Abstract
Oxidative stress is caused by an imbalance between reactive oxygen species (ROS) production and the ability of an organism to eliminate these toxic intermediates. Mutations in PTEN-inducible kinase 1 (PINK1) link mitochondrial dysfunction, increased sensitivity to ROS, and apoptosis in Parkinson's disease. Whereas PINK1 has been linked to the regulation of oxidative stress, the exact mechanism by which this occurs has remained elusive. Oxidative stress with associated mitochondrial dysfunction leads to cardiac dysfunction and heart failure (HF). We hypothesized that loss of PINK1 in the heart would have deleterious consequences on mitochondrial function. Here, we observed that PINK1 protein levels are markedly reduced in end-stage human HF. We also report that PINK1 localizes exclusively to the mitochondria. PINK1−/− mice develop left ventricular dysfunction and evidence of pathological cardiac hypertrophy as early as 2 mo of age. Of note, PINK1−/− mice have greater levels of oxidative stress and impaired mitochondrial function. There were also higher degrees of fibrosis, cardiomyocyte apoptosis, and a reciprocal reduction in capillary density associated with this baseline cardiac phenotype. Collectively, our in vivo data demonstrate that PINK1 activity is crucial for postnatal myocardial development, through its role in maintaining mitochondrial function, and redox homeostasis in cardiomyocytes. In conclusion, PINK1 possesses a distinct, nonredundant function in the surveillance and maintenance of cardiac tissue homeostasis.
Keywords: mitochondrial swelling, mitochonopathy, mitochondrial energetics
Heart failure (HF) is the inability of the heart to adequately pump blood to meet the demands of the body. It is the leading cause of morbidity and mortality in North America (1). The quality of life and the prognosis for this group of patients remains poor with 1-y survival rates less than 40% (1). Conventional pharmacological therapy, which only slows the progression of the disease by alleviating the workload of the heart, does not directly target the disease process itself. Alternatives to medical therapy are limited to transplantation or mechanical assist devices; approaches that are themselves associated with significant morbidity and mortality. As such, it is exceedingly important to discover alternate strategies that will effectively treat this disease entity.
Oxidative stress is created by the imbalance between production of reactive oxygen species (ROS) and the elimination of toxic intermediates by antioxidant systems. The heart with its high metabolic state and limited capacity for regeneration is particularly sensitive to oxidative stress. Upon exposure to ROS, the heart undergoes hypertrophic growth, a process that involves cell enlargement, myofibrillar disarray, and reexpression of fetal genes (2). Although cardiac hypertrophy is considered an initial adaptive response, prolonged hypertrophy is ultimately detrimental and leads to progressive HF.
The discovery of recessively inherited mutations in PTEN-inducible kinase 1 (PINK1), a highly conserved Parkinson's disease-susceptibility gene (3), provides the link between mitochondrial dysfunction and oxidative stress in Parkinson's disease (4–7). PINK1, a putative serine/threonine protein kinase, is ubiquitously expressed and localized to the mitochondria. Although the role of PINK1 in protecting against oxidative stress-induced cell death has been intensively studied in neuronal systems, its biological function in other tissues remains elusive (8).
Any alteration in mitochondrial homeostasis in the heart, the organ which has the largest mitochondrial content by far, should have deleterious consequences on cardiac function. This realization prompted us to investigate the role of cardiac PINK1. We hypothesized that if PINK1 is to regulate mitochondrial function in the heart, the loss of PINK1 will ultimately result in pathologic cardiac hypertrophy and left ventricular (LV) dysfunction. Here we show that protein levels of PINK1 in end-stage human HF were diminished, supporting a reciprocal relationship between PINK1 activity and HF. Although normal at birth, the PINK1−/− mice develop cardiac hypertrophy and LV dysfunction by 2 mo of age. This phenotype was accompanied by increased oxidative stress and mitochondrial dysfunction. Thus, our work provides compelling evidence in vivo that PINK1 is crucial in the maintenance of mitochondrial function and redox homeostasis in cardiomyocytes.
Results
Down-Regulation of PINK1 Protein Expression in End-Stage Human HF.
