Abstract
The East African hominin Paranthropus boisei was characterized by a suite of craniodental features that have been widely interpreted as adaptations to a diet that consisted of hard objects that required powerful peak masticatory loads. These morphological adaptations represent the culmination of an evolutionary trend that began in earlier taxa such as Australopithecus afarensis, and presumably facilitated utilization of open habitats in the Plio-Pleistocene. Here, we use stable isotopes to show that P. boisei had a diet that was dominated by C4 biomass such as grasses or sedges. Its diet included more C4 biomass than any other hominin studied to date, including its congener Paranthropus robustus from South Africa. These results, coupled with recent evidence from dental microwear, may indicate that the remarkable craniodental morphology of this taxon represents an adaptation for processing large quantities of low-quality vegetation rather than hard objects.
Keywords: C4 photosynthesis, C3 photosynthesis
The East African hominin Paranthropus boisei possessed large and low-cusped postcanine dentition, large and thick mandibular corpora, and powerful muscles of mastication, which are generally believed to be adaptations for a diet of nuts, seeds, and hard fruit (1–3). This notion emerged from interpretations of P. boisei’s morphology, but gained indirect support from dental microwear studies of its congener, Paranthropus robustus; these concluded that wear on the molars of South African Paranthropus was consistent with its having ingested and chewed small, hard food items, if not as primary resources, then at least as fallback foods (4–6). Although some have suggested that the craniodental morphology of P. boisei is consistent with the consumption of tough rather than hard foods (7, 8), this idea has been largely eschewed by most workers. Thus, when a recent study using dental microwear texture analysis revealed no evidence for the consumption of hard foods by P. boisei (9), it challenged decades of received wisdom, and underscored the need for independent lines of paleodietary evidence.
Stable carbon isotope analysis has proven a powerful tool for testing hypotheses about the diets of extinct herbivorous mammals (10, 11). It is based on the idea that carbon isotope compositions vary predictably between plant foods [e.g., plants using the C3 photosynthetic pathway (most dicotyledonous plants including trees, shrubs, forbs, herbs) and those using the C4 pathway (predominantly tropical grasses and sedges, which are monocotyledonous plants)], and further that dietary carbon remains locked in tooth enamel even after millions of years (10). Carbon isotope studies of P. robustus from South Africa indicated that it consumed some plants using C4 photosynthesis such as tropical grasses or sedges, but were also consistent with most of its dietary carbon (approximately 70%) having been derived from the C3 food items favored by extant chimpanzees (Pan troglodytes) such as tree fruits (12, 13). In contrast, stable isotopes measurements of two P. boisei specimens from Tanzania suggested a high component of C4 biomass in its diet (14), which would make its diet fundamentally distinct from all known modern or fossil hominoid taxa. However, it is unclear whether the data for these two individuals is characteristic of the species, or whether its diet varied over time and space, as the variability in previously analyzed hominin taxa is substantial (e.g., ref. 13).
We present stable isotope data for an additional 22 P. boisei individuals from central and northern Kenya that range between 1.9 and 1.4 million years in age, and extend the spatial range of the Tanzanian specimens by more than 700 km. These data inform not only our understanding of the diet of P. boisei, but given its occasional morphological similarities with other australopith taxa (e.g., Australopithecus afarensis, Australopithecus garhi, Australopithecus africanus), potentially our understanding of the role of diet in early hominin evolution.
Results
The δ13C values of P. boisei do not change over the half million years for which samples were available (r2 = 0.10). The average δ13C value for these samples was -1.3 ± 0.9‰ (24 teeth from 22 individuals), with a maximum value of +0.7‰ and a minimum value of -3.4‰ (Table 1). Using estimates of +2 and -12‰ for a “pure-C4 grazing” or “pure-C3 browsing” diet (14), the δ13C values for P. boisei correspond to a diet where C4 biomass comprises 77 ± 7%, with minimum and maximum values of 61 and 91%, respectively.
Table 1.
