilitating ligand release (Fig. S3). Equally possible, a previously observed weak point in the apoM barrel structure (between strands D and G) (28) could facilitate a lateral release of the ligand from the calyx upon docking to membranes or even to the S1P1 receptors. Although these scenarios currently remain hypothetical, r-apoM clearly is capable of delivering S1P to S1P1 receptors at the cell surface. It remains to be tested whether apoM is equally effective in delivering S1P to other S1P receptors (S1P2–5) or if the S1P binding to apoM provides receptor specificity that directs the biological effects of HDL-associated S1P.
In summary, our studies define apoM as a carrier of S1P in HDL and demonstrate that the HDL-associated apoM–S1P complex mediates vasoprotective actions on the endothelium. This signaling axis may be critical in normal vascular homeostasis and perturbed in vascular diseases.
Materials and Methods
Detailed descriptions of materials and methods are given in SI Materials and Methods.
Mice.
Apom-TgN, Apom-TgH, and Apom−/− mice were backcrossed at least seven times with C57B6/J mice. Mice were housed at the Panum Institute, University of Copenhagen, Copenhagen (17, 25).
Lipoproteins and S1P.
Human apoM+ HDL and apoM− HDL were isolated from human plasma with ultracentrifugation (1.063–1.21 g/L) followed by immunoaffinity chromatography on an anti-apoM monoclonal column (16). ApoM was quantified with ELISA (26). For gel filtration, 500-μL plasma samples from Apom-TgH (n = 5), Apom−/− (n = 7), and WT (n = 6) mice were separated on serially connected Superose 6 and Superose 12 10/300 GL columns (16). S1P was measured with HPLC (29) or liquid chromatography tandem MS (LC-MS/MS) (30).
Western Blotting.
Western blotting was done after separation in 10 or 12% SDS/PAGE gels with antibodies against human apoM, p44/42, phospho-p44/42, and phospho-Akt (Cell Signaling).
Crystal Structure of r-ApoM with S1P.
Human r-apoM was prepared as described (15). The r-apoM–S1P complex was prepared by adding S1P (dry powder) to a concentrated solution of r-apoM (13 mg/mL). Formation of the r-apoM–S1P complex could be monitored with isoelectric focusing (Fig. S8A). The r-apoM–S1P complex was purified by gel filtration to remove unbound S1P before crystallization using hanging-drop vapor diffusion. Data were collected to a resolution of 1.7 Å. The structure was solved using molecular replacement and refined to R-work and R-free of 19.1% and 22.1%, respectively (Table S1).
r-ApoM– and Albumin-Bound S1P.
S1P was dissolved in methanol. After evaporation, the S1P was redissolved by sonication in 20 mM Tris-HCl (pH 8.0) containing equimolar amounts of r-apoM (19) or fatty acid free BSA (Sigma A6003) and kept at 4 °C until use.
Cell Culture.
Passage 4–10 HUVEC were cultured as described (31). HEK293 cells stably expressing S1P1-GFP (21) were cultured in DMEM with 10% FBS. Confocal laser scanning microscopy was performed using a FluoView FV10i system (Olympus). Migration assays were performed using a 96-well chemotaxis chamber system (Neuroprobe) (32). VE-cadherin was visualized with immunofluorescence, nuclei with TO-PRO-3 dye, and F-actin with rhodamine phalloidin.
Vascular Permeability.
Mice were injected i.v. with Evans Blue (30 μg/g body weight). After 30 min the mice were anesthetized and perfused extensively with saline via the right ventricle to remove intravascular Evans Blue. The lung wet weights were measured, and Evans Blue was extracted in 1 mL formamide at 56 °C for 16 h. Evans Blue concentration was determined from the OD620 minus OD500 in the extract and a serial dilution of a standard.
Statistics.
Numerical differences were analyzed with two-tailed Student's t test.