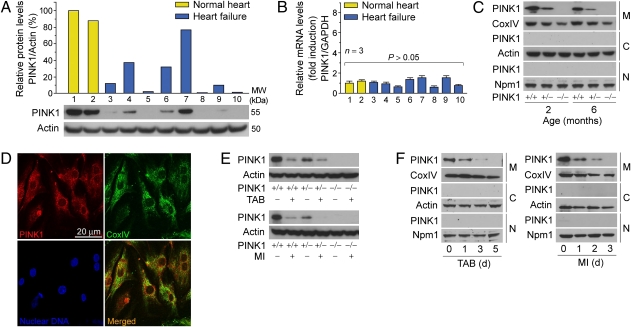
In the heart, mitochondria are not only the major source of ROS, they are also exquisitely sensitive to the damaging effects of oxidative stress, resulting in cell death (9). Increased oxidative stress has been well documented in patients with HF (10–13). Thus, we reasoned that PINK1 protein expression would be reduced in this patient population. LV tissue obtained from patients with end-stage HF and normal healthy controls were analyzed by Western blotting (Fig. 1A and Table 1) and quantitative real-time PCR (qRT-PCR) (Fig. 1B). There was a substantial decrease in PINK1 protein levels in LV samples from patients with end-stage HF compared with normal controls, irrespective of the etiology of their HF condition. In contrast, the variability in PINK1 mRNA levels was not significantly different, implying that PINK1 is regulated at the posttranscriptional level. These findings are compatible with our view that when exposed to chronic aberrant oxidative stress, the human heart undergoes maladaptive changes including impaired PINK1 protein expression.
Fig. 1.
Down-regulation of PINK1 protein expression in human end-stage HF. (A) Representative Western blot of PINK1 in total human LV extracts (Lower) with densitometric quantification (Upper). Lane 1 was set at 100%. MW, relative molecular weight. kDa, kilodalton. One result of two independent experiments. (B) Quantitative real-time PCR (qRT-PCR) analysis of PINK1 mRNA levels in LV tissues from human patients described in A. Lane 1 was set at onefold. Mean ± SEM. (C) Subcellular localization of PINK1 protein in adult mouse LV tissue. M, mitochondria; C, cytoplasm; N, nucleus; CoxIV, cytochrome c oxidase subunit IV; Npm1, nucleophosmin1. (D) Isolated neonatal mouse LV cardiomyocytes were fixed and costained with specific antibodies to PINK1 and mitochondrial CoxIV for indirect immunofluorescence confocal microscopic analysis. (E) PINK1 protein levels are susceptible to acute pressure overload (TAB) and ischemia (MI) for 24 h. (F) PINK1 is not redistributed to the cytoplasm or nucleus in response to increased hemodynamic load and hypoxic stress. Mice (10–12 wk) were subjected to TAB (Left) or MI (Right).
Table 1.
Patient characteristics of human LV samples
| Lane | Age (yr) | Sex | Etiology of HF syndrome |
| 1 | 38 | Female | Control |
| 2 | 38 | Female | Control |
| 3 | 36 | Male | Adriamycin |
| 4 | 62 | Male | Idiopathic |
| 5 | 25 | Male | Valvular |
| 6 | 60 | Male | Idiopathic |
| 7 | 65 | Male | Idiopathic |
| 8 | 51 | Male | Ischemic |
| 9 | 61 | Male | Ischemic |
| 10 | 49 | Female | Familial |
As in Fig. 1 A and B.
To elucidate its function in cardiomyocytes, we examined the localization of PINK1 by Western blot analysis of biochemically fractionated cellular extracts of murine adult ventricles. The mitochondrial localization of PINK1 was also investigated by confocal immunofluorescence microscopy in isolated mouse neonatal ventricular cardiomyocytes. We observed that the subcellular localization of PINK1 in cardiomyocytes was restricted exclusively to the mitochondrial compartment (Fig. 1 C and D). In murine models of HF, PINK1 protein levels were decreased within 24 h (Fig. 1E) and appreciable levels of cytoplasmic or nuclear PINK1 protein were never observed (Fig. 1F).
PINK1 Deficiency Disturbs the Normal Regulation of Mitochondrial Transmembrane Potential (ΔΨm) in Cardiomyocytes.
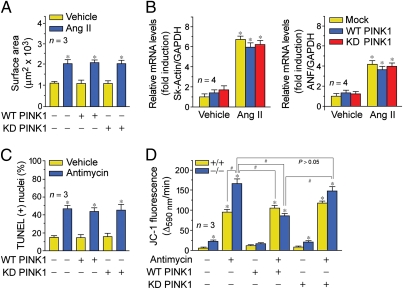
To demonstrate the impact of PINK1 on hypertrophic growth, we treated isolated neonatal rat ventricular cardiomyocytes with angiotensin II (Ang II; 100 nM) for 24 h. To determine whether PINK1 could prevent the development of hypertrophy in response to Ang II, isolated cardiomyocytes were transduced with wild-type (WT) and catalytically inactive, kinase-dead (KD) PINK1 lentiviruses. Although ectopic WT PINK1 alone did not induce hypertrophy, an increase in cell surface area and expression of fetal hypertrophic genes was seen when combined with Ang II. This response was unaffected by KD PINK1 (Fig. 2 A and B and Fig. S1A). We could not confirm the importance of kinase activity in this process due to lack of antibodies capable of immnoprecipitating endogenous PINK1 (Fig. S1B).