δ13C and δ18O of P. boisei from the Baringo Basin, Kenya (this study), the Turkana Basin, Kenya (this study) and from Olduvai Gorge and Peninj, Tanzania (14)
| Specimen | δ13C | δ18O | Tooth | Age range | Median age | Nominal % C4 |
| Baringo Basin | ||||||
| KNM-CH-302 | −1.3 | −1.5 | m-frag | > 1.42 | 1.42 | 76 |
| Turkana Basin | ||||||
| KNM-ER-810 | −3.4 | −3.3 | P3 | 1.77 | 1.77 | 61 |
| KNM-ER-818 | 0.7 | 1.9 | Lt M3 | 1.55–1.65 | 1.60 | 91 |
| KNM-ER-1804 | −1.2 | −0.7 | Lt M3 | 1.77 | 1.77 | 77 |
| KNM-ER-3887 | −1.6 | −2.9 | Rt M3 | 1.50–1.65 | 1.57 | 74 |
| KNM-ER-6080 | −2.2 | −0.6 | Rt M2 | 1.55–1.65 | 1.60 | 70 |
| KNM-ER-13750 | 0.2 | 0.5 | M-frag | 1.87 | 1.87 | 87 |
| KNM-ER-15940 | −1.1 | −0.6 | Lt M3 | 1.77 | 1.77 | 78 |
| KNM-WT-17396 | −1.9 | −3.1 | Lt M3 | 1.65–1.79 | 1.72 | 72 |
| KNM-WT-37100 | −1.8 | −1.5 | M2 or M3 | 1.65–1.79 | 1.72 | 73 |
| KNM-WT-37748 | −2.1 | Rt M3 | 1.65–1.79 | 1.72 | 71 | |
| KNM-ER-1171(C) | −0.6 | −1.9 | Lt M1 | 1.56–1.60 | 1.58 | 81 |
| KNM-ER-1469(A) | −2.3 | −0.1 | Lt M3 | 1.87–1.90 | 1.89 | 69 |
| KNM-ER-1479(A) | −2.3 | 0.2 | M3 | 1.87 | 1.87 | 69 |
| KNM-ER-1806(C) | −1.3 | −2 | Rt M3 | 1.85 | 1.85 | 76 |
| KNM-ER-3737(B) | −1.6 | −2.5 | Rt M1 | 1.56–1.60 | 1.58 | 74 |
| KNM-ER-3952(F) | −1.3 | 0.0 | Lt M3 | 1.87 | 1.87 | 76 |
| KNM-ER-729(A) | 0.0 | −0.7 | Lt P4 | 1.49–1.55 | 1.52 | 86 |
| KNM-ER-732(A) | −0.1 | −1.8 | P4 | 1.56–1.60 | 1.58 | 85 |
| KNM-ER-733(A) | −1.5 | −2.6 | Rt M3 | 1.49–1.55 | 1.52 | 75 |
| KNM-ER-733(D) | −0.5 | −2.2 | Lt P4 | 1.49–1.55 | 1.52 | 82 |
| KNM-ER-802(D) | −0.1 | −1.6 | Lt M1 | 1.56–1.60 | 1.58 | 85 |
| KNM-ER-802(G) | −1.9 | M3 | 1.56–1.60 | 1.58 | 72 | |
| KNM-ER-816(B) | −1.9 | −1.3 | Rt P4 | 1.77 | 1.77 | 72 |
| Olduvai | ||||||
| OH5 | −1.2* | — | Lt M2 | — | 1.82 | 77 |
| Peninj | ||||||
| NMT-W64-160 | 0.7* | — | Lt M2 | — | 1.62 | 81 |
| Average | −1.3 | 77 | ||||
| SD | 0.9 | 7 | ||||
| Number† | 24 | 24 |
*Data from ref. 14.
†Number of different individuals. KNM-ER-733 and KNM-ER-802 are represented by two teeth each from two different individuals. The average value for each individual was used to compute the overall average and standard deviation.
The attribution of specimens to Paranthropus boisei is as follows: KNM-CH 302 (ref. 48); the “East Rudolf” fossils from the Koobi Fora Formation (refs. 49 and 50 and references therein); and the “West Turkana” teeth from the Nachukui Formation (50, 51).
