Abstract
The colonic epithelium self-renews every 3 to 5 d, but our understanding of the underlying processes preserving wound healing from carcinogenesis remains incomplete. Here, we demonstrate that Nod-like receptor pyrin domain-containing protein 6 (NLRP6) suppresses inflammation and carcinogenesis by regulating tissue repair. NLRP6 was primarily produced by myofibroblasts within the stem-cell niche in the colon. Although NLRP6 expression was lowered in diseased colon, NLRP6-deficient mice were highly susceptible to experimental colitis. Upon injury, NLRP6 deficiency deregulated regeneration of the colonic mucosa and processes of epithelial proliferation and migration. Consistently, absence of NLRP6 accelerated colitis-associated tumor growth in mice. A gene-ontology analysis on a whole-genome expression profiling revealed a link between NLRP6 and self-renewal of the epithelium. Collectively, the integrity of the epithelial barrier is preserved by NLRP6 that may be manipulated to develop drugs capable of preventing adenoma formation in inflammatory bowel diseases.
Keywords: colonic myofibroblasts, colorectal cancer
Inflammatory bowel diseases (IBD), encompassing Crohn disease and ulcerative colitis, refer to a common relapsing-remitting inflammatory condition of the gastrointestinal tract that remains incurable (1). About 2.2 million Europeans and 1.4 million northern Americans are afflicted by IBD, the hallmarks of which include severe diarrhea, abdominal pain, fever, weight loss, bleeding, and malnutrition. The cumulative risk of colorectal cancer (CRC) is up to two times higher in IBD compared with that in the general population (2). Established risk factors for CRC in IBD include younger age at diagnosis, greater extent and duration of disease, family history of CRC, and coexisting primary sclerosing cholangitis. At the level of the inflammatory colorectal adenomas, several somatic mutations (e.g., overactivating mutations of the β-catenin) intrinsically contribute to carcinogenesis through activation of kinases, including ERK (3), and of transcription factors, including STAT3 (4). However, the cellular and molecular mechanisms whereby IBD patients may develop CRC remain unresolved.
The severity of colitis is an important determinant of colorectal neoplasia development in IBD (5, 6). Inflammatory cells that are infiltrating the tumor microenvironment contribute to the production of a variety of elicitors, including IL-1β, that subsequently alter genomic stability of epithelial cells (7). Biologically active IL-1β is secreted upon cleavage by an inflammatory cysteine protease, namely Caspase-1 (also known as IL-1β converting enzyme). At the molecular level, Caspase-1 interacts with its adaptor ASC (apoptosis-associated speck-like protein containing a caspase-recruitment domain) and with certain cytosolic Nod-like receptor pyrin domain-containing proteins (NLRPs), including NLRP6 (8). The latter (previously referred to as Pypaf-5) possess a Pyrin as a C-terminal effector domain that interacts with both ASC and Caspase-1 (9). In response to stress-associated molecular patterns, NLRP6, Caspase-1, and ASC are assembled to form a molecular platform that is referred to herein as the NLRP6-coupled inflammasome. Consistently, coexpression of NLRP6 and ASC has been shown to trigger secretion of the biologically active IL-1β through proteolytic cleavage by Caspase-1 and to promote activation of the transcription factor NF-κB in vitro (9). Recent findings shed light on the essential role of Caspase-1 in intestinal inflammation and carcinogenesis, as revealed by the increased susceptibility of mice deficient for Caspase-1 in experimental models of IBD and of CRC (10–13). However, the role of NLRP6 in tissue repair and intestinal tumorigenesis has yet to be determined.
Results
NLRP6 Is Essential for Wound Healing of the Intestinal Mucosa.