Fig. 2.
(A and B) PINK1 overexpression does not affect hypertrophic growth in response to Ang II. (A) Isolated neonatal rat LV cardiomyocytes were transduced with the indicated lentiviruses and incubated with Ang II (100 nM) for 48 h. Fixed cells were stained with antibodies to α-actinin. Mean ± SEM *P < 0.01 versus vehicle. (B) Analysis of hypertrophic marker gene expression (ANF, atrial natriuretic factor; Sk-actin, skeletal α-actin) by qRT-PCR. Mean ± SEM. *P < 0.01 versus mock vehicle. (C) Isolated neonatal mouse LV cardiomyocytes transduced with WT or KD PINK1 lentiviruses and incubated with antimycin (50 μM) for 6 h. Analysis of apoptosis of fixed cells by TUNEL immunofluorescence. Mean ± SEM. *P < 0.01 versus vehicle. (D) The mitochondrial membrane potential (ΔΨm) of PINK1−/− cardiomyocytes is susceptible to ROS-induced depolarization. Ectopic WT PINK1 rescues antimycin-induced decreases of ΔΨm in PINK1−/− cardiomyocytes. Cells, incubated with JC-1 (5 μg/mL), were treated with antimycin (50 μM). JC-1 emission at 535/595 nm was recorded at 1 reading/min for 30 min using a fluorescence spectrophotometer. The rate between two time points (Δemission at 595 nm/min) was calculated in the most linear range of decline for JC-1 fluorescence intensity. Mean ± SEM. *P < 0.01 versus vehicle.
To determine whether PINK1 plays a protective role against ROS-induced apoptosis, murine neonatal ventricular cardiomyocytes were treated with antimycin (50 μM). PINK1−/− cells showed a 30% increase in TUNEL-positive nuclei compared with vehicle-treated controls (Fig. 2C and Fig. S1C). We failed to observe statistically significant differences in cardiomyocytes transduced with either WT PINK1 or KD PINK1 lentiviruses.
To evaluate the consequences of PINK1 loss on mitochondrial function, we examined the integrity of mitochondrial transmembrane potential (ΔΨm) after acute oxidative stress in isolated primary mouse cardiomyocytes from PINK1−/−,+/+ animals. Cells were labeled with the fluorochrome JC-1 (2 μM) to monitor the ΔΨm and then exposed to antimycin (50 μM), which inhibits respiratory complex III, resulting in enhanced ROS production. We observed a ROS-dependent impairment of ΔΨm in PINK1−/− cardiomyocytes compared with littermate control cells at baseline in the absence of antimycin. This was denoted by a decrease in the intensity of JC-1 fluorescence. Antimycin induced a significant decrease in JC-1 emission in PINK1−/−,+/+ cardiomyocytes, although the degree of ΔΨm was more dramatic in the PINK1−/− cells (Fig. 2D). To further prove the requirement of PINK1 in this process, we reintroduced WT PINK1 and KD PINK1 in PINK1−/− cardiomyocytes. We observed that lentiviral transduction of WT PINK1 rescued the detrimental impact of antimycin on ΔΨm in PINK1-deficient cells. In contrast, KD PINK1 did not show such an effect. Whereas our results thus far exclude a role for kinase activity in cardiac hypertrophy, these data are supportive of our view that PINK1 kinase activity is important in the maintenance of normal mitochondrial function. Ideally the ability to demonstrate kinase activity of endogenous PINK1 is required as definitive experimental proof for its role in this process (Fig. S1B). The additional observation of baseline perturbations in mitochondrial membrane potential is of particular importance, reflecting the presence of enhanced ROS production and a probable state of oxidative stress in the PINK1−/− hearts.
PINK1 Knockout Mice Develop Autonomous Age-Dependent Cardiac Hypertrophy.