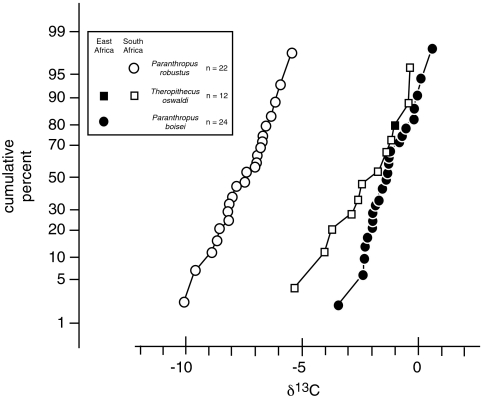
The carbon isotope composition of P. boisei is statistically indistinguishable from that of coeval grass-consumers from the region including Equidae (x = -0.4‰, SD = 0.9‰, n = 18), Suidae (x = -0.2‰, SD = 0.8‰, n = 10), and Hippopotamidae (x = -1.3‰, SD = 1.0‰, n = 23) (P > 0.05, ANOVA, Games–Howell), and highly different from C3 biomass-consuming Giraffidae (x = -12.5‰, SD = 0.8‰, n = 4, P < 0.0001). The δ13C values of contemporaneous equids, giraffids, hippopotamids, and suids are presented in Table S1. The diet of P. boisei differs significantly from that of contemporaneous P. robustus (x = -7.6‰, SD = 1.1‰, n = 18) (Fig. 1) in South Africa and early Homo throughout Africa (x = -7.8‰, SD = 1.5‰, n = 6) (P < 0.0001; data from refs. 13 and 14) (Fig. 2). The δ13C values of P. boisei are also starkly different from those reported for earlier taxa such as Ardipithecus ramidus (P < 0.0001) and Australopithecus africanus (P < 0.0001) (data from refs. 13 and 15). The data for other hominin species are presented in Table S2.
Fig. 1.
Cumulative frequencies of P. boisei from eastern Africa with contemporaneous P. robustus from southern Africa, and for T. oswaldi from both eastern and southern Africa. Multiple analyses from single individuals have been averaged.
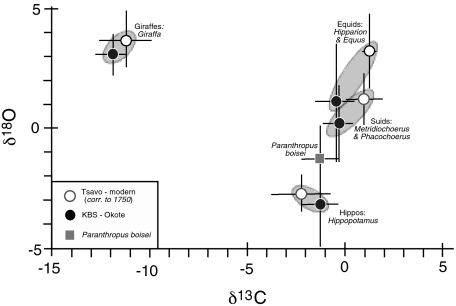
Fig. 2.
δ13C and δ18O values of P. boisei from the Turkana Basin compared with giraffes, suids, hippos, and equids from the KBS and Okote Members of the Koobi Fora Formation, and to their modern counterparts from the Tsavo region in Kenya.
Discussion
C4 Diet of P. boisei.
The carbon isotope composition of P. boisei is fundamentally different from that of all known living and fossil hominoids, which vary from nearly pure C3 consumers like gorillas (16) and chimpanzees (17, 18) to variable C3 consumers like A. africanus and P. robustus (12, 13). Indeed, the only known haplorrhine primate with a similar carbon isotope composition is the extinct grass-eating baboon Theropithecus oswaldi (x = -2.3‰, SD = 1.5‰, n = 12) (see Table S2 and references therein) from the same general time period. Despite a once broad distribution, the genus Theropithecus is now limited to a single species living in the highlands of Ethiopia. These observations suggest that the organisms with which P. boisei most likely competed for resources were not contemporaneous Homo, Papio (savanna baboons), or other frugivorous taxa, but probably C4 biomass consumers including equids, suids, hippos, Theropithecus, and some bovids.
The vast majority of C4 biomass is grass, although there can be large quantities of C4 sedges, especially in wetland environments such as the Okavango Delta today (19). A much smaller percentage of C4 dicots and crassulacean acid metabolism plants with similar carbon isotope compositions exists, although the typically low abundance of the former and low abundance and frequent toxicity of the latter make them unlikely to be regular targets for early hominins (20). Thus, it is almost certain that P. boisei had a diet focused on grasses, sedges, or both.
