Abstract
Amphibians highlight the global biodiversity crisis because ∼40% of all amphibian species are currently in decline. Species have disappeared even in protected habitats (e.g., the enigmatic extinction of the golden toad, Bufo periglenes, from Costa Rica). The emergence of a fungal pathogen, Batrachochytrium dendrobatidis (Bd), has been implicated in a number of declines that have occurred in the last decade, but few studies have been able to test retroactively whether Bd emergence was linked to earlier declines and extinctions. We describe a noninvasive PCR sampling technique that detects Bd in formalin-preserved museum specimens. We detected Bd by PCR in 83–90% (n = 38) of samples that were identified as positive by histology. We examined specimens collected before, during, and after major amphibian decline events at established study sites in southern Mexico, Guatemala, and Costa Rica. A pattern of Bd emergence coincident with decline at these localities is revealed—the absence of Bd over multiple years at all localities followed by the concurrent emergence of Bd in various species at each locality during a period of population decline. The geographical and chronological emergence of Bd at these localities also indicates a southbound spread from southern Mexico in the early 1970s to western Guatemala in the 1980s/1990s and to Monteverde, Costa Rica by 1987. We find evidence of a historical “Bd epidemic wave” that began in Mexico and subsequently spread to Central America. We describe a technique that can be used to screen museum specimens from other amphibian decline sites around the world.
Keywords: chytridiomycosis, emerging infectious disease, epizootic
The global biodiversity crisis, which predicts the sixth mass extinction in Earth's history (1), is often illustrated with examples from class Amphibia because ∼40% of all amphibian species are currently in decline (2). Habitat destruction, overexploitation for food and the pet trade, pollution and climate change all have been implicated, but an emerging infectious fungal disease, chytridiomycosis, has raised alarm because it has spanned taxonomic and geographical barriers, reaching areas of protected habitat and further compounding the effects on biodiversity loss attributed to other factors (3). This disease is caused by the chytridiomycete fungus Batrachochytrium dendrobatidis (Bd). Bd has a flagellated infective life stage called the zoospore that imbeds itself into the keratinized skin of amphibians causing hyperkeratosis, loss of skin function, osmoregulatory failure, and death (4–6). The emergence of Bd, described in 1999, has been definitively tied with collapse of amphibian populations in Australia (5), Panama (7), California (8), and Peru (9) and has been implicated in many declines that occurred decades ago (10, 11). Chytridiomycosis is unusual because multiple host species in at least one region have disappeared (7), apparently before density-dependent factors could slow the spread of disease (8, 12). Establishing the presence of Bd in museum specimens from vanished populations could be the key to uncovering the historical and geographical spread of this pathogen and would provide objective evidence of Bd emergence and subsequent Bd-driven amphibian decline. In this study, we use noninvasive sampling and molecular techniques to detect Bd in formalin-preserved specimens to investigate the role of Bd in two well-studied cases of enigmatic amphibian decline in Mesoamerica (i): the decline and disappearance of anurans from Costa Rica's Monteverde Reserve in the late 1980s (13, 14), and (ii) the decline and disappearance of plethodontid salamanders from the mountains of southern Mexico and western Guatemala in the 1970s and 1980s (15).
The sudden extinction of the golden toad (Bufo periglenes) and harlequin frog (Atelopus varius) from Costa Rica's Monteverde Reserve in the late 1980s (13, 14) are among the earliest and best-documented cases of enigmatic declines that have come to characterize the global amphibian crisis. The subsequent disappearance of 40% (20/49 species) of anurans from Monteverde's cloud forest (16) places the Monteverde declines among the most extreme cases of documented biodiversity loss in amphibian faunas. Various hypotheses have arisen regarding the cause of this decline, including the arrival of Bd to naïve amphibian populations in Monteverde as part of a southward-moving Bd wave (7) and climate-linked Bd emergence (13, 16, 17), with implications for worldwide Bd emergence. Remarkably, despite the central role that Bd has been hypothesized to play in these declines, no direct evidence has been reported of Bd emergence in Monteverde coincident with declines.
The declines of bolitoglossine salamanders (family: Plethodontidae) from the neotropics of southern Mexico and Guatemala (15, 17) are among the few records of enigmatic decline occurring in salamanders. As in Monteverde and in other cases of enigmatic decline from around the world, these salamander populations seem to have disappeared from montane environments despite the availability of suitable, protected habitat (11, 15). Although preliminary data from Rovito et al. (15) show that Bd currently exists in San Marcos, Guatemala, where plethodontid salamanders are known to have declined, interpretation of these data is difficult because many species have been extirpated.
In this study, we introduce a reliable noninvasive molecular technique that enables us to detect Bd in formalin-preserved amphibian specimens. Furthermore, we use this technique to examine amphibian specimens collected in areas of documented decline in Mexico, Guatemala, and Costa Rica. In addition, we present laboratory studies that investigate the susceptibility of two neotropical plethodontid species to Bd and demonstrate that Bd can cause mortality in these species.