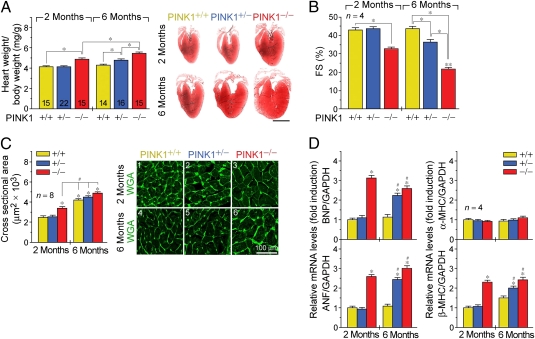
At 1 wk after birth, PINK1−/− mice do not exhibit significant differences in the heart/body weight (HBW) ratios, cardiomyocyte cross-sectional area, or expression of hypertrophic marker genes (Fig. S2 A–C). By 2 mo of age, we found normal cardiac function and no evidence of cardiac hypertrophy in the PINK1+/+ and PINK1+/− mice (Fig. 3 A–C). However, significantly increased HBW ratios and dramatic reductions in fractional shortening (FS) were observed in PINK1−/− mice. In particular, PINK1−/− animals developed cardiac hypertrophy with an average increase of 18% (P < 0.001) in HBW ratios compared with age-matched PINK1+/+, +/− controls. Morphometric analysis of cardiomyocyte cross-sectional areas revealed that genetic deletion of PINK1 resulted in a 148% (P < 0.01) increase in cell area indicating that PINK1 deficiency does not increase cardiomyocyte number. We noticed that LV function was compromised in 2-mo-old PINK1−/− mice as evidenced by a significant reduction in fractional shortening (33% compared with 43% in controls, P < 0.05; Fig. 3B). We also observed increased mRNA transcript levels of atrial natriuretic factor (ANF), brain natriuretic peptide (BNP) and β-myosin heavy chain (β-MHC), without altered expression of α-myosin heavy chain (α-MHC) (Fig. 3D). This change in fetal gene expression is reflective of pathological cardiac hypertrophy. At 6 mo of age, PINK1−/− mice exhibited a further increase in cardiac mass (P < 0.001 versus age-matched controls) and cardiomyocyte cross-sectional area with an incremental worsening in LV function (FS 22% compared with 44% in WT, P < 0.05; Fig. 3 A–C). Prototypical biochemical markers of cardiac hypertrophy were also elevated, indicating that these mice suffer from a classic HF phenotype (Fig. 3D). These results exclude a transient and possible developmental effect of PINK1 deficiency on cardiomyocyte size. We also compared the responses of PINK1 animals subjected to pressure overload, as a result of transaortic banding (TAB). After 4 wk of TAB, PINK1−/−, +/− mice exhibited exaggerated cardiac hypertrophy compared with WT controls (Fig. S3A–E), which is consistent with our concept that loss of PINK1 promotes pathological hypertrophic growth.
Fig. 3.
PINK1−/− mice develop age-dependent cardiac hypertrophy and LV dysfunction. (A) Heart/body-weight ratio (Left) and Masson stain (Right) of myocardial longitudinal sections. (Scale bar, 2 mm.) Mean ± SEM. *P < 0.05. Sample size is indicated inside respective bar. (B) Fractional shortening (FS) determined by echocardiography. Mean ± SEM. *P < 0.05. **P < 0.05 versus PINK1−/− 2 mo. (C) Cardiomyocyte size (Left) and representative immunofluorescence micrographs of wheat germ agglutinin–Alexa Fluor 488 conjugate (WGA) stained myocardial cross-sections (Right). Mean ± SEM. #P < 0.05. *P < 0.05 versus PINK1+/+ 2 mo. (D) Quantitative RT-PCR analysis of mRNA levels of hypertrophic marker genes normalized to GAPDH mRNA. The ratio of hypertrophic markers to GAPDH in PINK1+/+ hearts at 2 mo was arbitrarily set to 1. *P < 0.05 versus PINK1+/+ 2 mo. Mean ± SEM. #P < 0.05 versus PINK1+/+ 6 mo.
Although PINK1+/− mice were initially normal, by 6 mo of age they developed cardiac hypertrophy with an average increase of 11% in HBW ratios (P < 0.05 versus WT) and worsening of cardiac function (Fig. 3 A–C), demonstrating that PINK1 function is haploinsufficient in suppressing a signaling mechanism that conveys pathological hypertrophy. We infer from our data that loss of PINK1 induces spontaneous hypertrophy in the adult heart.