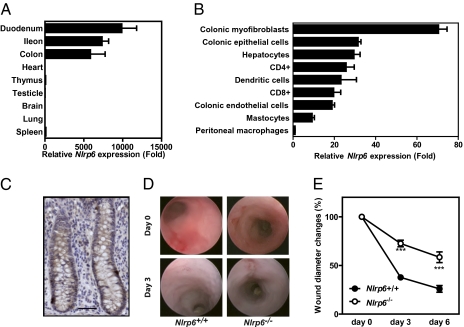
Gene expression analysis revealed that NLRP6 was primarily expressed by any part of the small and large intestine (Fig. 1A). In contrast to what is observed in the gut, the transcript level of Nlrp6 in the spleen, thymus, heart, lung, brain, and testicles was barely detectable by quantitative RT-PCR analysis (Fig. 1A). Besides its expression by peripheral blood mononuclear cells (9), NLRP6 was primarily expressed by colonic myofibroblasts (Fig. 1 B and C). The restricted expression pattern of NLRP6 led us to investigate whether NLRP6 may regulate pathways involved in re-epithelialisation of colon wounds. The mucosal regeneration after biopsy injury of the descending colon was assessed in Nlrp6-deficient and control animals (Fig. S1) by using a straight-type rigid miniature endoscope and 3-French biopsy forceps (14). Healing of oval-shaped wounds in the mucosa of the distal colon of control animals was recovered by 62% and by 74% at 3 and 6 d postinjury, respectively (Fig. 1 D and E). In contrast, an incomplete healing of the average surface lesion area was observed in Nlrp6-deficient mice over time (Fig. 1 D and E). Collectively, our results unveiled a key role of NLRP6 in mucosal wound healing.
Fig. 1.
Nlrp6 is primarily expressed by colonic myofibroblasts and is involved in tissue repair. (A and B) Relative Nlrp6 expression was determined by quantitative RT-PCR analysis and normalized using Actb (n = 4). (C) Nlrp6 was detected by immunohistochemistry in human colonic myofibroblasts. (Scale bar, 50 μm.) (D and E) The mucosal regeneration was assessed after biopsy injury of the descending colon of Nlrp6-deficient and control animals by using a straight-type rigid miniature endoscope and 3-French biopsy forceps. Wound diameter was assessed just after biopsy at days 0, 3, and 6 (n = 3).
NLRP6 Prevents Relapsing Colitis in Mice.
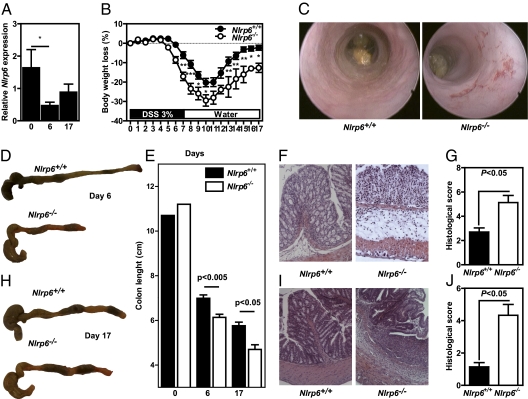
Given the important role of NLRP6 in tissue repair, we next hypothesized that NLRP6 may have a role in maintaining intestinal homeostasis using a validated experimental model of relapsing-remitting intestinal wounding (15). Wild-type and Nlrp6-deficient (Nlrp6−/−) mice were challenged with 3% dextran sodium sulfate (DSS) for 7 d followed by a 10-d period of regular drinking water. The sulfated polysaccharide DSS is known to recapitulate epithelial tissue disruption, potentially leading to microbial translocation that is frequently observed in IBD (16). Subsequently, the injured colonic mucosa is infiltrated by leukocytes that secrete endogenous activating signals of the inflammasome, including reactive oxygen species (17). Under this condition, a significant decreased expression of NLRP6 was observed after DSS exposure that was progressively restored during remission (Fig. 2A), suggesting a protective role of NLRP6 in intestinal inflammation. Surrogate markers of colitis-associated morbidity, including body weight loss, stool consistency, and rectal bleeding, were daily monitored. DSS-treated Nlrp6−/− mice exhibited a significant decrease of their initial body weight compared with similarly treated wild-type animals (Fig. 2B). In addition, Nlrp6-deficient mice showed persistent signs of colitis compared with controls, including hunched posture, rectal bleeding, and diarrhea. Endoscopy analysis of the DSS-challenged animals revealed overt signs of tissue damage with bleeding and ulcerations in the absence of NLRP6, as observed in IBD (Fig. 2C). Furthermore, the length of the diseased colon harvested from Nlrp6-deficient mice was significantly shorter than that observed in controls at day 6 (Fig. 2 D and E). Throughout the remission phase, immunohistochemical analysis showed enhanced infiltration of leukocytes that was accompanied by the formation of edema, as determined by the histological score (Fig. 2F). In addition, the disruption of the colonic architecture in Nlrp6−/− mice (Fig. 2 G and J) resulted in enhanced permeability (Fig. 3A). In contrast, the histological aspect of the colonic mucosa was normal in wild-type mice at the end of experimental procedure (Fig. 2I). Importantly, the enhanced shrinkage (Fig. 2E) and mucosal damage (Fig. 2 G and J) of the DSS-treated colon in Nlrp6−/− mice lasted even after 10 d of regular water. Taken together, these results demonstrate that a lack of functional NLRP6-coupled inflammasome renders mice prone to develop relapsing colitis.