Results
Noninvasive sampling methods (swab/Qiagen and swab/Prepman) of formalin-preserved amphibian specimens collected and preserved at the Museum of Vertebrate Zoology (MVZ) as far back as 1972 found Bd in 90 and 83% of samples, respectively, that were identified as positive by histology (Table 1). We measured the sensitivity of the recovery of DNA between replicate runs on real-time PCR (singlicate, duplicate, triplicate, and quadruplicate) and found that resulting Bd prevalences were 61.8, 78.1, 84.2, and 89.5% accurate in relation to histology, respectively (Table S1). Bd prevalence resulting from a single sample qPCR run was found to be significantly different from histology results (χ2 test, P = 0.00987), but prevalence resulting from duplicate or higher qPCR runs did not differ significantly from histology results (Table S1). We also found that estimated infection intensities (measured in zoospore equivalents) detected in museum specimens were not consistent with actual infection intensities (taken before animals were killed and fixed in formalin), and thus we only relied on positive versus negative results instead of reporting actual infection intensities from qPCR results (Table S2).
Table 1.
Comparison of recovery rates between histology and three methods using noninvasive sampling and molecular tools to detect Bd in formalin-preserved specimens (Genus: Batrachoseps)
| Year of collection | Total sampled | Histology, no. positives | Swab/Qiagen, no. positives | Swab/Prepman no. positives | Brush/Qiagen no. positives |
| 1971 | 7 | 0 | 3 | 0 | 1 |
| 1973 | 15 | 15 | 13 | 15 | 9 |
| 1974 | 3 | 3 | 3 | 2 | 3 |
| 1993 | 7 | 7 | 3 | 4 | 7 |
| 1995 | 3 | 1 | 1 | 0 | 0 |
| 2007 | 3 | 3 | 3 | 3 | 2 |
| Total | 38 | 29 | 26 | 24 | 22 |
| Recovery rate, no. positives/histology positives | 100% | 89.7% | 82.8% | 75.9% | |
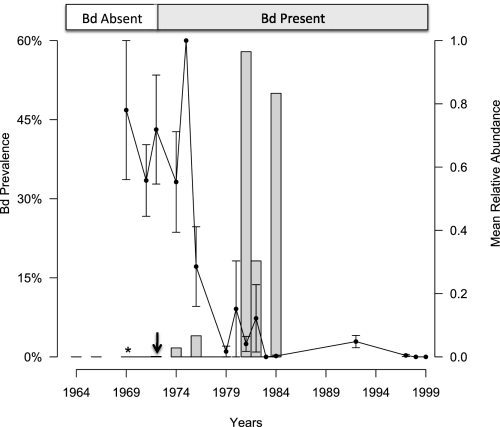
Using the swab/Prepman and swab/Qiagen techniques, we surveyed amphibian specimens collected in five neotropical amphibian communities from Mexico, Guatemala, and Costa Rica where amphibian declines occurred. A strong pattern of Bd emergence was found for all countries—Bd is absent over multiple years and is followed by the concurrent emergence in several species from each locality (Tables 2 and 3 and Tables S3 and S4). Furthermore, we found Bd emergence to coincide directly during the year of decline (1987) in Monteverde, Costa Rica (Table 3) and the absence of Bd in healthy populations (with exception in Cerro San Felipe, Mexico where specimens were not available for sampling before the earliest date of detection, 1974) followed by the emergence and presence of Bd in declining and impacted populations in Mexico (Fig. 1) and Guatemala.
Table 2.
Bd prevalence detected in museum specimens collected from Guatemala
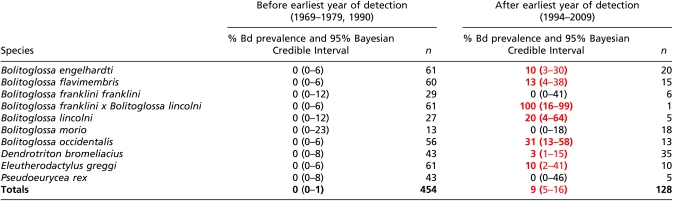 |
Red and bold font indicate positive Bd prevalence.
Table 3.