During pathological hypertrophy, a mismatch between the number of capillaries and the size of cardiomyocytes develops, contributing to the development of LV dysfunction (14, 15). At 2 and 6 mo of age, the number of microvessels were significantly smaller in PINK1−/− hearts than in PINK1+/+, +/− controls (Fig. S4A). Thus, inhibition of PINK1 exerted detrimental effects on cardiac performance, and these effects may be attributable in part to PINK1-dependent decreases in neovascularization.
Another hallmark of decompensated hypertrophy is a proliferative response of cardiac fibroblast, leading to the development of cardiac fibrosis (16). At 2 and 6 mo of age, morphometric quantification revealed significantly increased interstitial cardiac fibrosis in PINK1−/− mice (Fig. S4B), compared with WT animals at both time points examined. These results indicate that PINK1 regulates several key aspects of cardiac remodeling, including the protection and activation of disparate cell types involved in the production of extracellular matrix and angiogenesis, all of which contribute to the development and pathogenesis of HF.
Enhanced Oxidative Stress in PINK1 Knockout Mice.
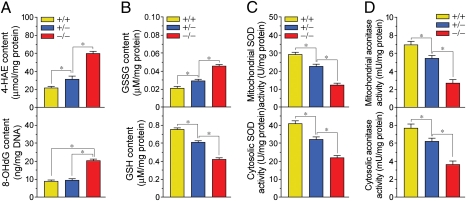
With the demonstration that PINK1 deficiency is accompanied by abnormal mitochondrial function and the development of HF, we expected that loss of PINK1 correlated with increased amounts of ROS being produced and an inability of the heart to eliminate these toxic intermediates. In keeping with this paradigm, we observed higher levels of cytotoxic 4-hydroxyalkenals (4-HAE), an indicator of ROS-dependent lipid peroxidation in PINK1−/− mice compared with WT (Fig. 4A, Upper). Similarly, levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a principal oxidative lesion that can cause base mispairing and mutations (17), were significantly greater (2.3-fold; P < 0.01) in PINK1−/− mice than their PINK1+/+, +/− counterparts (Fig. 4A, Lower). These data indicate that, whereas PINK1 is located within the mitochondria, it has far-reaching consequences in that its loss will ultimately have profound effects on cellular responses to oxidative stress.
Fig. 4.
Enhanced cardiac oxidative stress in PINK1−/− mice. (A) 4-hydroxyalkenals (4-HAE; Upper) and 8-hydroxy-2′-deoxyguanosine (8-OhdG; Lower), (B) oxidized glutathione (GSSG; Upper) and reduced glutathione (GSH; Lower) levels, (C) superoxide dismutase (SOD), and (D) aconitase activities, in total LV extracts from 2-mo-old PINK1 littermates. Mean ± SEM. *P < 0.05. n = 4.
To further substantiate our notion that PINK1 loss results in enhanced oxidative stress, we next measured the reduced glutathione/oxidized glutathione (GSH/GSSG) ratios and activity of superoxide dismutase (SOD), essential cardiac antioxidants (18). We observed that GSH/GSSG ratios and cytosolic and mitochondrial SOD activities were significantly lower in PINK1−/− mice compared with WT siblings (Fig. 4 B and C). In addition, both mitochondrial and cytoplasmic aconitase activities, alternate markers of oxidative stress (19, 20), were significantly decreased in PINK1−/− mice compared with WT animals (Fig. 4D). The noted statistically significant difference in oxidative stress observed between PINK1+/− and PINK1+/+ animals implies that PINK1 heterozygosity confers susceptibility to changes in the cardiac phenotype. We infer from this data that PINK1 loss results in enhanced ROS production, inhibiting the activity of several cellular detoxification networks residing in the cytosolic and mitochondrial compartments. In addition, the lack of PINK1 with abnormal increases in ROS levels may act synergistically to enhance the development of cardiac hypertrophy.
PINK1 Protects Cardiomyocytes from Apoptosis.
At low concentrations, ROS function as second messengers promoting cardiac hypertrophy, but at high concentrations, ROS are cytotoxic. Thus, we analyzed whether cardiac myocytes in 2-mo-old PINK1−/− were susceptible to cell death at baseline, as would be predicted if mitochondrial PINK1 is a negative regulator of mitochondrial ROS. We observed an induction of caspase-3 proteolytic activity and significantly increased numbers of TUNEL-positive cardiomyocyte nuclei in PINK1−/− hearts compared with PINK1+/+, +/− hearts (Fig. S4 C and D). We also detected significantly increased rates of cell death in TAB-treated PINK1−/− hearts (Fig. S5 A–C). These results support our view that PINK1 triggers cardiomyocytes apoptosis by oxidative stress.
