Abstract
Mobile ectothermic animals can control their body temperatures by selecting specific thermal conditions in the environment, but embryos—trapped within an immobile egg and lacking locomotor structures—have been assumed to lack that ability. Falsifying that assumption, our experimental studies show that even early stage turtle embryos move within the egg to exploit small-scale spatial thermal heterogeneity. Behavioral thermoregulation is not restricted to posthatching life and instead may be an important tactic in every life-history stage.
Fluctuating ambient temperatures pose a major challenge to many organisms because thermal regimes constrain performance in fitness-relevant activities as well as influence metabolic rates (1). Although a few species [mammals and birds, plus some reptiles, fishes, insects, and plants (2, 3)] can control their own body temperatures via metabolic heat production (endothermy), most organisms are ectothermic. Many ectotherms use behavioral tactics (such as sun-seeking and shade-seeking) to maintain their body temperatures within selected bounds (4, 5). Behavioral thermoregulation requires an ability to detect spatial thermal heterogeneity and to move to favorable sites. Accordingly, biologists have assumed that the (immobile) embryonic stage cannot thermoregulate behaviorally (6). The embryo is viewed as thermally passive, with its body temperatures during development determined by ambient conditions, modified only by maternal nest-site choice (in oviparous species) or maternal thermoregulation [in viviparous species (7)]. Our studies falsify that assumption, showing instead that turtle embryos actively move toward warmer regions within the egg.
Most reptiles lay eggs in underground nests, heated by sunlight falling on the ground above. Thermal conditions may vary over small spatial scales inside such a nest [e.g., eggs at the top vs. the bottom of the nest or the center vs. the periphery (8)]. Even within a single egg, the warmest part is likely to be that closest to the heat source [usually nearest the top of the nest, but sometimes the side if the nest is laid on steeply sloping ground (9)]. An embryo that was able to detect such thermal gradients and move to the warmest part of the egg thus might substantially affect its own temperature. Even a small thermal shift might be enough to accelerate development [and thus hasten hatching and increase likely hatchling survival (10)] and modify a wide range of fitness-relevant hatchling traits [e.g., body size, shape, sex, and locomotor ability (11, 12)]. Although reptile embryos thus could plausibly obtain a fitness benefit by behavioral thermoregulation, researchers have assumed that such tiny, poorly developed animals are incapable of achieving this feat.
Results
Our measurements of egg surface temperatures confirmed that the side closest to a heat source can be significantly warmer than the side farthest away (comparing upper, lateral, and bottom parts of eggs: Friedman ANOVA χ2 = 38.1, P < 0.0001). Mean temperature on the part of the shell closest to the heat source averaged almost 1 °C warmer than the other side of the same egg [means 28.7 °C ± 0.3 for upper surface (closest to heat source), 27.9 °C ± 0.2 for lower surface, and 28.3 °C ± 0.2 for lateral surface]. Thus, an embryo could modify its own developmental temperature if it was able to perceive such thermal gradients and move to exploit them.
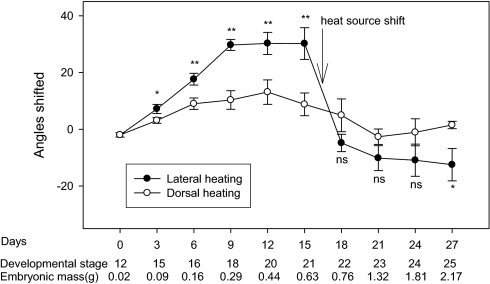
During laboratory incubation, turtle embryos did indeed move within their eggs to track the location of the heat source (Fig. 1). Embryos moved within a few days of the experiment's commencement and gradually moved farther and farther from their original positions. When we changed the location of the heat source, the embryos followed [Fig. 2: The results of ANOVA with treatment group and days since beginning of treatment as the factors were the following: within the first 15 d (heat from the left), interaction treatment × time F1,114 = 9.44, P < 0.003; within the second 15 d (heat from the right), treatment F1,89 = 10.48, P < 0.002].