Bd prevalence detected from amphibian specimens pre-decline (1967–1984) and during the year of decline (1987) in Monteverde, Costa Rica
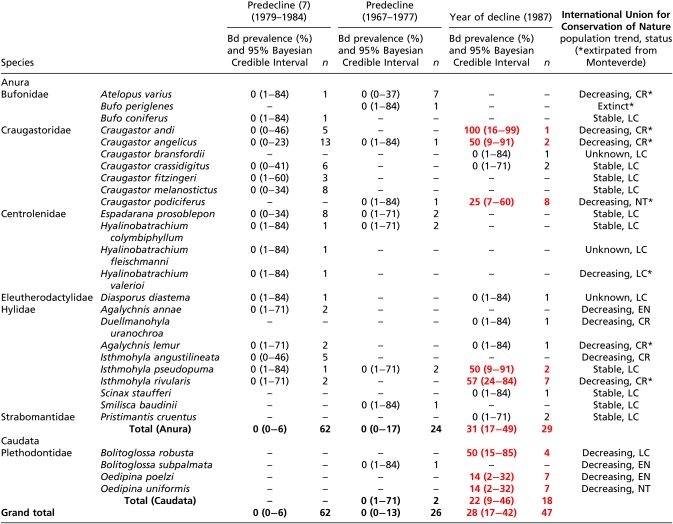 |
Positive Bd prevalence values are indicated by bold and red text. International Union for Conservation of Nature status is denoted as Least Concern (LC), Near Threatened (NT), Endangered (EN), or Critically Endangered (CR).
Fig. 1.
Timeline of mean relative salamander abundance (line) and mean Bd prevalence (bars) for all sites and species in Mexico. Asterisk indicates zero Bd prevalence for which sample sizes were large and probability of a false negative was low (<10%). Arrow indicates earliest year of Bd detection in Mexico (1972). The absence of Bd occurs during high abundance years and is followed by Bd emergence and increasing Bd prevalence that coincides with the marked decline of salamanders (15) at all sites in Mexico.
We further examined the strength of detection if Bd had been present at a prevalence of 5% (probability of a false negative). The emergence of Bd in Mexico, Guatemala, and Costa Rica, which is predicated on the verification of its absence before the earliest date of detection, was found to have low probability of a false negative for all countries: Mexico (1964–1971), 0.019; Guatemala (1969–1979), 8.1 × 10−11; and Costa Rica (1967–1984), 0.01. In Mexico, Bd was eventually detected in all species sampled except Pseudoeurycea unguidentis and Parvimolge townsendi, despite relatively large sample sizes for those two species (probability of a false negative: 0.081 and 0.12, respectively). In Guatemala, Bd was found in 7 out of 10 species sampled, with no Bd detected in Bolitoglossa franklini franklini, Pseudoeurycea rex, and Bolitoglossa morio; however, collections after 1994 (earliest date of detection in Guatemala) for all three species were small (probability of a false negative: 0.95, 0.77, and 0.40, respectively). From 20 anuran species that have disappeared from Monteverde Reserve, we tested 4 of these species during the year of decline and found 3 to be positive with Bd: Craugastor andi, Craugastor angelicus, and Isthmohyla rivularis. Three other species reported as disappeared were found to be Bd negative predecline; however, these specimens were not collected in 1987 (B. periglenes, A. varius, and Duellmanohyla uranochroa) and thus were not sampled during decline. Agalychnis lemur is reported to have disappeared from Monteverde, but is only represented by one specimen that did not test positive for Bd.
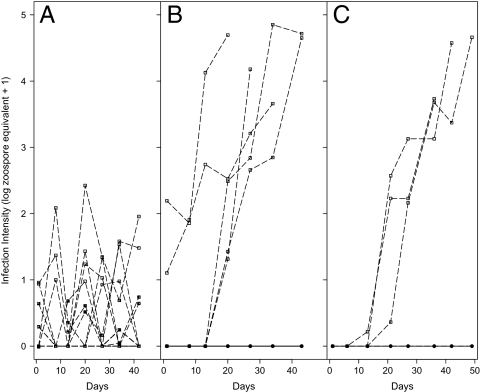
In the laboratory, we observed the effects of Bd infection in two species of wild-caught salamanders and one frog species from Mexico. All 10 frogs, Plectrohyla matudai, and 5 of 10 Pseudoeurycea leprosa contained Bd infections when collected in the field; all 6 Bolitoglossa rufescens were uninfected from the field and 3 were experimentally infected with Bd in the laboratory. Both species of salamanders, P. leprosa and B. rufescens, revealed high susceptibility to Bd infection, but the frog, P. matudai, was resistant to Bd infection (Fig. 2). All infected salamanders (five P. leprosa and three B. rufescens) rapidly increased in infection intensity and suffered mortality when average level of infection intensity of ∼10,000 ZE (zoospore equivalents) was reached (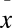 ± 1 SE) = 37,841 ± 7,111 zoospore equivalents × swab−1. All uninfected salamanders (negative controls; 10 P. leprosa and 3 B. rufescens) remained uninfected and healthy during the duration of the infection trial. In contrast to infected salamanders, all infected frogs (n = 10) maintained Bd infection at levels well below the lethal zone (<1,000 ZE) and also remained healthy for the duration of the trial.
± 1 SE) = 37,841 ± 7,111 zoospore equivalents × swab−1. All uninfected salamanders (negative controls; 10 P. leprosa and 3 B. rufescens) remained uninfected and healthy during the duration of the infection trial. In contrast to infected salamanders, all infected frogs (n = 10) maintained Bd infection at levels well below the lethal zone (<1,000 ZE) and also remained healthy for the duration of the trial.
