Abstract
The immediate early gene NUR77 (also called NGFI-B) is required for T cell antigen receptor-mediated cell death and is induced to very high levels in immature thymocytes and T cell hybridomas undergoing apoptosis. The Akt (PKB) kinase is a key player in transduction of anti-apoptotic and proliferative signals in T cells. Because Nur77 has a putative Akt phosphorylation site at Ser-350, and phosphorylation of this residue is critical for the transactivation activity of Nur77, we investigated whether Akt regulates Nur77. Coimmunoprecipitation experiments showed the detection of Nur77 in Akt immune complexes, suggesting that Nur77 and Akt physically interact. We further show that Akt specifically phosphorylates Ser-350 of the Nur77 protein within its DNA-binding domain in vitro and in vivo in 293 and NIH 3T3 cells. Because phosphorylation of Ser-350 of Nur77 is critical for its function as a transcription factor, we examined the effect of Akt on this function. By using luciferase assay experiments, we showed that phosphorylation of Nur77 by Akt decreased the transcriptional activity of Nur77 by 50–85%. Thus, we show that Akt interacts with Nur77 and inactivates Nur77 by phosphorylation at Ser-350 in a phosphatidylinositol 3-kinase-dependent manner, connecting the phosphatidylinositol 3-kinase-dependent Akt pathway and a nuclear receptor pathway.
The immediate-early gene NUR77 (also known as NGFI-B and TR3) is required for T cell antigen receptor-mediated apoptosis, and its expression is induced in T cell hybridomas and immature thymocytes undergoing cell death (1). Nur77 is an orphan nuclear receptor that activates transcription through a unique NGFI-B response element (NBRE; ref. 2). To date, three members of the orphan nuclear family have been identified, Nur77, Nor1, and Nurr1 (3). All three can bind to and transactivate through the same response element, NBRE (3). It has been shown that Nur77, Nor1, and Nurr1 can form heterodimers that synergistically enhance transcription from NBRE reporters (4). In thymocytes, Nor-1 is induced to very high levels upon T cell antigen receptor stimulation and has similar kinetics to Nur77 (1). Constitutive expression of Nur77 or Nor1 in thymocytes leads to massive apoptosis, suggesting the functional redundancy of these two family members (1). On the other hand, Nurr1 is undetectable in stimulated thymocytes (1). The transcriptional activity of Nur77 is controlled in part by phosphorylation of its Ser-350 residue located within the DNA-binding domain (5). Phosphorylation of Ser-350 has been shown to inhibit DNA binding of Nur77 in vitro (5). In PC12 cells, nerve growth factor-induced phosphorylation of Ser-350 resulted in a 60% decrease of Nur77 transactivating activity (6). The DNA-binding domains of Nor1 and Nurr1 show >80% identity to the DNA-binding domain of Nur77, including 100% identity of the residues surrounding Ser-350 of Nur77.
The protein kinase Akt (PKB) is a critical molecule in transduction of antiapoptotic and proliferative signals in T cells (7, 8). Activation of Akt by various growth and survival factors involves a phosphatidylinositol 3-kinase (PI-3K)-dependent membrane translocation step and a PDK1-mediated phosphorylation step at Thr-308 and Ser-473 (reviewed in ref. 7). Recent studies showed that Akt phosphorylates and regulates a variety of key targets important for cell survival and proliferation like Bad, Raf, and IKKα (9–11). Recently, we reported that Akt interacts with the Tcl1 oncogene product, which is activated in T cell leukemias (12). This interaction results in increased Akt kinase activity and in its nuclear translocation (12). To characterize this pathway further, we decided to identify nuclear targets of Akt specific for lymphoid cells. Because the residues surrounding Ser-350 of Nur77 (RGRLPS) clearly resemble the Akt phosphorylation site (RXRXXS; ref. 7), we considered Nur77 a good candidate for such targets. Thus, we investigated the possible phosphorylation and regulation of Nur77 by Akt.