Fig. 2.
NLRP6 prevents relapsing colitis in mice. Wild-type (Nlrp6+/+) and Nlrp6-deficient mice (Nlrp6−/−) were challenged with 3% DSS for 6 d or 7 d followed by a 10-d period of regular drinking water. (A) Nlrp6 expression was assessed by quantitative RT-PCR in colon from control wild-type mice (n = 4) and from animals treated with DSS for 6 d (n = 9) or for 7 d of DSS challenge followed by a 10-d period of regular water (n = 6). *P < 0.05. (B) Body loss of weight of Nlrp6+/+ (n = 10) and Nlrp6−/− (n = 5) mice was monitored. *P < 0.05, **P < 0.01, ***P < 0.001. (C) Endoscopic analysis was made before necropsy to observe the tissue damage after 6 d of DSS treatment. Representative photographs of dissected Nlrp6+/+ and Nlrp6−/− mice at day 6 (D) and day 17 (H). (E) The colon length was measured at day 6 (from nine Nlrp6+/+ and six Nlrp6−/− mice) and day 17 (from seven Nlrp6+/+ and three Nlrp6−/− mice). The histological score was evaluated on serial colonic sections stained with H&E at day 6 (from nine Nlrp6+/+ and six Nlrp6−/− mice) (F and G) and day 17 (from seven Nlrp6+/+ and three Nlrp6−/− mice) (I and J).
Fig. 3.
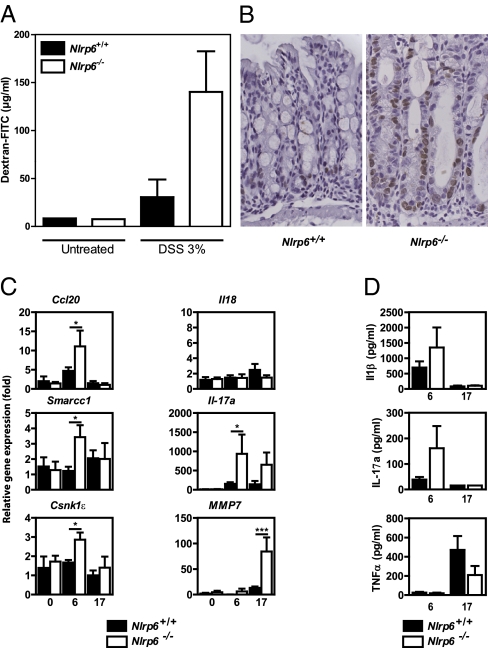
Incomplete wound healing in the colon of Nlrp6-deficient mice result in enhanced cell cycle arrest, intestinal permeability, and inflammatory response. (A) FITC-dextran concentration in sera from DSS-treated Nlrp6+/+ (n = 2) and Nlrp6−/− (n = 4) mice. (B) Immunohistochemical detection of BrdU incorporation was performed in the colon of DSS-treated animals after a 1-h pulse-chase. (C) Relative gene expression and (D) cytokine concentration were determined at day 0 (from four Nlrp6+/+ and three Nlrp6−/− mice), day 6 (from nine Nlrp6+/+ and six Nlrp6−/− mice), and day 17 (from seven Nlrp6+/+ and three Nlrp6−/− mice) by quantitative RT-PCR and ELISA analysis, respectively.
NLRP6 Controls Epithelial Cell Organization and Proliferation upon Injury.