Fig. 2.
Results from laboratory trials monitoring Bd infection for one neotropical frog, (A) Plectrohyla matudai, and two neotropical plethodontid salamanders, (B) Pseudoeurycea leprosa and (C) Bolitoglossa rufescens. Dotted lines with square points represent Bd-infected individuals and solid lines with circle points represent Bd-uninfected individuals. All infected salamanders (five P. leprosa and three B. rufescens) increased infection intensity rapidly over time and suffered mortality. The average infection intensity of animals that died was (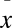 ± 1 SE) = 37,841 ± 7,111 zoospore equivalents × swab−1. Uninfected salamanders (10 P. leprosa and three B. rufescens) remained healthy and Bd negative over time. For P. matudai, all individuals (n = 10) were infected but persisted with low levels of infection.
± 1 SE) = 37,841 ± 7,111 zoospore equivalents × swab−1. Uninfected salamanders (10 P. leprosa and three B. rufescens) remained healthy and Bd negative over time. For P. matudai, all individuals (n = 10) were infected but persisted with low levels of infection.
Discussion
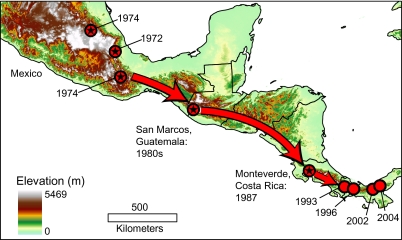
Our results indicate that the chytrid pathogen emergence in plethodontid salamanders in Mexico and Guatemala, and in salamanders and frogs at Monteverde, Costa Rica, is coincident with the amphibian community collapse and extinctions that occurred at these localities, providing direct evidence for the hypothesis that Bd played a major role in these declines. Furthermore, our data corroborate a pattern of temporal and spatial spread of Bd previously posited by Lips et al. (7, 18), which we now are able to extend much further northwest, from southern Mexico in the early 1970s, reaching Guatemala by the 1980s/1990s, and spreading to Monteverde, Costa Rica by 1987 (Fig. 3). The absence of Bd in all countries before first detection, despite multiyear sampling, runs counter to the idea that the pathogen was present in these environments and emerged in response to climatic changes (16).
Fig. 3.
Map of the spatial-temporal spread of Bd southward from Mexico (1970s) to Guatemala (1980s) to Monteverde, Costa Rica (1987), and further through lower Central America (1993–2004). Red circles with stars represent Bd emergence points from our data and plain red circles represent previously published points of Bd emergence in lower Central America from Lips et al. (7).
The pattern elucidated here of the arrival and emergence of the chytrid pathogen in concert with amphibian declines was best documented in two other cases of Bd-driven amphibian decline (7, 8). In these studies, the absence of Bd in healthy populations was well documented through multiyear sampling periods followed by documentation of mass mortality and population decline directly caused by a Bd epizootic outbreak. The spatial-temporal spread of Bd we document from Mexico to Costa Rica is further supported by previously published accounts of Bd-linked amphibian decline in Mexico and Costa Rica. In southern Mexico, the decline of 19–48% of anuran fauna is documented to have occurred in the mid- to late-1970s and early 1980s, and these declines were posited to be Bd driven because Bd was found during the most recent surveys (18). These results are consistent with the decline of plethodontids in southern Mexico (15), which was previously unexplained, and the emergence of Bd in the early 1970s that we report in this study. Furthermore, histological examination of amphibian specimens documented Bd in amphibians collected in 1986 from a location about 75 km east-southeast of Monteverde; Bd thus had emerged close to Monteverde just 1 y before we find Bd in amphibians at Monteverde when the declines there began (19). We detected the earliest record of Bd in Monteverde from a small but extremely valuable collection of specimens collected from this reserve in 1987 and obtained only through extraordinary permission (specimen collection from Monteverde was not permitted between 1984 and 1987); we did not detect Bd in samples collected between 1967 and 1977 in our study, and additional histological sampling by Lips et al. (7) also did not detect Bd in specimens collected between 1979 and 1984. The exact timing of Bd emergence in Guatemala during the 1980s is not possible because studies in the relevant region were suspended because of social disruptions that made field studies hazardous during that decade. Given the lack of Bd in earlier years and the presence in later years and the pattern of Bd emergence along with the timing of amphibian decline in Guatemala, we infer that epizootic spread from Mexico to Costa Rica would place the emergence of Bd in Guatemala in the 1980s.