Materials and Methods
DNA Constructs, Transfection, Glutathione S-transferase (GST) PullDown, Immunoprecipitation, and Western Blotting.
Full-length NUR77 cDNA was cloned into a pEGFP-N1 vector (CLONTECH; GFP, green fluorescent protein) by using standard protocols. The AKT-hemagglutinin (HA) construct has been described (12). NIH 3T3 and 293 cells were grown in RPMI medium 1640 or modified Eagle's medium. Transfections other than luciferase assay experiments (see below), cell lysate preparations, immunoprecipitations, and Western Blot analysis were carried out as described (12). Nuclear and cytoplasmic extracts were prepared as described (13). For the work shown in Fig. 3B, NIH 3T3 cells were starved overnight, and the cells were treated with platelet-derived growth factor (PDGF; 100 ng/ml) for 1 h or with Wortmannin (200 nM) for 30 min followed by PDGF for 1 h and lysed. Antibodies used were anti-HA, clone 11 (Babco, Richmond, CA); anti-GFP (Roche Molecular Biochemicals); anti-Akt, anti-phospho-Ser-473-Akt, and anti-CREB (New England Biolabs); Anti-Crk (Transduction Laboratories, Lexington, KY); anti-Tcl1 (12) and anti-phospho-Nur77 (6). GST pulldown was performed as described (12). GST-Akt, GST-PH-domain, and GST alone were purchased from Upstate Biotechnology (Lake Placid, NY).
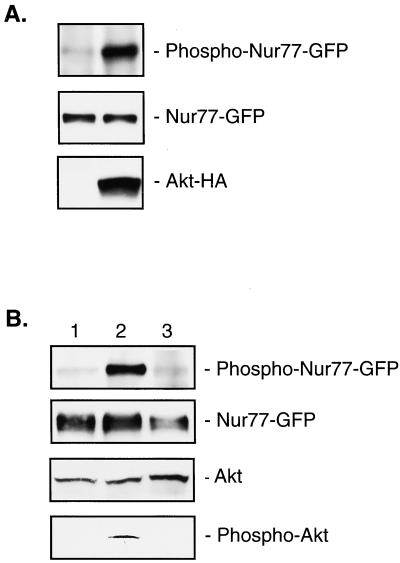
Figure 3.
Akt phosphorylates Nur77 in vivo. (A) 293 cells were transfected with NUR77-GFP and empty vector (left lane), or NUR77-GFP and AKT-HA (right lane). Nur77 was immunoprecipitated with anti-GFP antibody (Top and Middle). Western blotting was carried out with anti-phospho(S350)-Nur77 (Top), anti-GFP (Middle) or anti-HA (Bottom) antibodies. (B) NIH 3T3 cells were transfected with NUR77-GFP, starved overnight, untreated (lane 1), treated with PDGF (100 ng/ml) for 1 h (lane 2), or treated with Wortmannin (200 nM) for 30 min followed by treatment with PDGF for 1 h (lane 3). Nur77 was immunoprecipitated with anti-GFP antibody (Top and Upper Middle). Western blotting was carried out with anti-phospho(S350)-Nur77 (Top), anti-GFP (Upper Middle), anti-Akt (Lower Middle), or anti-phospho-Akt (Bottom) antibodies.
In Vitro Phosphorylation.
The DNA-binding domain of Nur77 (amino acids 264–365) was cloned into a pGEX-4T-1 vector (Amersham Pharmacia). The GST-fusion protein was isolated according to the manufacturer's recommendations. Activated Akt was purchased from Upstate Biotechnology. In vitro phosphorylation was carried out by using an Akt kinase assay kit (New England Biolabs) with the following modifications: 200 ng of activated Akt, 20 μCi (1 Ci = 37 GBg) of [γ-32P]ATP, and 2 μM ATP were used in each reaction.
Luciferase Assay.