Given the increased susceptibility of Nlrp6-deficient mice in an experimental model of IBD, we next sought to determine whether NLRP6 might control colonocyte proliferation and migration upon injury. Immunohistochemical detection of 5 BrdU incorporation was performed in the colon of DSS-treated animals after 1-h pulse-chase. Cycling epithelial cells at the crypt and around the wounds was perceptibly enhanced when NLRP6 was absent (Fig. 3B). In addition, cell migration within the lower half of the epithelial invagination was affected by the absence of functional NLRP6, indicating that the latter may suppress intestinal epithelial cell proliferation upon injury. In an attempt to determine the NLRP6-dependent gene program involved in maintenance of intestinal homeostasis, RNA was next extracted from the colon of DSS-treated animals and gene expression was assessed by quantitative RT-PCR analysis. Following injury by DSS, the levels of transcripts for molecules involved in cell proliferation, including the tumor-suppressor gene Casein kinase ε (Csnk1ε) that stabilizes β-catenin, was found to be up-regulated in the colon of Nlrp6−/− mice compared with treated wild-type animals (Fig. 3C). Similarly, the expression of molecules that are primarily expressed within the proliferating epithelial population of the colonic crypts, including SMARRC1 (18), was significantly enhanced compared with that in control mice (Fig. 3C). Of note, intestinal tumorigenesis is linked to abnormal expression of Csnk1ε (19, 20) and of SMARRC1, which is a member of the switch/sucrose nonfermentable chromatin remodeling complex (21). In addition, an enhanced mRNA and protein expression of IL-17A was observed in the colon of Nlrp6−/− mice (Fig. 3 C and D). Likewise, the colonic mucosa Nlrp6−/− mice showed enhanced transcript levels of molecules involved in the Th17-mediated pathway, including Chemokine (C-C motif) ligand 20 (Ccl20) and matrix metalloproteinase-7. In contrast, transcription of several genes encoding proinflammatory cytokines, including IL-1β, were similar between the diseased colon of wild-type and that of Nlrp6-deficient animals (Fig. 3D). Consistently, the use of interleukin-1 receptor antagonist (namely anakinra) failed to protect Nlrp6−/− mice from enhanced DSS-induced colitis, as evidenced by body weight loss (Fig. S2A) and signs of rectal bleeding and diarrhea (Fig. S2B). At autopsy, the macroscopic appearance of the colon of Nlrp6−/− was indistinguishable from that of anakinra-treated Nlrp6−/− mice upon DSS injury. Collectively, the efficiency of the process of wound healing requires functional NLPR6 by regulating several processes involved in proliferation of the adjacent epithelia and in Th17 function. In accordance with our findings, disease severity in mice deficient for two additional components of the NLRP6-coupled inflammasome, namely Caspase-1 and ASC, was similar to that observed in Nlrp6−/− mice after DSS injury of the colonic epithelial barrier (10, 11, 13).
NLRP6 Is a Negative Regulator of Colorectal Tumorigenesis.
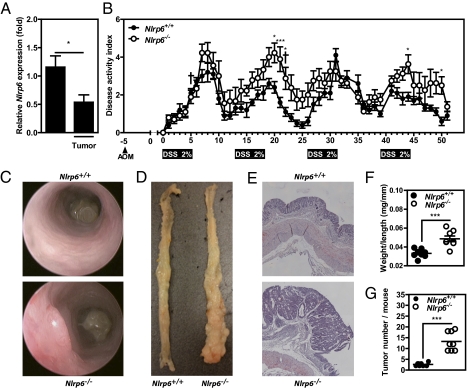
Given the key pathophysiological role of colonic myofibroblast in wound healing and colorectal carcinogenesis, we next reasoned that NLRP6 may prevent the development of tumors in chronically inflamed colon. Similarly to what we observed in DSS-treated mice (Fig. 2A), NLRP6 expression was lowered at the level of the adenocarcinoma compared with that in nontumoral colonic sections (Fig. 4A), suggesting that NLRP6 may be a negative regulator of intestinal tumorigenesis. To test this hypothesis, wild-type and Nlrp6-deficient mice were subjected to a well-characterized experimental model of colitis-associated CRC (22). Briefly, animals were first administered azoxymethane (AOM) by intraperitoneal injection followed by repeated oral administration of DSS. AOM is an alkylating procarcinogen causing formation of O6-methylguanine adducts by the colon, promoting G-to-A transition during DNA replication. Mice were challenged with four rounds of DSS for a period of 5 d interspersed with 7 d access to drinking water. Under this condition, Nlrp6-deficient mice lost significantly more body weight during the second round of DSS, which was accompanied by more severe clinical signs of colitis, including rectal bleeding and diarrhea, compared with wild-type animals (Fig. 4B). Although none of the treated wild-type mice died, about 20% of Nlrp6-deficient mice were moribund during the DSS-AOM regimen and needed euthanasia at days 5 and 22 for ethical reasons, further indicating that mutant mice are more sensitive to DSS. Consistently, endoscopy analysis of mice revealed the presence of adenomas within the colon of Nlrp6-deficient mice (Fig. 4C). At necropsy, higher weight-to-length ratios were observed in Nlrp6-deficient mice compared with those of controls because of accelerated tumor growth when NLRP6 was absent (Fig. 4 D and F). The tumor burden was also significantly higher at the end of the colon of Nlrp6-deficient mice compared with controls (Fig. 4G).