Previous studies investigating Bd in archival specimens used histological methods (7, 20), but this technique is time consuming and destructive to specimens. Because of this, a previous study used a noninvasive PCR assay to detect Bd in six formalin-preserved specimens but was unsuccessful (40). We were able to recover Bd DNA at a relatively high rate of success (83–90%) and we believe the success of our technique may result from (i) the very short length (146 bp) of the target sequence for Bd amplification, (ii) the presence of many copies per Bd cell of the ITS-1 region being targeted in our assay (41), and (iii) recovery of many cells of Bd in our swabbing technique because Bd grows on the skin surface of the host. Similar PCR recovery techniques have been able to extract DNA from specimens that are 10–85 y old (21). Additionally, molecular techniques have been used to detect human diseases from formalin-fixed tissues for some time; for example, Lyme disease (22) and Borna disease virus (23). Our results agree with previously published literature on the recovery of DNA from archival specimens and suggest that these techniques should be used for the detection of other zoonotic diseases and also for the discovery of potential symbionts that may affect disease dynamics (e.g., Janthinobacterium lividum) (24). Our study also highlights the importance of continued specimen collection, given that the time series of museum specimens represents an invaluable repository of information on demographic patterns, pathogens, associated endosymbionts, and other ecological information.
We also examined the question of whether Bd, described as an aquatic fungus (6), could cause lethality in neotropical plethodontids, which are terrestrial and direct-developing amphibians. Previous studies have reported mortality from Bd infection in plethodontids through laboratory investigation of Batrachoseps from California (25) and in the field, with reports of dead Oedipina and Bolitoglossa (8, 26). The presence of Bd we report in field-collected animals as well as in historical specimens provides additional evidence that neotropical plethodontids are hosts of the chytrid pathogen. Results from our infection trials demonstrate that under laboratory conditions, the disease, chytridiomycosis, quickly develops in individuals harboring Bd infection and is highly lethal in at least two species of neotropical plethodontids—P. leprosa and B. rufescens.
Additionally, our infection trials reveal a pattern of mortality in infected individuals that agrees with “Vredenburg's 10,000 Zoospore Rule,” which describes mortality in individuals (27) and population extinctions in the wild (8) when average infection intensities reached ∼10,000 ZE. In our laboratory study, all infected salamanders died at high infection intensities [(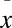 ± 1 SE) = 37,841 ± 7,111 zoospore equivalents × swab−1]. Resistant species, such as the P. matudai we report here, persist with low infection levels (<1,000 ZE) in the laboratory. These infection intensities are close to those reported in nonsusceptible (enzootic) wild populations of Rana sierrae infected with Bd (12). Further investigation of other suspected susceptible and nonsusceptible species is needed to determine whether Bd infection intensity can predict susceptibility of amphibian hosts to Bd. Despite the high susceptibility to Bd infection found for P. leprosa and B. rufescens in our laboratory trials, interpretation of these results should be done cautiously, because responses to Bd infection may differ under field conditions. In the laboratory, manipulation of climatic variables significantly affects host responses to Bd infection and survivability (25, 28).
± 1 SE) = 37,841 ± 7,111 zoospore equivalents × swab−1]. Resistant species, such as the P. matudai we report here, persist with low infection levels (<1,000 ZE) in the laboratory. These infection intensities are close to those reported in nonsusceptible (enzootic) wild populations of Rana sierrae infected with Bd (12). Further investigation of other suspected susceptible and nonsusceptible species is needed to determine whether Bd infection intensity can predict susceptibility of amphibian hosts to Bd. Despite the high susceptibility to Bd infection found for P. leprosa and B. rufescens in our laboratory trials, interpretation of these results should be done cautiously, because responses to Bd infection may differ under field conditions. In the laboratory, manipulation of climatic variables significantly affects host responses to Bd infection and survivability (25, 28).
In both Mexico and Guatemala, Bd was not detected in three species despite (i) good sample sizes (n ≥ 15, probability of a false negative = 0.46)—Pseudoeurycea unguidentis, Parvimolge townsendi, and B. morio and (ii) presence of Bd in heterospecifics at the site. The absence of Bd in these species may indicate variability in susceptibility to Bd between species; variation in Bd prevalence between species has also been reported in Panama (29). Additionally, Rovito et al. (15) reported a disproportionate decline in terrestrial specialists over arboreal specialists and microhabitat generalists in Guatemalan salamander populations. A possible explanation for the discrepancy in the decline of terrestrial specialists may be explained by differences in population density between terrestrial specialists and arboreal specialists/microhabitat generalists. In the 1970s, terrestrial species in both Mexico and Guatemala were found in high abundance and high densities, and arboreal and microhabitat generalists were found at lower abundances and densities (15, 30). Thus, the pattern seen among habitat specialists may be following a density-dependent host–pathogen dynamic where Bd outbreak operates independently from differences in host susceptibility or virulence in Bd strains (12). From this model, an epizootic outbreak occurs in a population when host densities permit Bd transmission rates to surpass the critical threshold leading to an outbreak (12). Thus, arboreal specialists and microhabitat generalists, even if experiencing rare cases of mortality in individuals due to Bd infection, could persist on a population level due to low population densities and low transmission rates. In contrast, terrestrial specialists occurring in high densities would have high transmission rates, resulting in a Bd outbreak and population collapse.