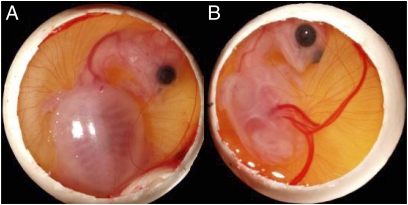
Fig. 1.
Typical positions of embryonic Chinese soft-shelled turtle (P. sinensis) inside eggs that were exposed to heat sources from above (A) or at the left side (B).
Fig. 2.
Shifts in the position of embryonic turtles (P. sinensis) inside eggs that were heated either from above or from the side. Changes in the embryo's location within its egg are shown by angular deviations of the embryo's body (measured at the point where the neck joins the carapace) from an uppermost-in-egg position (where all embryos were located at the beginning of the trials). Data on mean developmental stages and embryonic masses are given below the x axis. The embryos moved toward the heat source and tracked the heat source position when it was changed from the left side to the right side on day 15 of the experiment. Asterisks show levels of statistical significance (ns, nonsignificant; *P < 0.05; **P < 0.01).
In seminatural nests, embryos moved within their eggs toward the closest sun-heated ground surface. In eggs inside nests buried in the side of a sloping bank (and thus experiencing heat from the side and not the top of the nest), embryos moved farther from their original positions over the course of the experiment than did embryos in eggs inside nests on flat ground (and thus exposed to heating from above) (F2,33 = 43.44, P < 0.0001). Mean displacement angles were close to zero (0.9° ± 1.3) for eggs exposed to heating from above, but greater in eggs in sloping-bank nests if the embryos began incubation near the upper part of the egg (15.0° ± 1.2) and even greater in sloping-bank nests in which embryos initially were on the side of the egg farthest from the sun-heated ground surface (22.2° ± 1.2).
Discussion
Embryonic turtles “bask” inside their eggs, falsifying the assumption that behavioral thermoregulation is possible only for posthatching stages of the reptile life history. Remarkably, even very small and poorly developed embryos (e.g., averaging <0.1 g on day 3 of the laboratory experiment) (Fig. 2) were able to detect thermal differentials within the egg and move to exploit that small-scale heterogeneity. To our knowledge, no previous study has looked for this ability, presumably because embryos were thought incapable of such behavior.
Behavioral thermoregulation by reptile embryos may well enhance offspring fitness. In our study, movements by embryos enabled them to maximize heat gain from their surroundings and thus increase their body temperatures. Even for small eggs like those of Pelodiscus sinensis (mean egg mass <5 g), the thermal differentials between the warmest and coldest parts of the eggs can reach up to 0.8 °C, enough to induce a considerable variation in the embryonic development rate and thus the incubation period (about 4.5 d) (13). First, warmer incubation conditions accelerate developmental rate and thus hasten hatching, a trait likely to be under strongly positive selection in many reptile populations (14). Reduced incubation periods also may minimize the embryo's exposure to risks of nest predation or lethal thermal or hydric extremes. Second, the long-term effect of incubation temperature on hatchling performance and growth in this species (12, 13) as well as in many other reptile taxa (11) suggests that embryonic thermoregulation could enhance hatchling fitness via modifications to a range of phenotypic traits. Third, behavioral thermoregulation may minimize thermal differentials among eggs within a single large nest, thereby facilitating synchronous hatching [which may limit predation by diluting an individual's risk of predation (15)]. Fourth, embryos might be able to avoid lethally (or deleteriously) high local temperatures by moving to cooler regions of the egg during periods of dangerously high temperatures.