Fig. 4.
Nlrp6−/− mice develop more tumors than control after experimental chronic inflammation in an experimental model of colorectal tumorigenesis. Five days after AOM administration (at 8 mg/kg), Nlrp6+/+ (n = 8) and Nlrp6−/− (n = 8) mice were subjected to four rounds of 2% DSS for 5 d interspersed with 7-d access to regular drinking water mice. (A) Nlrp6 expression was assessed by quantitative RT-PCR in tumoral and nontumoral resection specimen. *P < 0.05. (B) Disease activity index, including body weight loss, presence of rectal bleeding, and stool consistency, was measured daily. *P < 0.05, ***P < 0.001. (C) Endoscopic analysis of mice at day 49. Representative photographs of dissected colon (D) and of H&E staining of paraformaldehyde-fixed tissue (E) at necropsy. Values represent the weight/length ratio (F) and the number of tumor (G) per colon of each Nlrp6+/+ and Nlrp6−/− mice. ***P < 0.001.
Genetic Ablation of NLRP6 Alters Expression of Paracrine Factors Involved in Colonic Epithelial Proliferation.
To further characterize how NLRP6 may negatively regulate intestinal tumorigenesis, we next performed transcriptional profiling of tumoral (T) and nontumoral (NT) biopsies that were procured from Nlrp6+/+ and Nlrp6−/− treated with the DSS-AOM regimen. We were able to identify a set of 905 genes that were induced or repressed greater than twofold independently of NLRP6 (Fig. 3 A and B). Of this gene set, an ontology-based analysis identified a significant enrichment of genes involved in inflammation mediated by the chemokine and cytokine signaling pathway, including the Chemokine (C-C motif) ligand 24 (Ccl24) (Fig. S4). In contrast, we did not observe any NLRP6-dependent changes of the transcript abundance of the IL-1 family member IL-18 and of prostaglandin-endoperoxide synthase 2. Consistent with our findings, activation of Wnt through the Adenomatous polyposis coli (APC)min mutation triggers colorectal development independently of the IL1R-mediated signaling pathway (3). Importantly, a Venn diagram revealed that the expression of certain genes was influenced by the NLRP6 genotype, as shown in Fig. S3A. Within the set of 1,884 genes that were solely differentially expressed in Nlrp6-deficient mice, a significant overrepresentation of paracrine factors of the p53 Wnt and Notch signaling pathways was observed, supporting a role of NLRP6 in regulation of intestinal crypt cell proliferation (Fig. S3C). Several genes encode agonists of the Wnt-signaling pathway, among which the dickkopf-related protein 2 (Dkk2) and Wingless-type mouse mammary tumor virus integration site family, member 3 (Wnt-3), 5b (Wnt-5b), and 6 (Wnt-6) (23), fell in this category. Notably, the microarray analysis clearly revealed an overexpression of the latter genes in tumoral resection specimens of Nlrp6-deficient mice compared with wild-type treated animals. Of interest, the expression of a set of Wnt-target genes, particularly the proto-oncogene Mycl1, was enhanced in the absence of NLRP6 (Fig. S3D). As observed for SMARRC1, it is worth noting that Mycl1 was primarily expressed in epithelial cells that are located at the base of the crypt, further supporting the regulatory role of NLRP6 in self-renewal of the epithelium. Sporadic and familial CRC tumorigenesis in humans are often caused by Wnt-activating mutations, including oncogenic forms of β-catenin–encoding gene, namely Ctnnb1. We therefore screened for mutations in exon 3 of Ctnnb1 (24). Consistent with our gene-expression profiling, de novo mutation that either affects nuclear localization of the β-catenin (Gly34Glu) or that alters phosphorylation of the β-catenin by GSK-3 β (Asp32Gly) were found de novo in four of eight tumoral tissues isolated from Nlrp6−/− mice compared with only one Gly34Glu allele in wild-type mice. Collectively, our comprehensive approach provides a mechanism whereby NLRP6 may limit epithelial cell depolarization and tissue disintegration upon injury.