The minimal thymidine kinase promoter (base pairs −81 to + 51) in the proximity of four NBRE elements (TTTTAAAAGGTCATGC) was cloned into a pGL3 vector (Promega). Transfection of 293 cells was carried out with the following constructs: cytomegalovirus–β-galactosidase (Promega, 1 μg); reporter construct (0.5 μg); NUR77-GFP (5 ng); AKT-HA or empty vector (0.5 μg). Luciferase activity was measured 48 h after transfection, normalized according to β-galactosidase activity, and adjusted to the effect of AKT-HA on the basal promoter. All experiments were carried out in duplicate and repeated three times with consistent results.
Results
Nur77 Physically Interacts with Akt.
We first examined whether Nur77 and Akt would physically interact. We cotransfected 293 embryonic kidney cells with AKT tagged with the HA epitope and NUR77 tagged with the GFP epitope; coimmunoprecipitation experiments with anti-HA and anti-GFP antibodies were carried out. Fig. 1A, lane 1 shows the detection of Nur77 in Akt immune complexes precipitated with the anti-HA antibody. In the complementary immunoprecipitation experiment, Akt coimmunoprecipitated with Nur77 (as demonstrated by Western blotting) with an anti-HA antibody (Fig. 1A, Lower, lane 3), indicating that Nur77 does indeed physically interact with Akt.
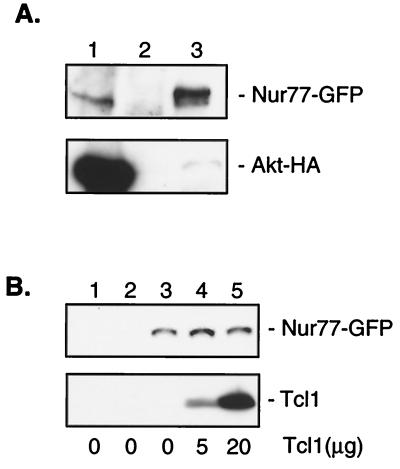
Figure 1.
Nur77 interacts with Akt. (A) 293 cells were cotransfected with NUR-77-GFP and AKT-HA. Immunoprecipitations were carried out with anti-HA antibody (lane 1), mouse IgG (lane 2), or anti-GFP antibody (lane 3). Western blotting was performed with anti-GFP antibody (Upper) or anti-HA antibody (Lower). (B) 293 cells were transfected with NUR-77-GFP. GST pulldowns were carried out with 5 μg of GST alone (lane 1), GST-PH-domain (lane 2), or GST-Akt (lanes 3–5). The indicated amount of recombinant Tcl1 was added to each tube. Western blotting was performed with anti-GFP and anti-Tcl1 antibody.
We have shown elsewhere (12) that Akt interacts with Tcl1 through Akt's PH domain. Both Nur77 and Tcl1 play important roles in normal and neoplastic T cell function (1, 12, 14). In fact, as mentioned above, Nur77 is involved in T cell apoptosis, and the activation of the TCL1 gene causes T cell leukemia (14). Therefore, we hypothesized that Nur77 and Tcl1 might work in the same pathway. To evaluate this possibility, we examined whether both Nur77 and Tcl1 interact with the PH domain of Akt and whether Tcl1 affects the interaction of Nur77 and Akt. For this purpose, we transfected 293 cells with NUR77-GFP and carried out GST pulldown experiments with GST alone, GST-Akt-PH domain, and full-length GST-Akt as bait, with and without the addition of recombinant Tcl1. Fig. 1A shows that, unlike Tcl1, Nur77 did not bind to the PH-domain of Akt (Fig. 1B, lane 2) but interacted only with full-length Akt (Fig. 1B, lanes 3–5). Increasing amounts of Tcl1 bound to Akt did not affect the quantity of Nur77-GFP protein bound to Akt (Fig. 1B, lanes 3–5). This result suggests that Tcl1 does not synergize or interfere with the interaction between Nur77 and Akt.