Clearly, the interactions between embryonic reptiles and their thermal environment are more complex, and less passive, than has been envisaged in previous research. In some reptile species, metabolic heating by embryos can elevate nest temperatures (16); in other taxa, thermal acclimation can adjust developmental rates to local thermal conditions (16, 17). Thus, the developmental rates of embryo reptiles are not passive consequences of maternally enforced decisions about the temperatures that the embryo will experience before hatching. Instead, facets of embryo behavior and physiology combine to allow even tiny embryos to control aspects of their own prehatching environment. The embryo is not simply a work in progress, but is a functioning organism with surprisingly sophisticated and effective means of affecting its own destiny.
Methods
Soft-shelled turtles (P. sinensis) from eastern China lay their eggs (mean clutch size = 16, mean egg mass = 4.6 g) inside shallow nests dug into the soil on riverbanks [range 6–11 cm from soil surface to uppermost egg (18)]. On steeply sloping banks, the closest proximity to the sun-warmed surface of the ground (and, hence, the warmest position within a given egg) is on the lateral rather than uppermost surface of an egg (18). Embryos of this species thus might be able to elevate their own incubation temperatures by moving within the egg. To test the hypothesis that an embryo will shift its position according to the direction of heat source, we incubated eggs of this species both in the laboratory and in simulated nests in the field. We obtained freshly laid eggs from a commercial turtle farm, located the embryo's position in each egg by candling (transmitted light), and marked that location on the eggshell with a pencil. All embryos were close to the uppermost surface of the egg at this stage. To quantify any thermally induced relocation of an embryo during our experiments, we removed part of the eggshell to locate the embryo beneath and then measured the angular deviation of the embryo's midpoint (i.e., the point where the neck meets the carapace, an obvious morphological feature) from its original position. The eggs were incubated under two sets of conditions:
i) A total of 260 eggs were incubated individually in 80-mL jars containing moist vermiculite (−220 kPa). Heating mats were used to deliver heat either to the top of the jar or to the side of the jar, creating optimal incubation temperatures (around 28 °C) for the eggs (13). In all cases, embryos were in the uppermost part of the egg (as determined by candling) when the trials commenced. To assess thermal heterogeneity within a single egg, we monitored temperatures at the uppermost, lateral, and bottom surfaces of 20 eggs in the “top-heated” treatment at 30-s intervals using 40-gauge thermocouples (TCTTT140; Temperature Controls Pty Ltd) connected to a data-taker (DT-80, Datataker Pty Ltd). In the “side-heating” treatment, we began with all heating mats on the left side of the jars, but shifted them to the right side of the jars after 15 d. We dissected 13 eggs from each treatment every 3 d to determine the position of the embryos (eggs that contained dead embryos were excluded in later analyses, so our sample sizes per treatment ranged from 10 to 13).
ii) A total of 540 eggs were incubated in groups of 15 in 36 simulated nests buried in soil at a field site near our laboratory. One-third of these nests were dug into flat ground (such that the uppermost part of each egg was closest to the sun-heated ground surface), with the eggs positioned at the beginning of the experiments such that each embryo was uppermost within its egg (as determined by candling). The other 24 nests were dug into a steep slope, such that the lateral surface of each egg was closest to the sun-heated ground surface. In half of these sloping-bank nests, the eggs were initially positioned such that embryos were in the uppermost part of their egg. In the other half of the sloping-bank nests, all eggs were initially positioned such that the embryos were on the side of the egg farthest from the sun-heated soil surface (45° from the uppermost part of their eggs). After 20 d, we excavated the nests and opened the eggs to determine the position of the embryos within their eggs.