Discussion
The colon is renewed every 3 to 5 d (25). IBD is thought to result from a defective wound healing from overt inflammatory response to certain commensals in the colon of genetically predisposed individuals. Following injury, the self-renewal of the epithelial barrier is controlled by a coordinated molecular machinery that began to be identified. We thereby undertook an in vivo approach based on inactivating NLRP6. We provided compelling evidence for an important role of NLRP6 in self-renewal of the intestinal epithelium. Importantly, absence of NLRP6 resulted in defective wound healing in response to trauma that were triggered either by biopsy or by exposure to DSS, perhaps through a failure of enterocytes to activate STAT3 (26). The discovery of a NLR controlling colonic myofibroblast function and interaction with adjacent epithelial progenitors at the bottom of the crypts was unexpected.
NLRP6 expression was down-regulated in inflamed colons and adenomas in mice. Consistently, an essential role of NLRP6 in intestinal homeostasis was demonstrated. Notably, NLRP6 deficiency resulted in enhanced epithelial expression of molecules, including Csnk1ε and SMARCC1, which may enhance proliferation of the dysplatic epithelium. Unlike NLRP6 and Caspase-1, the role of the NLRP3-coupled inflammasome in intestinal homeostasis and tumorigenesis remains controversial in mice (10, 11, 13, 27, 28), suggesting a differential role between the NLRP3- and NLRP6-coupled inflammasomes. Noteworthy, we failed to observe any changes in secretion of IL-1β and any anti-inflammatory effect of anakinra in Nlrp6-deficient mice that may account for their vulnerability to DSS.
Finally, we identified the NLRP6-coupled inflammasome as a negative regulator in colonic myofibroblast of intestinal tumorigenesis that mirrors the phenotype of mice deficient for two other NLRP6-interacting molecules, namely Caspase-1 and ASC (11). Importantly, colonic myofibroblast is a radio-sensitive cellular compartment within the stem cell niche of the colon. In addition, colonic myofibroblast is a major source of modulators of the Wnt signaling pathway that is essential in synchronizing the behavior of the colonic epithelium in response to injury. Notably, the Wnt signaling pathway governs about 80 genes that are involved in proliferation, upward migration, and differentiation (29). It is worth noting that inhibiting Csnk1ε down-regulates the activity of the Wnt/β-catenin signaling pathway that is overactivated in a large proportion of patients with CRC (19). Similarly, alteration of the expression of the transcription factor Smarrc1 is linked to deregulation of the Wnt/β-catenin signaling pathway and to disease severity in CRC (18). Herein, an enhanced expression of paracrine factors that alter the Wnt/β-catenin signaling pathway was also observed within tumors of Nlrp6-deficient mice. Given that genetic ablation of the cytosolic adaptor MyD88 rescued APC deficiency in the colon (30), the regulatory role of NLRP6 on signaling pathways mediated through MyD88 remains now to be assessed. Of note, clinical investigations revealed that NLRP6 expression and ASC methylation may represent unique molecular markers of carcinogenesis (31, 32). Collectively, we provide evidence that NLRP6 is involved in maintenance of the integrity of the epithelial barrier, suggesting that continuous stimulation of NLRP6 may represent a promising therapeutical strategy in IBD and CRC.
Materials and Methods
Generation of Nlrp6-Deficient Mice.
Nlrp6−/− mice were generated through homologous recombination by using the Lex-1 ES cells that are derived from the 129SvEvBrd strain. A gene-targeting vector with a neomycin-resistance cassette was constructed to replace the first two exons of Nlrp6 (Fig. S1A). The latter is required to encode the Pyrin domain of NLRP6, which is essential for recruiting ASC and subsequently for activating Caspase-1 (9). The mRNA sequence of Nlrp6 gene is reported in GenBank accession no. JF810536. Genotyping of positive ES clones was accomplished by Southern blotting analysis. Nlrp6-deficient mice (Nlrp6−/−) were produced at the expected Mendelian ratio by crossing heterozygous animals. Genotyping of mouse tail DNA was performed to confirm the presence of the wild-type and targeted alleles (Fig. S1B). Nlrp6−/− animals were viable and were backcrossed onto a C57BL6/J background. Nlrp6−/− mice showed no gross abnormalities when bred under specific pathogen-free environment. The absence of Nlrp6 mRNA in Nlrp6−/− animals was confirmed by quantitative RT-PCR (Fig. S1C).
Induction of Relapsing-Remitting Colitis and Inflammation-Driven Colon Carcinogenesis.