Although we now have evidence for the presence of Bd in montane populations of neotropical plethodontids, the question remains of how Bd, an “aquatic fungus,” came to infect and be maintained in these fully terrestrial and high-elevation species. It is still unknown how Bd moves across the landscape, but possible pathways include movement by birds, humans, water, or wind (11). We think that on a local scale it is more likely that Bd is spread by amphibian hosts (8). For example, many terrestrial salamanders that do not breed in water share moist microhabitats (e.g., bromeliads) with more aquatic and motile frog species. Zoospores have also been reported in bromeliads containing amphibians (31). Bd-resistant amphibian hosts have been previously suggested as agents of spread. For example, introduced bullfrogs (Rana catesbeiana), which are effective carriers of the fungus (32), may transmit Bd to new areas (33). From our infection trials, we found Bd resistance in P. matudai, a highly mobile species known to breed in water and overlap with salamanders in bromeliads. We propose that P. matudai may be one of the many resistant species potentially mediating the spread of Bd across geographical distances and among taxa that do not have aquatic larval phases such as the neotropical bolitoglossine salamanders.
Climate change has been invoked as the trigger for amphibian declines in Monteverde in a number of studies (34–36). In Europe, a study suggested a link between climate change and Bd emergence (37). Whereas our results do not rule out a role for climate change, we have found no necessary connection between climate change and the particular extinction events at our study sites. We suggest that the emergence and spread of a pathogen into naïve host populations can explain amphibian declines in the neotropics as other studies have also posited (7, 38, 39). The spatial-temporal pattern of Bd spread we describe, using a Bd PCR assay on museum specimens coincident with larger Bd-related amphibian declines occurring throughout Central America, indicates that Bd played a major role in these declines, regardless of other contributing factors. Our Bd PCR assay could be used to determine whether Bd was associated with other enigmatic amphibian declines that occurred historically and may help delineate the spread of Bd throughout the world's amphibians.
Materials and Methods
Noninvasive Sampling of Formalin-Preserved Specimens.
We investigated the effectiveness of noninvasive sampling techniques in combination with molecular methods to detect Bd in formalin-preserved amphibian specimens. We sampled a total of 38 specimens from six species of the genus Batrachoseps collected between 1971 and 2007 in Northern California. All specimens had been previously examined for Bd using histology (25), which determined that 29 specimens were Bd positive and 9 were Bd negative.
We tested two noninvasive sampling methods on formalin-preserved museum specimens used previously but unsuccessfully (40): skin swab (41) and brush (Oral-b interdental refill brush) (40). To decrease chances of contamination by errant skin pieces or other floating zoospores in preservation jars, each individual specimen was rinsed with 70% EtOH before sampling and gloves were rinsed or changed between animals. Brushes and swabs were stroked 30 times over the ventral surface of salamanders from neck to vent, stored in 1.5-mL microcentrifuge tubes, and kept at 4 °C until processing.
We extracted swabs using two comparative extraction methods: Prepman Ultra (41) and Qiagen DNeasy blood and tissue kit. Qiagen DNeasy extractions were used according to their tissue extraction protocol, with a variation in final elution volume to 40 μL of AE buffer. Brush samples were extracted using Qiagen DNeasy blood and tissue kit, also with a final elution volume of 40 μL AE buffer. All extractions were diluted 1:10 in 0.25× TE buffer and run in triplicate on real-time PCR following Boyle et al. (41) along with positive controls at dilution levels of 0.1, 1.0, 10, and 100 ZE. Raw genomic output from real-time PCR was multiplied by 80 to account for dilution during extraction, resulting in a relative infection intensity measured in terms of ZE. Samples were regarded as being Bd positive if one out of three replicates returned a positive result (>0.1 ZE). False positives are rare when working with Bd and real-time PCR (42), but negative controls were run to ensure against false positives. Real-time PCR results were compared with results from histological examination to determine accuracy in Bd detection. Recovery rate was calculated as the percentage of Bd positives resulting from our noninvasive sampling technique out of a total of 29 histology-confirmed Bd positives.
Sampling Specimens from Mexico, Guatemala, and Costa Rica.
For specimen sampling from Monteverde, Costa Rica, we used the swab/Qiagen method to sample a total of 26 specimens from 9 different species collected predecline (1967, 1976, and 1977) and 48 specimens from 16 different species collected during the first reported year of decline (1987). We sampled all specimens using synthetic cotton swabs (41). To decrease chances of contamination by errant skin pieces or other floating zoospores in preservation jars, each individual specimen was rinsed with 70% EtOH before sampling and gloves were rinsed or changed between animals. Frogs were swabbed on the ventral surface, including the inner thighs, abdomen, and between toes. Salamanders were swabbed on the ventral surface from neck to vent. We averaged 30 strokes per individual to standardize sampling. Swabs were extracted using a Qiagen DNeasy blood and tissue kit, and run in triplicate on Taqman real-time PCR using the same methods as described above (41, 42).