It has recently been suggested that sedges were an important hominin resource because they are often found in the riverine woodlands favored by many savanna primates and because their tubers are a potentially high energy resource for which tool-wielding hominins would have had little competition (14, 21). Nevertheless, sedges often utilize C3 photosynthesis, are not widely distributed in many habitats, and might have been of dubious nutritional value without cooking (22, 23). Moreover, there is no record of any large mammal feeding on sedges to such an extent. Regardless, if P. boisei was a wetland sedge specialist, it would suggest an extremely limited distribution in ancient landscapes with important implications for our understanding of early hominin biology and biogeography.
Grasses, in contrast, are widely abundant and use the C4 photosynthetic pathway in most African savanna environments; they are utilized extensively by taxonomically diverse mammals including the primate Theropithecus. A reason for thinking that grass blades were not consumed by P. boisei is that the low occlusal relief of its cheek teeth is the opposite of what might be expected for a consumer of leaves. Folivores tend to have great occlusal relief, whereas flat teeth are usually found in hard-object consumers among frugivorous primates (1). Nevertheless, the dental microwear complexity profiles of P. boisei and Theropithecus are similar, suggesting a diet with comparable mechanical properties (24). Although the high anisotropy (directionality) of Theropithecus molar microwear is very different from that of P. boisei, it has been suggested that this results from different constraints posed by their dentognathic morphology rather than diet (24). In other words, it is possible that they were utilizing similar foods but chewing them in different ways.
P. boisei cheek teeth display notable gradients of gross wear, resulting in large, deeply excavated dentine exposures, and in this regard, they are similar to other australopith species (e.g., A. afarensis and A. africanus) that also possess low tooth cusps with thick enamel. Thus, like other australopiths, P. boisei undoubtedly had a diet that consisted of foods with abrasive qualities—the gross wear is as likely due to repetitive loading of phytolith-rich tough foods as exogenous grit. Thus, either grass or sedge consumption and/or exogenous grit might well have contributed to P. boisei’s notable wear gradient.
Of perhaps greater moment than its potential specific similarities, the microwear of P. boisei molars, which shows remarkable uniformity over time from about 2.3 Ma to about < 1.4 Ma (9, 24), stands in stark contrast to the wear fabrics exhibited by primate hard-object consumers. Indeed, there is no evidence beyond the anecdotal [e.g., the broken left first permanent molar crown in the KNM-ER 729 P. boisei mandible (8) and the observation that a couple of P. boisei molars show antemortem enamel chipping (25)] that these food items were hard.
Paleoenvironment of P. boisei.
Previous interpretations of the environmental conditions of P. boisei are varied and include closed wet habitats (26), scrub woodland to arid shrubland (27), and semiarid savanna associated with woodlands and gallery forest (28). The oxygen isotope composition of P. boisei and contemporaneous mammal tooth enamel provides further information about its water utilization and environment (29). Fig. 2 shows the δ18O and δ13C values for the water-dependent hippopotamus, the water-independent browser Giraffa, and two grazers (equids and suids) from the Kay Behrensmeyer Site (KBS) and Okote members (Table S1); it also shows the δ18O and δ13C values for their modern counterparts in the Tsavo region in Kenya (Table S3). These data suggest that P. boisei was very water dependent based on its δ18O values, which are more negative than those of coeval suids, equids, and giraffids. The similarity in 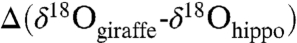 suggests that the environment in the Turkana Basin in KBS-Okote time had a water deficit (30) similar to that in the Tsavo region today (mean annual temperature = 25 °C; mean annual precipitation = 550 mm per year). Δ47 values from paleosols in the Turkana region indicate soil temperatures between 30 and 37 °C, indicating high mean temperatures and an open habitat in the Koobi Fora and Nachukui regions where these fossils were found (31).
suggests that the environment in the Turkana Basin in KBS-Okote time had a water deficit (30) similar to that in the Tsavo region today (mean annual temperature = 25 °C; mean annual precipitation = 550 mm per year). Δ47 values from paleosols in the Turkana region indicate soil temperatures between 30 and 37 °C, indicating high mean temperatures and an open habitat in the Koobi Fora and Nachukui regions where these fossils were found (31).
Overall, the stable isotope evidence from paleosols and tooth enamel is compatible with semiarid savanna with riparian woodlands or with woodlands associated with lakes (32). Grasses or sedges would have been highly available in such an environment.