All animal studies were approved by the local investigational review board of the Institut Pasteur of Lille. Animal experiments were performed in an accredited establishment (N° B59-108) according to governmental guidelines N°86/609/CEE. Age- and sex-matched animals were housed five per cages and had free access to a standard laboratory chow diet in a temperature-controlled SPF environment and a half-daylight cycle exposure. Relapsing-remitting colitis was induced by giving mice 3% (wt/vol) DSS (TdB Consultancy) for a period of 6 to 8 d followed by normal drinking water for 2 to 10 d. DSS was dissolved in drinking water and changed every 3 d. Signs of morbidity, including body weight, stool consistency, and occult blood or the presence of macroscopic rectal bleeding gross, were monitored daily as previously described (15). At specific time points throughout the course of the challenge, mice were autopsied to assess the severity of the disease by measurement of colon lengths and by histological scoring. To assess the role of NLRP6 in development of inflammation-associated tumorigenesis of the colon, mice were challenged intraperitoneally with AOM (8 mg/kg body weight; Sigma-Aldrich) 5 d before one or four regimens that consist in a 5-d period of 2% DSS treatment (wt/vol) followed with a week of regular water, as previously described (22). The distal colon located 1-cm above the anal canal was dissected out and cut transversally. Tissue specimens were collected and kept frozen until further quantification of transcript and protein levels. One piece of colon was fixed in 4% paraformaldehyde and embedded in paraffin for blinded immunohistochemistry analysis by two investigators. Histological scoring of H&E-stained sections take into account the level of inflammatory cell infiltration and the epithelial damage, as previously described (15).
Mouse Endoscopy.
The presence of ulcerations and of adenomas within the colon was monitored by using the Coloview high resolution mouse endoscopic system (Karl-Storz), as described previously (15, 22).
Immunohistochemistry.
For NLRP6 immunostaining, we used a polyclonal goat antibody [NLRP6 (F-20): sc-50636; Santa Cruz Biotechnology]. Full-thickness sections were processed for peroxidase immunostaining using the Dako Laboratories system following the manufacturer's recommendations. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections using the streptavidin-biotin-peroxydase method in a Dakocytomation AutoStainer. Sections were first deparaffinized and rehydrated. Antigen retrieval was performed by incubating the slides in Tris-citrate buffer pH 6.0 for 20 min at 97 °C (PT Link; Dakocytomation). Endogenous peroxydase activity was blocked by incubation in 3% hydrogen peroxide for 10 min. The polyclonal goat anti-NLRP6 (dilution: 1/25) was incubated on slides for 30 min at temperature room. Biotinylated secondary antibody was a polyclonal rabbit anti-goat (Dakocytomation). Sections were incubated with 3,3′-diaminobenzidine substrate (Dako Laboratories) for 1 min before the reaction was stopped in distilled water and counterstained with hematoxylin. Withdrawal of the primary antibody and replacement with a nonspecific antibody were used as negative controls. The BrdU incorporation assay was used to determine the proliferation rate of colonic epithelial cells on formalin-fixed colonic sections according to the manufacturer's protocol (BD Biosciences). Briefly, colon specimens were dissected out 1 h after intraperitoneal injection with BrdU (120 mg/kg) and fixed in formalin until further labeling of S-phase cells.
Microarray and Gene-Ontology Analysis.
Nontumoral (NT) and tumoral (T) colonic resection specimens from wild-type and Nlrp6−/− animals were dissected out and stored at −80 °C in RNAlater (Ambion, Applied Biosystems), until extraction of total RNA accordingly to manufacturer's instructions (Qiagen). The quality of the extracted RNA was confirmed by Agilent 2100 Bioanalyzer using RNA Nano 6000 (Agilent Technologies). The 4 × 44 K Whole Mouse Genome Oligo Microarrays (Agilent Technologies) was used to determine the gene-expression profile of two biological replicates from all four stages (i.e., nontumoral and tumoral resection specimens from wild-type and Nlrp6−/− animals). For each labeling, 2 μg of total RNA per sample were engaged in the synthesis of a fluorescent probe labeled with Cy5 or Cy3 fluorophores. A 2 × 2 factorial experimental design and a dye-swap strategy were used. After a within-array loess normalization (33), raw data were analyzed with the LIMMA package (34) and sets of differentially expressed genes were filtered for a P value < 0.01 and a limit log fold-change > 1 by using moderated t-statistic with empirical Bayes shrinkage of the SEs. Statistics were corrected for multiple testing using a false-discovery rate approach. A gene-ontology analysis using Panther was next performed on up- and down-regulated genes that are referred in Unigene.