Akt Phosphorylates Nur77 in Vitro and in Vivo.
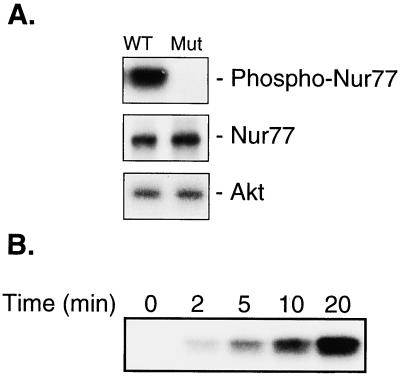
Because Nur77 contains a putative Akt phosphorylation site, we investigated whether Nur77 is phosphorylated by Akt at Ser-350. In phosphorylation experiments in vitro, we used a GST-linked Nur77-DNA-binding domain (amino acids 264–365) containing the wild-type Akt binding site (RGRLPS), or a mutated Akt binding site (WGRLPS). Fig. 2A shows that Akt specifically phosphorylates Ser-350 of the wild-type Nur77 DNA-binding domain (lane 1) but not the DNA-binding domain containing the mutated site (lane 2). Fig. 2B shows the phosphorylation of Nur77 DNA-binding domain by Akt in a time-dependent manner.
Figure 2.
Akt phosphorylates Nur77 in vitro. (A) Phosphorylation of wild-type (WT) Nur77 DNA-binding domain but not mutant domain by Akt. Unphosphorylated Nur77 and Akt were detected by Western blotting with anti-GST and anti-Akt antibody, respectively. (B) Time course of phosphorylation of Nur77 by Akt.
Because Akt phosphorylates Nur77 in vitro, we proceeded to determine whether this phosphorylation also occurs in vivo. We transfected 293 cells with NUR77-GFP and AKT, or with NUR77-GFP and the empty vector. The amount of Nur77 was detected by using anti-GFP antibody, and the amount of phospho-Ser-350-Nur77 was detected by using an anti-Phospho-Ser-350 antibody (Fig. 3A). In the presence of overexpressed Akt, the amount of phosphorylated Nur77 increased >10-fold. In the similar experiment, overexpression of inactive K179 M Akt mutant (7) did not show any effect on phosphorylation of Nur77 (not shown). This result suggests that Akt phosphorylates Nur77 in vivo.
The activation of Akt by insulin and various growth and survival factors such as PDGF involves a PI-3K-dependent membrane translocation step that is caused by the binding of the PH domain to D3 phosphoinositides and a PDK-1-mediated phosphorylation step at Thr-308 and Ser-473 of Akt (7, 15). Wortmannin, a PI-3K inhibitor, completely inhibits the activation of Akt (15, 16). The activation of Akt by treatment with PDGF in NIH 3T3 cells is a model system routinely used to assay phosphorylation of various Akt targets (7, 12). Thus, we proceeded to determine whether phosphorylation of Nur77 by Akt is PI-3K-dependent. NIH 3T3 cells were transfected with NUR77-GFP and serum-starved overnight. Cells were then treated with PDGF or Wortmannin and PDGF; the amount of phosphorylated Nur77 was detected with anti-Nur77(P-Ser-350) antibody. Fig. 3B shows that PDGF treatment significantly increases the phosphorylation of Nur77 by Akt and that treatment with Wortmannin completely inhibits this effect. As expected, Akt was activated (i.e., phosphorylated at Ser-473) by PDGF, and this effect was inhibited by Wortmannin (Fig. 3B). Thus, Nur77 is phosphorylated by endogenous Akt in NIH 3T3 cells at Ser-350 in a PI-3K-dependent manner.