Using the swab/Prepman method, we examined formalin-preserved specimens that were part of reported declines in Mexico and Guatemala (15) for the presence of Bd. We tested 615 specimens from Guatemala collected between 1969 and 2010 and 537 specimens from Mexico collected between 1964 and 2009 for the presence of Bd. No field studies were conducted in Guatemala between 1979 and 1990, so no samples exist for this time period. We attempted to achieve a sample size of 30 individuals per species, per locality, per year—when possible—to achieve a minimum detection prevalence of 5% (8); for later sampling years where species were rare, we sampled all individuals available.
Bd prevalence was calculated for each locality by species and year along with posterior distributions calculated using Bayesian probability for 95% credible intervals (43). For instances of zero Bd prevalence, we also calculated the probability of a false negative on the basis of a true Bd prevalence of 5% in the population using the formula (1 − 0.05)S, where S represents sample size. Mean relative salamander abundance (Fig. 1) was based on data from Rovito et al. (15) (1969 estimates were calculated using the number of collectors listed in the MVZ database) and was calculated by first normalizing the data relative to each species' highest abundance (relative salamander abundance) and then averaged by year to find mean relative salamander abundance.
Lab Infection Trials.
In 2008, six uninfected B. rufescens and 10 infected P. matudai were collected from Chiapas, Mexico. In 2009, 15 P. leprosa were collected from Puebla, Mexico. All animals were individually housed and imported live to the animal care facility at San Francisco State University (SFSU), where they were individually housed in plastic 5-L containers with lids and moist paper towels. Each animal was checked daily by animal care staff; containers where changed and animals were fed live crickets once a week during the entire length of the experiment (7 wk). During the 7-wk infection trial, all frogs and salamanders were swabbed once a week and monitored for health. For the infection trial, three B. rufescens were infected with Bd, whereas the remaining three served as negative controls. Infection with Bd was achieved by housing each B. rufescens together with one Bd-positive P. matudai in a small Tupperware with 0.5-inches of double distilled H2O for 1 h a day for 5 consecutive days. All frogs (P. matudai) were housed in 10-L plastic rat containers that were lined with moist paper towels and also included a small water dish (100 mL). Frogs were fed crickets once a week, and cages were misted daily and changed every 2 wk. Swabbing of salamanders consisted of 30 strokes on the ventral side of the animal, from neck to vent. Swabbing of frogs consisted of a total of 30 strokes with 20 strokes across the ventral side of the body including down each thigh, with 10 strokes distributed among toe webbing (30 strokes total). Swabs were stored in microcentrifuge tubes and kept at 4 °C until processing. Swabs were extracted using Prepman Ultra protocol and run in singlicate on Taqman real-time PCR (41). Identical methods for calculating infection intensity and determining positive versus negative individuals used for formalin-preserved animals (described above) were also used for live animals in laboratory infection trials.
Supplementary Material
Acknowledgments
We thank T. Papenfuss for encouragement and helpful comments. We thank C. Spencer and the University of California Berkeley Museum of Vertebrate Zoology and the Biology Department at the University of Texas at Arlington for access to specimens; G. Parra-Olea, C. Vásquez-Almazán, A. Muñoz-Alonso, and J. C. Windfield-Perez for assistance in collection of specimens and laboratory animals; J. Savage for identifying juvenile frog specimens; A. Swei, C. Briggs, J. Parra, B. Chicana, L. Torres, C. Singer, and R. Figueroa for assistance in specimen sample collection and laboratory analysis. We thank permitting agencies in Guatemala, Mexico, and Costa Rica. SFSU Institutional Animal Care and Use Committee (A8-006). S.M.R. was funded by National Science Foundation (NSF) Grant DEB 1026393. T.L.C. and the V.T.V. laboratory were funded by NSF Grant EF-0723563.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9323.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105538108/-/DCSupplemental.