South Africa vs. East Africa: P. robustus vs. P. boisei.
Why the difference in carbon isotope composition between Paranthropus in eastern and southern Africa? If these congeners had similar biology then it could be argued that the difference represents a generalist genus eating different things in disparate regional environments. However, most herbivorous taxa do not show evidence of diet change between the regions (compare ref. 33 with ref. 34), including Theropithecus (Table S2). Moreover, there is little reason to believe that the potential environments of P. boisei in East Africa were homogenous over time and space, or that its habitat was so different from that of P. robustus that the carbon isotope compositions of their diets did not overlap. In fact, a variety of paleoenvironments have been reconstructed for P. boisei that clearly overlap with those of P. robustus (27, 28). Most studies, however, have emphasized open and well-watered habitats for P. boisei [e.g., deltaic environments and/or edaphic grasslands (26, 27, 35)]. P. boisei and P. robustus carbon isotope values could also differ if both were principally sedge consumers and there was a differential distribution of C3 and C4 sedges. Indeed, there is some evidence that C4 sedges are more common in East African habitats today (23).
Given current evidence, however, the simplest explanation is adaptive divergence between the eastern and southern African P.aranthropus populations, with the former focusing on grasses or sedges and the southern population consuming a more traditional hominoid diet that included tree fleshy fruits, as well as variable C4 resources. In short, P. robustus had an expanded dietary repertoire relative to extant apes that included C4 resources, whereas P. boisei had completely abandoned the presumed ancestral diet (C3-based foods) to focus on a resource abundant in savanna and wetland environments.
Implications for Craniodental Adaptations.
These results might also have broader significance for our understanding of australoptith craniodental adaptations. Earlier taxa such as A. anamensis and A. afarensis exhibit, albeit in an incipient state, craniodental features that have been surmised to indicate a diet that consisted of hard objects (36–39) Traditional thinking has been that such masticatory adaptations permitted hominins to colonize increasingly seasonal and open environments (37). However, recent dental microwear studies suggest that the mechanical properties of A. afarensis (and A. anamensis) diets were nearly identical to those of P. boisei (9, 24, 40–42). If this is so, could it be that the australopith masticatory package represents an adaptation to C4 resources such as grasses or sedges? The similarity in dental microwear fabrics among the eastern African australopiths, all of which lack any evidence for hard-object food consumption (9, 24, 40–42), is consistent with the notion that their craniodental morphology could reflect “repetitive loading” rather than hard-object consumption (7, 8, 43). If this is borne out, it would suggest our understanding of early hominin ecology and biomechanics needs rethinking, but must ultimately wait on stable isotope data from earlier hominins.
Conclusions
Carbon isotope data show that P. boisei had a diet primarily of C4 resources, most likely grasses or sedges, over a wide range of time (> 0.5 Ma) and space (Turkana, Baringo, Natron, and Olduvai regions). These data are irreconcilable with the idea of P. boisei having eaten foods even broadly similar to those of African apes. They are also inconsistent with the notion that P. boisei ate nuts or hard fruits preponderantly, and also suggest that Paranthropus in eastern Africa (P. boisei) and southern Africa (P. robustus) had very different diets, a notion also supported by dental microwear (6, 9). In sum, this study suggests that the prevailing ideas based on morphological and biomechanical considerations are at least partly in error, and that our understanding of the dietary basis of masticatory differentiation within the hominin lineage may require revision.
Methods
Samples were obtained from the National Museums of Kenya. Approximately 2 mg of powder was obtained using a high-speed rotary drill; P. boisei enamel from broken tooth surfaces were sampled so that information concerning morphology was not compromised. Powdered samples were treated with 0.1 M buffered acetic acid for 30 min to remove secondary carbonates (see Table S4). Samples were reacted with 105% phosphoric acid at 90 °C in silver capsules and analyzed on an isotope ratio mass spectrometer following cryogenic separation of CO2; results are reported using the standard per mil (‰) notation with Vienna Pee Dee Belemnite as the standard for both oxygen and carbon isotope measurements. Corrections for temperature-dependent isotope fractionation in oxygen were made using modern and fossil internal reference materials that had been reacted at 25 °C (44). For comparative purposes, modern mammals have had their δ13C values adjusted to compensate for recent changes in atomospheric δ13C values (45–47).