Gene-Expression Analysis.
To confirm microarray data, isolated RNA were reverse-transcribed with the High-Capacity cDNA Archive kit (Applied Biosystems), according to the manufacturer's instructions. The resulting cDNA (equivalent to 5 ng of total RNA) was amplified using the SYBR Green real-time PCR kit and detected on a Stratagene Mx3005P (Agilent Technologies). RT-PCR was performed with the forward and reverse primers (sequences available upon request) that were designed using Primer express software, version 1.0 (Applied Biosystems). On completion of the PCR amplification, a DNA melting curve analysis was carried out to confirm the presence of a single amplicon. Actb was used as an internal reference gene to normalize the transcript levels. Relative mRNA levels (2-ΔΔCt) were determined by comparing (i) the PCR cycle thresholds (Ct) for the gene of interest and Actb (ΔCt) and (ii) ΔCt values for treated and control groups (ΔΔCt).
Statistics.
Data were analyzed using Prism4.0 (GraphPad Software). The nonparametric Kruskal-Wallis test with Dunn's multiple comparison test or the parametric one-way ANOVA test with Bonferroni's multiple comparison test were used. Values represent the mean of normalized data ±SEM. Asterisks signify a significant difference of P < 0.05.
Supplementary Material
Acknowledgments
We thank J. Bolen for a generous supply of mutant mice and K. Jambou for excellent technical assistance in managing the colony of Nlrp6-deficient mice. This work was supported by grants from the Fondation pour la Recherche Médicale, the Association pour la Recherche sur le Cancer, and from the European Union-FEDER (Grants ARCir and CPER).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100981108/-/DCSupplemental.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Loftus EV., Jr Colombel JF, Sandborn WJ. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn's disease in population-based cohorts. Inflamm Bowel Dis. 17:471–478. doi: 10.1002/ibd.21417. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldner MJ, et al. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207:2855–2868. doi: 10.1084/jem.20100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Rutter MD, et al. Cancer surveillance in longstanding ulcerative colitis: Endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–1816. doi: 10.1136/gut.2003.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 9.Grenier JM, et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002;530(1-3):73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- 10.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seno H, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 16.Sedman PC, et al. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–649. doi: 10.1016/0016-5085(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 17.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 18.Andersen CL, et al. Dysregulation of the transcription factors SOX4, CBFB and SMARCC1 correlates with outcome of colorectal cancer. Br J Cancer. 2009;100:511–523. doi: 10.1038/sj.bjc.6604884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SY, et al. CK1epsilon is required for breast cancers dependent on beta-catenin activity. PLoS ONE. 2010;5:e8979. doi: 10.1371/journal.pone.0008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuja TJ, Lin F, Osann KE, Bryant PJ. Somatic mutations and altered expression of the candidate tumor suppressors CSNK1 epsilon, DLG1, and EDD/hHYD in mammary ductal carcinoma. Cancer Res. 2004;64:942–951. doi: 10.1158/0008-5472.can-03-2100. [DOI] [PubMed] [Google Scholar]
- 21.Roberts CW, Orkin SH. The SWI/SNF complex—Chromatin and cancer. Nat Rev Cancer. 2004;4:133–142. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- 22.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr Biol. 2000;10:1611–1614. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- 24.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 25.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 26.Herrera VL, Bagamasbad P, Didishvili T, Decano JL, Ruiz-Opazo N. Overlapping genes in Nalp6/PYPAF5 locus encode two V2-type vasopressin isoreceptors: Angiotensin-vasopressin receptor (AVR) and non-AVR. Physiol Genomics. 2008;34(1):65–77. doi: 10.1152/physiolgenomics.00199.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 29.Van der Flier LG, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317(5834):124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed FE, Vos P. Molecular markers for human colon cancer in stool and blood identified by RT-PCR. Anticancer Res. 2004;24:4127–4134. [PubMed] [Google Scholar]
- 32.Yokoyama T, et al. Methylation of ASC/TMS1, a proapoptotic gene responsible for activating procaspase-1, in human colorectal cancer. Cancer Lett. 2003;202(1):101–108. doi: 10.1016/j.canlet.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Yang YH, et al. Normalization for cDNA microarray data: A robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth GK, Yang YH, Speed T. Statistical issues in cDNA microarray data analysis. Methods Mol Biol. 2003;224:111–136. doi: 10.1385/1-59259-364-X:111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.