PINK1 Knockout Mice Develop Mitochondrial Dysfunction.
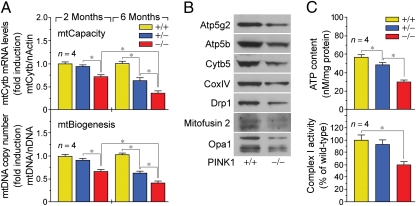
From our observations that (i) PINK1 is localized to the mitochondrial compartment, and (ii) PINK1−/− mice exhibit enhanced oxidative stress, we surmised that the baseline number and capacity of mitochondria are also decreased in PINK1−/− mice. Impaired mitochondrial (mt) biogenesis is a key marker of physiologic stress or genetic mutations. Mitochondria have multiple circular genomes (mtDNA) that are replicated independently from the nuclear genome (nDNA). To assess mitochondrial biogenesis, the mtDNA copy number was determined by qPCR of the mt-gene cytochrome b (Cytb) normalized to levels of a nuclear-encoded single-copy gene, β-actin. The ratio of mtDNA to nDNA in PINK1+/+ hearts was arbitrarily set to 1. At 2 mo of age, we noticed a marked reduction (39%; P < 0.05) in mitochondrial copy number in PINK1−/− hearts, compared with littermate controls (Fig. 5A, Lower). We also used mitochondrial gene transcription as a surrogate for mitochondrial capacity. The mtDNA encodes 13 core polypeptides involved in oxidative phosphorylation in addition to 22 tRNA and 12S and 16S rRNA genes for mitochondrial protein synthesis. The mRNA expression levels of the mitochondrial Cytb gene were measured by qRT-PCR and corrected for the transcript expression of nuclear β-actin. The ratio of transcript levels of mtCytb to N-actin in PINK1+/+ mice was arbitrarily set to 1. We found that the mRNA levels of Cytb were also reduced in the PINK1−/− hearts, compared with littermate controls (Fig. 5B Upper). In addition, at 6 mo of age, a progressive deterioration of mtDNA replication and mt gene expression were evident in both PINK1−/− and PINK1+/− mice (Fig. 5A).
Fig. 5.
Impaired cardiac mitochondrial biogenesis and bioenergetics in PINK1−/− mice. (A) Impaired mitochondrial (mt) biogenesis in 2-mo-old PINK1−/− mice (Lower). mtDNA copy number of mt-gene cytochrome b (Cytb) normalized to levels of nuclear encoded single-copy gene β-actin. We used mt-gene transcription as a surrogate for mt-capacity (Upper). mRNA expression levels of the mtCytb gene were measured by qRT-PCR and corrected for transcript expression of nuclear β-actin. Mean ± SEM. *P < 0.05. (B) PINK1 deficiency induces global down-regulation of mitochondrial protein expression in cardiac ventricles of 2-mo-old mice. (C) Impaired ATP production (Upper) and reduced complex I (NADH dehydrogenase) activity in total LV extracts from 2-mo-old PINK1−/− mice (Lower). Mean ± SEM. *P < 0.05.
Having shown that there is a reduction in mitochondrial DNA replication and mitochondrial gene expression, we reasoned that mitochondrial protein expression would also be affected with the loss of PINK1. Western blot analysis of mitochondrial extracts demonstrates the down-regulation of several factors involved in diverse processes such as oxidative phosphorylation (ATP synthases: Atp5g2, Atp5b; cytochrome oxidases: Cytb5; CoxIV), mitochondrial fission (Drp1), and mitochondrial fusion (mitofusin 2, Opa1) in PINK1−/− hearts compared with PINK1+/+ hearts (Fig. 5B). These data support the notion that PINK1 deficiency globally affects protein levels of factors important in the maintenance of mt-biogenesis and bioenergetics.
To determine whether ultrastructural changes in mitochondria within the PINK1−/− cardiomyocytes were present, we used transmission electron microscopy. Gross abnormalities in the mitochondrial structure were not observed in PINK1−/− cardiomyocytes; rather, a significant increase in cross-sectional area was noted in the PINK1−/− cells, compared with PINK1+/+ cardiomyocytes (93 ± 13 μm2 and 82 ± 6 μm2 in PINK1−/− and PINK1+/+ cardiomyocytes, respectively, n = 150–200; P < 0.01). Therefore, the mitochondria are larger in size, a finding typical of mitochondrial swelling.