The intracellular localization of Akt is primarily cytoplasmic (12, 17). Nur77, on the other hand, may be localized in the cytoplasm or in the nucleus in different cells (2, 18). A very recent report (18) demonstrated that the intracellular localization of Nur77 is regulated in part by the phosphorylation of Ser-105 in the N-terminal part of the molecule. Phosphorylation of this residue is induced by nerve growth factor through the mitogen-activated protein kinase pathway, and was shown to cause a change in conformation that resulted in the exposure of the nuclear export signal on the surface of the protein and consequent transient export of Nur77 to the cytoplasm (18). Therefore, the export of Nur77 to the cytoplasm could result in Nur77 being available for phosphorylation by cytoplasmic Akt.
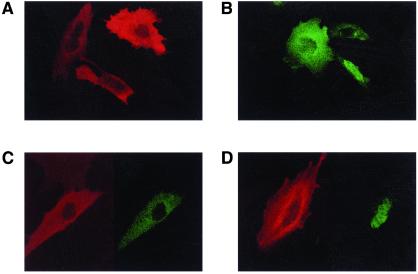
To confirm that phosphorylation of Nur77 by Akt occurs in the cytoplasm, we first examined the intracellular localization of Nur77 and Akt in the same cells. We cotransfected NIH 3T3 cells with NUR77-GFP and AKT-HA and detected the location of both proteins by immunofluorescence. Fig. 4 A and B shows that Nur77 is localized either in the nucleus or in the cytoplasm, whereas Akt is located only in the cytoplasm. The expression of Nur77 and Akt in the same cells altered neither the cytoplasmic localization of Akt nor the cytoplasmic or nuclear expression of Nur77 (Fig. 4 C and D). In some cells, Nur77 was found in both the nucleus and the cytoplasm (not shown).
Figure 4.
Intracellular localization of Nur77-GFP and Akt-HA. NIH 3T3 cells were transfected with NUR77-GFP (green) and/or AKT-HA (red). (A and B) Intracellular localization of Akt-HA alone and Nur77-GFP alone (respectively). (C and D) Intracellular localization of Akt-HA and Nur77-GFP (respectively) cotransfected to the same cells.
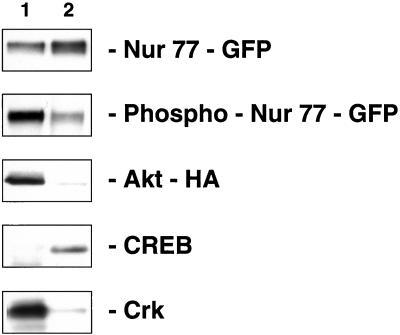
To determine the intracellular distribution of phosphorylated Nur77, we transfected 293 cells with NUR77-GFP and AKT-HA and isolated nuclear and cytoplasmic protein extracts of these transfected cells. Fig. 5 shows that, although Nur77 is distributed approximately equally between the nucleus and the cytoplasm, phosphorylated Nur77 is located mostly in the cytoplasm, suggesting that phosphorylation of Nur77 by Akt occurs in the cytoplasm. In these experiments, as expected, Akt was found almost exclusively in the cytoplasm, and control proteins CREB (19) and Crk (20) were found in the nucleus and the cytoplasm, respectively (Fig. 5).
Figure 5.
Intracellular distribution of Nur77. Transfection of 293 cells was carried out with NUR77-GFP, and nuclear and cytoplasmic lysates were prepared. The amount of cytoplasmic and nuclear extracts (lanes 1 and 2) represents the same number of cells. Lane 1, cytoplasmic extract; lane 2, nuclear extract. Western blotting was performed with anti-GFP, anti-phospho-Nur77, anti-HA, anti-CREB, or anti-Crk antibodies as indicated.
Akt Inhibits the Transcriptional Activity of Nur77.