Supplementary Material
Acknowledgments
We thank Karen Rasmussen and Charlotte Wandel and Bernd Gardill for technical assistance, Uwe Müller for help during diffraction data collection, and Dr. Kevin R. Lynch for the gift of VPC44116. This work was supported by Grant 07143 from the Swedish Research Council and by grants from the Söderberg's Foundation and the Swedish Heart-Lung Foundation (to B.D.), by Grants 09-06452/FSS (to L.B.N.) and 09-073571/FSS (to C.C.) from the Danish National Research Council, by grants from the Rigshospitalet Research Council (to L.B.N.) and from the Sonderfonds of the University Erlangen-Nuremberg (to Y.A.M.), and by Grants HL-67330, HL-70694, and HL89934 from the National Institutes of Health (to T.H.).
Footnotes
The authors declare no conflict of interest.
Data deposition: Crystallography, atomic coordinates, and structure factors for the S1P-apoM crystal structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2YG2).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103187108/-/DCSupplemental.
References
- 1.Garcia JG, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura T, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 3.Aoki S, et al. Sphingosine 1-phosphate-related metabolism in the blood vessel. J Biochem. 2005;138:47–55. doi: 10.1093/jb/mvi100. [DOI] [PubMed] [Google Scholar]
- 4.Nofer JR, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: Signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik JH, Chae Ss, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- 9.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 10.Camerer E, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 12.Venkataraman K, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argraves KM, et al. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem. 2008;283:25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu N, Dahlbäck B. A novel human apolipoprotein (apoM) J Biol Chem. 1999;274:31286–31290. doi: 10.1074/jbc.274.44.31286. [DOI] [PubMed] [Google Scholar]
- 15.Christoffersen C, et al. The signal peptide anchors apolipoprotein M in plasma lipoproteins and prevents rapid clearance of apolipoprotein M from plasma. J Biol Chem. 2008;283:18765–18772. doi: 10.1074/jbc.M800695200. [DOI] [PubMed] [Google Scholar]
- 16.Christoffersen C, et al. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J Lipid Res. 2006;47:1833–1843. doi: 10.1194/jlr.M600055-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Christoffersen C, et al. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J Biol Chem. 2008;283:1839–1847. doi: 10.1074/jbc.M704576200. [DOI] [PubMed] [Google Scholar]
- 18.Wolfrum C, Poy MN, Stoffel M. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med. 2005;11:418–422. doi: 10.1038/nm1211. [DOI] [PubMed] [Google Scholar]
- 19.Sevvana M, et al. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J Mol Biol. 2009;393:920–936. doi: 10.1016/j.jmb.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 20.Morales-Ruiz M, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 21.Liu CH, et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oo ML, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 23.Awad AS, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 24.English D, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 25.Christoffersen C, et al. Opposing effects of apolipoprotein m on catabolism of apolipoprotein B-containing lipoproteins and atherosclerosis. Circ Res. 2010;106:1624–1634. doi: 10.1161/CIRCRESAHA.109.211086. [DOI] [PubMed] [Google Scholar]
- 26.Axler O, Ahnström J, Dahlbäck B. An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J Lipid Res. 2007;48:1772–1780. doi: 10.1194/jlr.M700113-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Ahnström J, et al. Levels of apolipoprotein M are not associated with the risk of coronary heart disease in two independent case-control studies. J Lipid Res. 2008;49:1912–1917. doi: 10.1194/jlr.M700471-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Sevvana M, et al. Mouse ApoM displays an unprecedented seven-stranded lipocalin fold: Folding decoy or alternative native fold? J Mol Biol. 2010;404:363–371. doi: 10.1016/j.jmb.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 29.He X, Huang CL, Schuchman EH. Quantitative analysis of sphingosine-1-phosphate by HPLC after napthalene-2,3-dicarboxaldehyde (NDA) derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:983–990. doi: 10.1016/j.jchromb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 30.Bielawski J, et al. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2009;579:443–467. doi: 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]
- 31.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- 32.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.