The healthy adult heart has an extensive capacity to generate ATP in an effort to match the energy demand to the variability in cardiac workload. Oxidative stress-induced impairment of respiratory activity and decline in ATP metabolism is detrimental for the contractile function and viability of cardiomyocytes (21, 22). Consistent with these data, we observed a significant decrease (48%; P < 0.01) in ATP levels in total LV samples derived from PINK1−/− mice compared with PINK1+/+, +/− animals (Fig. 5C, Upper). Next, we measured the activity of the mtDNA-encoded oxidative phosphorylation complex I by an immunocomplex in vitro assay. The biochemical measurement of respiratory chain function revealed reduced complex I activity (60%; P < 0.01) in PINK1−/− mice compared with PINK1+/+, +/− controls (Fig. 5C, Lower). Therefore, decreases in mt-bioenergetics do occur concomitantly with decreases in mt-biogenesis.
Discussion
We present several noteworthy observations from our genetic deletion studies supporting the importance of PINK1 in the murine heart during postnatal development. In particular, PINK1−/− mice develop LV dysfunction with a reduced fractional shortening and evidence of pathological cardiac hypertrophy as early as 2 mo of age. Associated with this baseline cardiac phenotype we have shown that the PINK1−/− mice have higher degrees of cardiomyocyte apoptosis with fibrosis and reciprocal reduction in capillary density. In addition, the PINK1−/− mice have greater levels of oxidative stress as reflected by higher levels of lipid peroxidation, DNA damage, and decreased aconitase activity in conjunction with reduced activity of several cytoplasmic and mitochondrial antioxidative systems. In response to biomechanical stress, the PINK1−/− mice developed a further exaggeration in the degree of cardiac hypertrophy compared with their littermate controls. Of clinical relevance is the finding that protein levels of PINK1 in end-stage human HF were also diminished, supporting a reciprocal relationship between PINK1 activity and HF. These findings all support our view that PINK1 is indispensable for normal heart function. However, it is important to remember that the generation of the PINK1−/− mice is not done as a conditional heart-specific knockout and that the influence of PINK1 loss on the vascular bed and other organs, which may contribute to the phenotypic changes in the heart, has not been studied.
Conceivably, the mitochondrial localization of PINK1 is of great importance for normal cardiac function. This is reflected in the degree of severity of the mitochondrial defect observed in the PINK1−/− mice. More specifically, we observed that loss of PINK1 resulted in (i) increased sensitivity to ROS-dependent depolarization of the mitochondrial membrane potential, (ii) decreased oxidative phosphorylation, (iii) reduced mitochondrial replication, (iv) mitochondrial swelling, and (v) global alterations in expression of mitochondrial proteins. However, we cannot specifically identify the mechanism that confers an increased susceptibility of the PINK1−/− mice in developing their baseline cardiac phenotype. Our analysis of the neonatal litters excludes the possibility of mitochondrial dysfunction resulting from an abnormal redox state in PINK1−/− mice. Rather, we favor the scenario in which inactivation of PINK1 is a primary event in HF progression. PINK1 loss constitutes a mitochondrial defect that establishes a pathological imbalance in ROS production, leading to derangements in mitochondrial bioenergetics. With the generation of abnormal ROS levels within the mitochondria, a cascade of events is initiated, including a decrease in ΔΨm, activation of the mitochondrial permeability transition pore (mPTP), and efflux of protons causing a decline in mitochondrial bioenergetics. The resulting mitochondrial damage may also further translate to the activation of signaling pathways involved in the development of cardiac hypertrophy and subsequent reduced contractility.
Indeed, this premise is supported by observations made by others, in that a role for PINK1 has been linked to the maintenance of mitochondrial function (6, 7, 23, 24). However, if we are to understand exactly how PINK1 affects mitochondria, the identity of potential substrates and/or interacting partners within the mitochondrion would be important to define if a kinase activity is indeed required for PINK1 function. Recently, the mitochondrial chaperone proteins TRAP1 and Omi/HrtA2 have been proposed as possible substrates (25, 26). In this model, Omi is phosphorylated in a PINK1-dependent manner, although whether Omi functions as a direct PINK1 substrate in vivo remains elusive. Previous work from our group has shown that FOXO3a regulates the transcription of PINK1 (27) in transformed cells and the activation of FOXO3a protects against ROS-induced apoptosis by up-regulating the expression of SOD and catalase (28, 29). This antioxidative function of FOXO3a may explain how PINK1 is regulated during cardiac oxidative stress. In conclusion, our work provides genetic proof in vivo for the importance of PINK1 as a mitochondrial protein functioning to regulate the complex interplay between mitochondrial function, ROS production, and hypertrophic signaling in the heart.