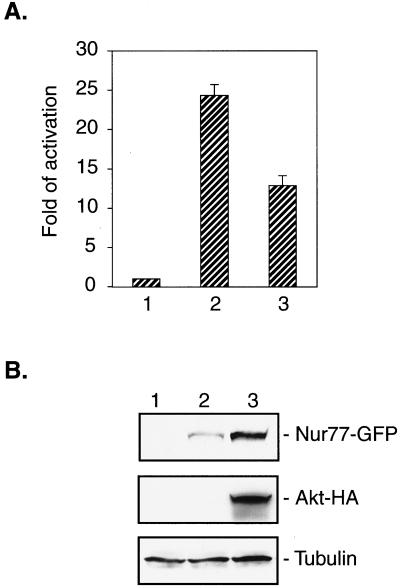
Previous reports showed that the phosphorylation of Ser-350 of Nur77 induced by nerve growth factor in PC12 cells decreased the transcriptional activity of Nur77 (6) and that Nur77 cannot bind DNA in vitro on phosphorylation of Ser-350 (5). Therefore, we investigated whether phosphorylation of Nur77 by Akt would affect the transactivating activity of Nur77. To this purpose, we carried out a reporter gene assay with the luciferase reporter gene under the control of the minimal thymidine kinase promoter in the proximity of four NBRE elements. We cotransfected 293 cells with the reporter construct, NUR77-GFP, and AKT or empty vector instead of AKT (Fig. 6). The resulting luciferase activity was measured and adjusted by using an internal β-galactosidase standard. Fig. 6A shows that phosphorylation of Nur77 by Akt decreased the transcriptional activity of Nur77 by ≈50%. Although an equal amount of the Nur77 construct was used for each transfection, a much higher level of Nur77 was expressed when NUR77 was cotransfected with AKT compared to cotransfection with the vector (Fig. 6B). Considering this difference in expression of Nur77, the actual decrease of transcriptional activity caused by phosphorylation by Akt was as high as 85%. These experiments were also carried out with the NUR77-HA construct, with similar results (not shown).
Figure 6.
Phosphorylation by Akt inhibits function of Nur77 as transcriptional activator. Transfection of 293 cells was carried out as described in Materials and Methods. (A) Bar 1, the activity of the basal promoter in 293 cells was set as 1; bar 2, the promoter activity in 293 cells transfected with NUR77-GFP and empty vector; bar 3, the promoter activity in 293 cells transfected with NUR77-GFP and AKT-HA. (B) The expression of Nur77-GFP (Top), Akt-HA (Middle), and tubulin (Bottom) in the cell lysates used in A.
Discussion
In this report, we demonstrate that Nur77 is a target of Akt protein kinase. Our results show that Akt interacts with Nur77, phosphorylates Nur77 in vitro and in vivo, and inactivates Nur77 in its function as a transcription factor in a PI-3K-dependent manner. Our previous report (12) showed that Akt and Tcl1 function in the same pathway. Because activation of TCL1 is a causal event in the development of T cell leukemia (14), and also because Tcl1 affects Akt enzymatic activity, it is logical to speculate that specific targets of Akt in T cells do exist. Nur77 is a good candidate for such targets for the following two reasons. First, Nur77 is expressed specifically in T cells and induced during T cell apoptosis (1). Second, Akt inactivates Nur77 in its function, corresponding to the fact that Akt is a prosurvival factor (7). In our experiments, we used overexpression of both proteins to determine that phosphorylation of Nur77 by Akt occurs in the cytoplasm. On the other hand, it is possible that, in cells of hematopoietic origin where Tcl1 and Nur77 are expressed at lower levels and where Tcl1 translocates Akt to the nucleus, the phosphorylation of Nur77 by Akt occurs in the nucleus.
In luciferase reporter experiments, the decrease of Nur77 transcriptional activity by Akt occurs, by definition, in the nucleus. However, downstream, when Nur77 and Akt were overexpressed in the same cells, the majority of phosphorylated Nur77 is located in the cytoplasm (Fig. 5). There are several possible explanations of this observation. First, excess of Nur77 present in transfection experiments described in Fig. 5 could influence the cellular localization of phospho-Nur77. Second, and more importantly, it is likely that there are downstream biochemical steps, such as additional phosphorylation/dephosphorylation or dimerization events (4, 18), which could play a role in the observed Akt-dependent inhibition of Nur77 transcriptional activity and which may affect its intracellular localization.