Treated and untreated fossil enamel from four samples were analyzed to test for the presence of exogenous carbonate. Fossil enamel samples were treated with 0.1 M buffered acetic acid for 30 min, rinsed four times with ultrapure (Milli-Q) distilled water, and dried overnight at 60 °C. Treated and untreated samples were analyzed back to back for δ13C and δ18O on an isotope ratio mass spectrometer. Carrara carbonate standards were used to convert voltages measured on the major mass Faraday cup (44) into CO2 yields (μmol/mg). Two of four untreated samples, ER-810-UNT and ER-1806C-UNT, had CO2 yields of 1.34 and 1.81 μmol/mg, respectively, indicating the presence of exogenous carbonate (Table S4). Their treated counterparts had yields of 0.75 and 0.59 μmol/mg, respectively, which fall within the range for modern enamel samples. Carbon isotope values between treated and untreated samples differed by up to 2.7‰ (Table S4). Based on the results from the four pilot samples, all remaining samples were treated as described above.
Supplementary Material
Acknowledgments.
We thank Frank Brown for discussions and Nick van der Merwe and Bernard Wood for reviews of the manuscript. Research was funded by the National Science Foundation (Grant BCS 0621542) and the University of Colorado Dean’s Fund for Excellence. We thank the National Museums of Kenya for permission for this study.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9319.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104627108/-/DCSupplemental.
References
- 1.Kay RF. The nut-crackers—A new theory of the adaptations of the Ramapithecinae. Am J Phys Anthropol. 1981;55:141–151. [Google Scholar]
- 2.Lucas PW, Corlett RT, Luke DA. Postcanine tooth size and diet in anthropoid primates. Z Morphol Anthropol. 1986;76:253–276. [PubMed] [Google Scholar]
- 3.Wood B, Constantino P. Paranthropus boisei: Fifty years of evidence and analysis. Am J Phys Anthropol. 2007;134(Suppl 45):106–132. doi: 10.1002/ajpa.20732. [DOI] [PubMed] [Google Scholar]
- 4.Grine FE. Dental evidence for dietary differences in Australopithecus and Paranthropus: A quantitative analysis of permanent molar microwear. J Hum Evol. 1986;15:783–822. [Google Scholar]
- 5.Grine FE, Kay RF. Early hominid diets from quantitative image analysis of dental microwear. Nature. 1988;333:765–768. doi: 10.1038/333765a0. [DOI] [PubMed] [Google Scholar]
- 6.Scott RS, et al. Dental microwear texture analysis reflects diets of living primates and fossil hominins. Nature. 2005;436:693–695. doi: 10.1038/nature03822. [DOI] [PubMed] [Google Scholar]
- 7.Jolly CJ. The seed eaters: A new model of hominid differentiation based on a baboon analogy. Man. 1970;5:5–26. [Google Scholar]
- 8.Walker AC. Diet and teeth. Dietary hypotheses and human evolution. Philos Trans R Soc Lond B Biol Sci. 1981;292:57–64. doi: 10.1098/rstb.1981.0013. [DOI] [PubMed] [Google Scholar]
- 9.Ungar PS, Grine FE, Teaford MF. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS One. 2008;3:e2044. doi: 10.1371/journal.pone.0002044. 10.1371/journal.pone.0002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee-Thorp JA, van der Merwe NJ. Carbon isotope analysis of fossil bone apatite. S Afr J Sci. 1987;83:712–715. [Google Scholar]
- 11.Cerling TE, Harris JM, Leakey MG. Browsing and grazing in modern and fossil proboscideans. Oecologia. 1999;120:364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 12.Lee-Thorp JA, van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27:361–372. [Google Scholar]
- 13.Sponheimer M, Lee-Thorp JA. Hominin palaeodiets: The contribution of stable isotopes. In: Henke W, Tattersall I, editors. Handbook of Paleoanthropology. Berlin: Springer-Verlag; 2007. pp. 555–586. [Google Scholar]