Materials and Methods
The generation of the PINK1 gene-deficient mice was previously described (30). Age-matched syngenic male PINK1+/+, +/−, −/− mice (10–12 wk, 22–26 g) were used in this study. All experiments used littermate controls of matched age and sex and in accordance with approved institutional animal care guidelines of the University Health Network (AUP 1772; Canadian Council in Animal Care). Functional analysis was done at various time points after birth and included 2D echocardiograhic, biochemical, histological, and immunofluorescent assessment. Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge M. Badiwala and J. Joseph for their assistance in the procurement of the human LV samples. This work was supported by grants awarded by the Canadian Institutes of Health Research (to T.W.M.). F.B. is the recipient of the Canadian Institutes of Health Research Phase I Clinician-Scientist Award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106291108/-/DCSupplemental.
References
- 1.Braunwald E, Bristow MR. Congestive heart failure: Fifty years of progress. Circulation. 2000;102(20, Suppl 4):IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 2.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 3.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 4.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 5.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas KJ, Cookson MR. The role of PTEN-induced kinase 1 in mitochondrial dysfunction and dynamics. Int J Biochem Cell Biol. 2009;41:2025–2035. doi: 10.1016/j.biocel.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthier A, et al. PINK1 displays tissue-specific subcellular location and regulates apoptosis and cell growth in breast cancer cells. Hum Pathol. 2011;42:75–87. doi: 10.1016/j.humpath.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J. 1993;14:1493–1498. doi: 10.1093/eurheartj/14.11.1493. [DOI] [PubMed] [Google Scholar]
- 11.Borchi E, et al. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta. 2010;1802:331–338. doi: 10.1016/j.bbadis.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Yücel D, et al. Increased oxidative stress in dilated cardiomyopathic heart failure. Clin Chem. 1998;44:148–154. [PubMed] [Google Scholar]
- 13.Khaper N, Kaur K, Li T, Farahmand F, Singal PK. Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction. Mol Cell Biochem. 2003;251:9–15. [PubMed] [Google Scholar]
- 14.Marcus ML, et al. Alterations in the coronary circulation in hypertrophied ventricles. Circulation. 1987;75:I19–I25. [PubMed] [Google Scholar]
- 15.Rakusan K, du Mesnil de Rochemont W, Braasch W, Tschopp H, Bing RJ. Capacity of the terminal vascular bed during normal growth, in cardiomegaly, and in cardiac atrophy. Circ Res. 1967;21:209–215. doi: 10.1161/01.res.21.2.209. [DOI] [PubMed] [Google Scholar]
- 16.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: Involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 17.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 18.Li Y, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 19.Gardner PR. Superoxide-driven aconitase FE-S center cycling. Biosci Rep. 1997;17:33–42. doi: 10.1023/a:1027383100936. [DOI] [PubMed] [Google Scholar]
- 20.Gardner PR, Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- 21.Singal PK, Gupta M, Randhawa AK. Reduced myocardial injury due to exogenous oxidants in pressure induced heart hypertrophy. Basic Res Cardiol. 1991;86:273–282. doi: 10.1007/BF02190607. [DOI] [PubMed] [Google Scholar]
- 22.Maslov MY, et al. Altered high-energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure-overload hypertrophy mice. Am J Physiol Heart Circ Physiol. 2007;292:H387–H391. doi: 10.1152/ajpheart.00737.2006. [DOI] [PubMed] [Google Scholar]
- 23.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 24.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alnemri ES. HtrA2 and Parkinson's disease: Think PINK? Nat Cell Biol. 2007;9:1227–1229. doi: 10.1038/ncb1107-1227. [DOI] [PubMed] [Google Scholar]
- 26.Plun-Favreau H, et al. What have PINK1 and HtrA2 genes told us about the role of mitochondria in Parkinson's disease? Ann N Y Acad Sci. 2008;1147:30–36. doi: 10.1196/annals.1427.032. [DOI] [PubMed] [Google Scholar]
- 27.Mei Y, et al. FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc Natl Acad Sci USA. 2009;106:5153–5158. doi: 10.1073/pnas.0901104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan WQ, Wang K, Lv DY, Li PF. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem. 2008;283:29730–29739. doi: 10.1074/jbc.M805514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundaresan NR, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.