Nur77 and the other two members of the gene family, Nor1 and Nurr1, have highly homologous DNA-binding domains (3, 4) that include the Akt phosphorylation site. Therefore, it is likely that, similarly to Nur77, Nor1 and Nurr1 can also be phosphorylated and inactivated by Akt. Altogether, our data demonstrate that Akt interacts with Nur77, phosphorylates it at Ser-350 in vitro and in vivo, and inactivates Nur77 in its function as transcription factor. Thus, Nur77 is a target molecule that transmits antiapoptotic and prosurvival signals in the PI-3K dependent Akt pathway. The role of PI-3K and Akt in signaling through the T cell antigen receptor is well known. Our finding that Nur77 is inactivated by Akt in a PI-3K-dependent manner raises the possibility that Akt and PI-3K are also involved in negative selection in the immune system.
Acknowledgments
We thank Philip Tsichlis for helpful discussion. This work was supported by National Institutes of Health Grant CA81534 (to C.M.C.), a Special Fellowship of the Leukemia and Lymphoma Society (to Y.P.), and a Kimmel Scholar Award (to F.B.).
Abbreviations
- TCR
T cell antigen receptor
- PI-3K
phosphatidylinositol 3-kinase
- GFP
green fluorescent protein
- HA
hemagglutinin
- GST
glutathione S-transferase
- PDGF
platelet-derived growth factor
References
- 1.Cheng L E-C, Chan F K-M, Cado D, Winoto A. EMBO J. 1997;16:1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis I J, Hazel T G, Chen R-H, Blenis J, Lau L F. Mol Endocrinol. 1993;7:953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen R F, Granas K, Johnsen H, Rolseth V, Sterri S. J Mol Neurosci. 1995;6:249–255. doi: 10.1007/BF02736784. [DOI] [PubMed] [Google Scholar]
- 4.Maira M, Martens C, Philips A, Drouin J. Mol Cell Biol. 1999;19:7549–7557. doi: 10.1128/mcb.19.11.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirata Y, Kiuchi K, Chen H C, Milbrandt J, Guroff G. J Biol Chem. 1993;268:24808–24812. [PubMed] [Google Scholar]
- 6.Katagiri Y, Hirata Y, Milbrandt J, Guroff G. J Biol Chem. 1997;272:31278–31284. doi: 10.1074/jbc.272.50.31278. [DOI] [PubMed] [Google Scholar]
- 7.Chan T O, Rittenhouse S E, Tsichlis P N. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok C L, Gil-Gomez G, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady H J. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann S, Moelling K. Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 11.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. Nature (London) 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 12.Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, Russo G, Tsichlis P, Croce C M. Proc Natl Acad Sci USA. 2000;97:3028–3033. doi: 10.1073/pnas.040557697. . (First Published March 14, 2000; 10.1073/pnas.040557697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber E, Matthias P, Muller M M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virgilio L, Lazzeri C, Bichi R, Nibu K, Narducci M G, Russo G, Rothstein J L, Croce C M. Proc Natl Acad Sci USA. 1998;95:3885–3889. doi: 10.1073/pnas.95.7.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P N. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 16.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N N, Franke T F, Bellacosa A, Datta K, Gonzalez-Portal M E, Taguchi T, Testa J R, Tsichlis P N. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 18.Katagiri Y, Takeda K, Yu Z X, Ferrans V J, Ozato K, Guroff G. Nat Cell Biol. 2000;2:435–440. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K K, Gonzalez G A, Biggs W H, Montminy M R. Nature (London) 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- 20.Jin S, Zhai B, Qiu Z, Wu J, Lane M D, Liao K. J Biol Chem. 2000;275:34344–34352. doi: 10.1074/jbc.M004927200. [DOI] [PubMed] [Google Scholar]