Acknowledgments
We thank B. J. Sun, Y. Wang, and B. Sun for their help in the laboratory. This work was supported by Natural Science Foundation of China Grant 30970362, “One Hundred Person Project” and Knowledge Innovation Program of the Chinese Academy of Sciences Grant KSCX2-EW-Z-4, Zhejiang Provincial Foundation of Natural Science Grant G5080004 (to W.-G.D.), and the Australian Research Council (R.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Johnston IA, Bennett AF. Animals and Temperature: Phenotypic and Evolutionary Adaptation. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 2.Patino S, Grace J, Banziger H. Endothermy by flowers of Rhizanthes lowii (Rafflesiaceae) Oecologia. 2000;124:149–155. doi: 10.1007/s004420050001. [DOI] [PubMed] [Google Scholar]
- 3.Ruben J. Evolution of endothermy in mammals, birds and their ancestors. In: Johnston IA, Bennett AF, editors. Animals and Temperature: Phenotypic and Evolutionary Adaptation. Cambridge, UK: Cambridge University Press; 1996. pp. 347–376. [Google Scholar]
- 4.May ML. Insect thermoregulation. Annu Rev Entomol. 1979;24:313–349. [Google Scholar]
- 5.Huey RB. Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough FH, editors. Biology of the Reptilia. Vol. 12. New York: Academic Press; 1982. pp. 25–91. [Google Scholar]
- 6.Tattersall GJ, Cadena V, Skinner MC. Respiratory cooling and thermoregulatory coupling in reptiles. Respir Physiol Neurobiol. 2006;154:302–318. doi: 10.1016/j.resp.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Shine R. Is increased maternal basking an adaptation or a pre-adaptation to viviparity in lizards? J Exp Zoolog A Comp Exp Biol. 2006;305:524–535. doi: 10.1002/jez.a.291. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman RA, Lott DB. Thermal, hydric and respiratory climate of nests. In: Deeming DC, editor. Reptilian Incubation: Environment, Evolution and Behaviour. Nottingham, UK: Nottingham University Press; 2004. pp. 15–43. [Google Scholar]
- 9.Pike DA, Webb JK, Shine R. Nesting in a thermally challenging environment: Nest-site selection in a rock-dwelling gecko, Oedura lesueurii (Reptilia: Gekkonidae) Biol J Linn Soc Lond. 2010;99:250–259. [Google Scholar]
- 10.Olsson M, Shine R. The seasonal timing of oviposition in sand lizards (Lacerta agilis): Why early clutches are better. J Evol Biol. 1997;10:369–381. [Google Scholar]
- 11.Shine R. Adaptive consequences of developmental plasticity. In: Deeming DC, editor. Reptilian Incubation: Environment, Evolution and Behaviour. Nottingham, UK: Nottingham University Press; 2004. pp. 187–210. [Google Scholar]
- 12.Ji X, Chen F, Du WG, Chen HL. Incubation temperature affects hatchling growth but not sexual phenotype in the Chinese soft-shelled turtle, Pelodiscus sinensis (Trionychidae) J Zool (Lond) 2003;261:409–416. [Google Scholar]
- 13.Du WG, Ji X. The effects of incubation thermal environments on size, locomotor performance and early growth of hatchling soft-shelled turtles, Pelodiscus sinensis. J Therm Biol. 2003;28:279–286. [Google Scholar]
- 14.Warner DA, Shine R. Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia. 2007;154:65–73. doi: 10.1007/s00442-007-0809-9. [DOI] [PubMed] [Google Scholar]
- 15.Colbert PL, Spencer RJ, Janzen FJ. Mechanism and cost of synchronous hatching. Funct Ecol. 2010;24:112–121. [Google Scholar]
- 16.Zbinden JA, Margaritoulis D, Arlettaz R. Metabolic heating in Mediterranean loggerhead sea turtle clutches. J Exp Mar Biol Ecol. 2006;334:151–157. [Google Scholar]
- 17.Du WG, Warner DA, Langkilde T, Robbins T, Shine R. The physiological basis of geographic variation in rates of embryonic development within a widespread lizard species. Am Nat. 2010;176:522–528. doi: 10.1086/656270. [DOI] [PubMed] [Google Scholar]
- 18.Wang ZJ. Reproductive habit of the Chinese soft-shelled turtle (Trionyx sinensis) Chin J Zool. 1963;5:33–34. [Google Scholar]