References
- 1.Barnosky AD, et al. Has the Earth's sixth mass extinction already arrived? Nature. 2011;471:51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 2.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 3.Wake DB, Vredenburg VT. Colloquium paper: Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voyles J, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 5.Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longcore JE, Pessier AP, Nichols DK. Batrachocytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- 7.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catenazzi A, Lehr E, Rodriguez LO, Vredenburg VT. Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conserv Biol. 2011;25:382–391. doi: 10.1111/j.1523-1739.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- 10.Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 11.Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009;63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- 12.Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA. 2010;107:9695–9700. doi: 10.1073/pnas.0912886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump ML, Hensley FR, Clark KL. Apparent decline of the golden toad: Underground or extinct? Copeia. 1992;(2):413–420. [Google Scholar]
- 14.Pounds JA, Crump ML. Amphibian declines and climate disturbance: The case of the golden toad and the harlequin frog. Conserv Biol. 1994;8:72–85. [Google Scholar]
- 15.Rovito SM, Parra-Olea G, Vásquez-Almazán CR, Papenfuss TJ, Wake DB. Dramatic declines in neotropical salamander populations are an important part of the global amphibian crisis. Proc Natl Acad Sci USA. 2009;106:3231–3236. doi: 10.1073/pnas.0813051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pounds JA, Fogden MPL, Savage JM, Gorman GC. Tests of null models for amphibian declines on a tropical mountain. Conserv Biol. 1997;11:1307–1322. [Google Scholar]
- 17.Parra-Olea G, Garcia-Paris M, Wake DB. Status of some populations of Mexican salamanders (Amphibia: Plethodontidae) Rev Biol Trop. 1999;47:217–223. [Google Scholar]
- 18.Lips KR, Mendelson Iii JR, Muñoz-Alonso A, Canseco-Marquez L, Mulcahy DG. Amphibian population declines in montane southern Mexico: Resurveys of historical localities. Biol Conserv. 2004;119:555–564. [Google Scholar]
- 19.Puschendorf R, Bolanos F, Chaves G. The amphibian chytrid fungus along an altitudinal transect before the first reported declines in Costa Rica. Biol Conserv. 2006;132:136–142. [Google Scholar]
- 20.Ouellet M, Mikaelian I, Pauli BD, Rodrigue J, Green DM. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv Biol. 2005;19:1431–1440. [Google Scholar]
- 21.Schader C, Lalanych K. DNA, PCR and formalized animal tissue: A short review and protocols. Org Divers Evol. 2003;3:195–205. [Google Scholar]
- 22.Wienecke R, Neubert U, Volkenandt M. Molecular detection of Borrelia burgdorferi in formalin-fixed, paraffin-embedded lesions of Lyme disease. J Cutan Pathol. 1993;20:385–388. doi: 10.1111/j.1600-0560.1993.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorg I, Metzler A. Detection of Borna disease virus RNA in formalin-fixed, paraffin-embedded brain tissues by nested PCR. J Clin Microbiol. 1995;33:821–823. doi: 10.1128/jcm.33.4.821-823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris RN, et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009;3:818–824. doi: 10.1038/ismej.2009.27. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein SB. An aquatic disease on a terrestrial salamander: Individual and population level effects of the amphibian Chytrid fungus, Batrachochytrium dendrobatidis, on Batrachoseps attenuatus (Plethodontidae) Copeia. 2009;2009:653. [Google Scholar]
- 26.Lips KR, Green DE, Papendick R. Chytridiomycosis in wild frogs from southern Costa Rica. J Herpetol. 2003;37:215–218. [Google Scholar]
- 27.Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ. Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg's “10,000 Zoospore Rule.”. PLoS ONE. 2011;6:e16708. doi: 10.1371/journal.pone.0016708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longo AV, Burrowes PA, Joglar RL. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis Aquat Organ. 2010;92:253–260. doi: 10.3354/dao02054. [DOI] [PubMed] [Google Scholar]
- 29.Brem FMR, Lips KR. Batrachochytrium dendrobatidis infection patterns among Panamanian amphibian species, habitats and elevations during epizootic and enzootic stages. Dis Aquat Organ. 2008;81:189–202. doi: 10.3354/dao01960. [DOI] [PubMed] [Google Scholar]
- 30.Wake DB, Lynch JF. The distribution, ecology, and evolutionary history of plethodontid salamanders in tropical America. Nat Hist Mus Los Angeles Co. 1976;25:1–65. [Google Scholar]
- 31.Cossel JO, Jr, Lindquist ED. Batrachochytrium dendrobatidis in arboreal and lotic water sources in Panama. Herpetolog Rev. 2009;40:45–47. [Google Scholar]
- 32.Daszak P, et al. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetolog J. 2004;14:201–207. [Google Scholar]
- 33.Hanselmann R, et al. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol Conserv. 2004;120:115–119. [Google Scholar]
- 34.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 35.Pounds JA. Climate and amphibian declines. Nature. 2001;410:639–640. doi: 10.1038/35070683. [DOI] [PubMed] [Google Scholar]
- 36.Pounds JA, et al. Ecology : Pounds et al. reply. Nature. 2007;447:E5–E6. [Google Scholar]
- 37.Bosch J, Carrascal LM, Durán L, Walker S, Fisher MC. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc Biol Sci. 2007;274:253–260. doi: 10.1098/rspb.2006.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lips KR, Diffendorfer J, Mendelson JR, III, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:e72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soto-Azat C, Clarke BT, Fisher MC, Walker SF, Cunningham AA. Non-invasive sampling methods for the detection of Batrachochytrium dendrobatidis in archived amphibians. Dis Aquat Organ. 2009;84:163–166. doi: 10.3354/dao02029. [DOI] [PubMed] [Google Scholar]
- 41.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 42.Hyatt AD, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ. 2007;73:175–192. doi: 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- 43.Edwards W, Lindman H, Savage LJ. Bayesian statistical inference in statistical research. Psychol Res. 1963;70:193–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.