- 14.van der Merwe NJ, Masao FT, Bamford MK. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S Afr J Sci. 2008;104:153–155. [Google Scholar]
- 15.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326:87–93. [PubMed] [Google Scholar]
- 16.Levin NE, et al. Quade J, Wynn JG, editors. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. Geol Soc Am Special Paper. 2008;446:215–234. [Google Scholar]
- 17.Schoeninger MJ, Iwaniec UT, Nash LT. Ecological attributes recorded in stable isotope ratios of arboreal prosimian hair. Oecologia. 1998;113:222–230. doi: 10.1007/s004420050372. [DOI] [PubMed] [Google Scholar]
- 18.Smith CC, Morgan ME, Pilbeam D. Isotopic ecology and dietary profiles of Liberian chimpanzees. J Hum Evol. 2010;58:43–55. doi: 10.1016/j.jhevol.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Ellery WN, Ellery K, Rogers KH, McCarthy TS. The role of Cyperus papyrus L. in channel blockage and abandonment in the northeastern Okavango Delta, Botswana. Afr J Ecol. 1995;33:25–49. [Google Scholar]
- 20.Peters CR, Vogel JC. Africa’s wild C4 plant foods and possible early hominid diets. J Hum Evol. 2005;48:219–236. doi: 10.1016/j.jhevol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Conklin-Brittain NL, Wrangham RW, Smith CC. Ungar PS, Teaford MF. Human Diet: Its Origin and Evolution. Westport: Bergin & Garvey; 2002. A two-stage model of increased dietary quality in early hominid evolution: The role of fiber; pp. 61–76. [Google Scholar]
- 22.Schoeninger MJ, Murray S, Bunn HT, Marlett JA. Composition of tubers used by Hadza foragers of Tanzania. J Food Compost Anal. 2001;14:15–25. [Google Scholar]
- 23.Stock WD, Chuba CK, Verboom GA. Distribution of South African C3 and C4 species of Cyperaceae in relation to climate and phylogeny. Austral Ecol. 2004;29:313–319. [Google Scholar]
- 24.Ungar PS, Krueger KL, Blumenschine RJ, Njau J, Scott RS. Dental microwear texture analysis of hominins recovered by the Olduvai Landscape Paleoanthropology Project, 1995–2007. J Hum Evol. 2011 doi: 10.1016/j.jhevol.2011.04.006. in Press. [DOI] [PubMed] [Google Scholar]
- 25.Constantino PJ, et al. Tooth chipping can reveal the diet and bite forces of fossil hominins. Biol Lett. 2010;6:826–829. doi: 10.1098/rsbl.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shipman P, Harris JM. Habitat preference and paleocecology of Australopithecus boisei in eastern Africa. In: Grine FE, editor. Evolutionary History of the “Robust” Australopithecines. New York: Aldine de Gruyter; 1988. pp. 343–381. [Google Scholar]
- 27.Reed KE. Early hominid evolution and ecological change through the African Plio-Pleistocene. J Hum Evol. 1997;32:289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- 28.Feibel CS, Harris JM, Brown FH. Palaeoenvironmental context for the Late Neogene of the Turkana Basin. In: Harris JM, editor. Koobi Fora Research Project. Vol. 3. Oxford: Clarendon Press; 1991. pp. 321–346. [Google Scholar]
- 29.Kohn MJ, Schoeninger MJ, Valley JW. Herbivore tooth oxygen isotope compositions: Effects of diet and physiology. Geochim Cosmochim Acta. 1996;60:3889–3896. [Google Scholar]
- 30.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. Stable isotopes as a proxy for paleoaridity. Proc Natl Acad Sci USA. 2006;103:11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passey BH, Levin NE, Cerling TE, Brown FH, Eiler J. High temperature environments of human evolution in East Africa based on bond ordering in paleosol carbonates. Proc Natl Acad Sci USA. 2010;107:11245–11249. doi: 10.1073/pnas.1001824107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerling TE, et al. Comment on the paleoenvironment of Ardipithecus ramidus. Science. 2010;328:1105-d. doi: 10.1126/science.1185274. 10.1126/science.1185274. [DOI] [PubMed] [Google Scholar]
- 33.Cerling TE, Harris JM, Passey BH. Diets of East African Bovidae based on stable isotope analysis. J Mammal. 2003;84:456–471. [Google Scholar]
- 34.Sponheimer M, et al. Diets of Southern African Bovidae: Stable isotope evidence. J Mammal. 2003;84:471–479. [Google Scholar]
- 35.Behrensmeyer AK. The habitat of Plio-Pleistocene hominids in East Africa: Taphonomic and microstratigraphic evidence. In: Jolly C, editor. Early Hominids of Africa. New York: St. Martin’s Press; 1978. pp. 165–189. [Google Scholar]
- 36.White TD, Suwa G, Simpson S, Asfaw B. Jaws and teeth of Australopithecus afarensis from Maka, Middle Awash, Ethiopia. Am J Phys Anthropol. 2000;111:45–68. doi: 10.1002/(SICI)1096-8644(200001)111:1<45::AID-AJPA4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.White TD, et al. Asa Issie, Aramis and the origin of Australopithecus. Nature. 2006;440:883–889. doi: 10.1038/nature04629. [DOI] [PubMed] [Google Scholar]
- 38.Macho GA, Shimizu D, Jiang Y, Spears IR. Australopithecus anamensis: A finite- element approach to studying the functional adaptations of extinct hominins. Anat Rec. 2005;283A:310–318. doi: 10.1002/ar.a.20175. [DOI] [PubMed] [Google Scholar]
- 39.Kimbel WH, Delezene LK. “Lucy” redux: A review of research on Australopithecus afarensis. Am J Phys Anthropol. 2009;140(Suppl 49):2–48. doi: 10.1002/ajpa.21183. [DOI] [PubMed] [Google Scholar]
- 40.Grine FE, Ungar PS, Teaford MF. Was the Early Pliocene hominin ‘Australopithecus’ anamensis a hard object feeder? S Afr J Sci. 2006;102:301–310. [Google Scholar]
- 41.Grine FE, Ungar PS, Teaford MF, El Zaatari S. Molar microwear in Praeanthropus afarensis: Evidence for dietary stasis through time and under diverse paleoecological conditions. J Hum Evol. 2006;51:297–319. doi: 10.1016/j.jhevol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Ungar PS, Scott RS, Grine FE, Teaford MF. Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis. Philos Trans R Soc Lond B Biol Sci. 2010;365:3345–3354. doi: 10.1098/rstb.2010.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hylander WL. Incisor size and diet in anthropoids with special reference to Cercopithecidae. Science. 1975;189:1095–1098. doi: 10.1126/science.808855. [DOI] [PubMed] [Google Scholar]
- 44.Passey BH, Cerling TE, Levin NE. Temperature dependence of acid fractionation for modern and fossil tooth enamels. Rapid Commun Mass Spectrom. 2007;21:2853–2859. doi: 10.1002/rcm.3149. [DOI] [PubMed] [Google Scholar]
- 45.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120:347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 46.Francey RJ, et al. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus. 1999;51:170–193. [Google Scholar]
- 47.Keeling RF, Piper SC, Bollenbacher AF, Walker SJ. Trends: A Compendium of Data on Global Change. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; 2010. Monthly atmospheric 13C/12C isotopic ratios for 11 SIO stations. [Google Scholar]
- 48.Gowlett JAJ, Harris JWK, Walton D, Wood BA. Early archaeological sites, hominid remains and traces of fire from Chesowanja, Kenya. Nature. 1981;294:125–129. doi: 10.1038/294125a0. [DOI] [PubMed] [Google Scholar]
- 49.Wood BA. Koobi Fora Research Project, Vol 4: Hominid Cranial Remains. Vol. 4. Oxford: Clarendon Press; 1991. [Google Scholar]
- 50.Brown B, Brown FH, Walker A. New hominids from the Lake Turkana Basin, Kenya. J Hum Evol. 2001;41:29–44. doi: 10.1006/jhev.2001.0476. [DOI] [PubMed] [Google Scholar]
- 51.Prat S, Brugal JP, Roche H, Texier PJ. Nouvelles découvertes de dents d’hominidés dans le membre Kaitio de la formation de Nachukui (1,65-1,9 Ma), Ouest du lac Turkana (Kenya) Comptes Rendus Palevol. 2003;2:685–693. [New discoveries of hominid teeth from the Kaito Member, Nachukui Formatioin, West Turkana (French)] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.