Abstract
Emerging evidence indicates that NADPH oxidase (NOX) and its reactive oxygen species (ROS) products modulate a variety of cellular events, including proliferation, differentiation, and apoptosis. In this study, we investigated the functions of NOX2 and ROS in immune modulation using NOX2 knockout (KO) mice. Interestingly, NOX2 KO mice spontaneously developed arthritis with onset at 6–7 wk of age and high incidence (60%) at 15–18 wk of age. Arthritis severity in NOX2 KO mice was proportionally increased with age and higher in females than in males. Bone destruction was confirmed by microcomputed tomography scanning and histological analyses of joints. Inflammatory factors, including TNF-α, IL-1β, and RANKL, and serum level of anti–type II collagen IgG were significantly increased in NOX2 KO mice. In addition, NOX2 deficiency perturbed the immune system upon aging. NOX2 KO mice demonstrated preferred development of CD11b+Gr-1+ myeloid cells with profound production of proinflammatory cytokines and augmented expression of IL-17 through the activation of STAT3 and RORγt in vivo. NOX2 deficiency increased differentiation of effector Th cells in vitro and decreased CD25+FoxP3+ Treg cells both in vitro and in vivo. Furthermore, adoptive transfer of NOX2-deficient CD4+ T cells into RAG KO mice increased arthritic inflammation compared with WT cells. These results demonstrated that NOX2 deficiency affected the development of CD11b+ myeloid cells and Th17/Treg cells, and thus promoted inflammatory cytokine production and inflammatory arthritis development, strongly supporting a crucial role for ROS generation in the modulation of Th17/Treg cell development and its related inflammatory immune response upon aging.
Keywords: rheumatoid arthritis, myeloid-derived suppressor cell
Reactive oxygen species (ROS), including superoxide, hydrogen peroxide, and hydroxyl radical, are normally produced in the process of cellular energy production and eliminated by antioxidant enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and peroxiredoxins (Prxs). In addition to ROS generation by the mitochondrial electron transport chain, the NADPH oxidase (NOX) complex produces a large amount of ROS in the plasma membrane and cytosol (1). Because ROS have the potential to damage DNA, lipids, and proteins, they are considered the leading cause of many inflammatory diseases, including cancer, atherosclerosis, and arthritis, as well as degenerative diseases (2).
The NOX complex was originally identified in neutrophils and macrophages, which kill microbes by producing ROS, and is often referred to as phagocyte NADPH oxidase (PHOX) (3). PHOX was first cloned in human chronic granulomatous disease (CGD) and referred to as NOX2 or gp91phox. In addition, the membrane-associated protein p22phox and cytosolic proteins p47phox, p40phox, p67phox, and the small GTPase Rac were discovered as subunits of the NOX complex. NOX2 constitutively associates with p22phox and is activated by subsequently recruiting p47phox, p40phox, and p67phox to the NOX2/p22phox complex in the membrane (4). When Rac finally interacts with the NOX2 complex, NOX2 generates superoxide by transporting electrons from intracellular NADPH to oxygen (5). Recently, several NOX family members have been implicated in the regulation of a variety of cellular functions, such as senescence, apoptosis, thyroid hormone synthesis, blood pressure regulation, and vestibular organogenesis (3, 6).
The importance of the NOX2 complex in innate immunity against bacterial and fungal infections is manifested in CGD, in which patients commonly inherit abnormalities of NOX2, p22phox, p47phox, or p67phox and display persistent inflammation in many tissues, including lung, liver, kidney, and colon (7, 8). Interestingly, mice lacking p47phox or gp91phox were lethally susceptible to microbial infection, developed granulomatous inflammation similar to symptoms in human CGD patients (9, 10), and demonstrated intensified chondrocyte destruction and joint inflammation in an experimental arthritis model (11–13). Inactivation of p22phox also resulted in clinically CGD-like immune defects in mice due to the inability of phagocytes to produce bacteria-killing ROS (14). In addition to phagocytes, T cells produce ROS through NOX2 activation (15, 16). ROS determine the reactivity and disease susceptibility of T cells (17, 18). Abnormal T-cell function is a common feature of autoimmune diseases, including lupus erythematosus, IgA nephropathy, arthritis, and autoimmune pulmonary disease (19), and frequently occurs in CGD patients (20). In particular, CD4+ T helper (Th) cells are critical mediators of immunological homeostasis and autoimmune response modulation by generating different effector Th subsets (Th1, Th2, and Th17) and regulatory T (Treg) cells (21, 22). Effector Th1, Th2, and Th17 cells produce signature cytokines (IFN-γ, IL-4, and IL-17, respectively) through the action of specific transcription factors (T-bet, GATA-3, and RORγt). Whereas IFN-γ and IL-17 are involved in infectious and autoimmune responses, Th2 cytokines IL-4, IL-5, and IL-13 control allergic immunity. Treg cells are also generated from CD4+ T cells by activation of FoxP3 upon TGFβ stimulation, and these cells suppress the development and function of effector Th cells (23, 24). Imbalance between Th1/Th17 and Treg cells is closely associated with autoimmune disease. Previous studies of NOX2-deficient mice have reported increased arthritis development and enhanced Th17 and IFN-γ production, but only upon stimulation with chicken type 2 collagen (CII) and complete Freund's adjuvant (CFA), and found evidence that NOX2-deficient dendritic cells produced higher levels of TGF-β and IL-6 than WT cells, thus resulting in enhanced TH17 development in NOX2 KO mice (11, 13). However, the effects of NOX2 deficiency on intrinsic IL-17 production between WT and KO T cells have not yet been determined.
In the current study, we investigated the impact of NOX2 deficiency on physiological changes upon aging. NOX2-deficient mice spontaneously developed arthritis in paws and joints, exhibiting age- and sex-related changes, and increased inflammatory cytokine levels. In addition, we observed that NOX2 and its own ROS products were involved in the control of Th17 and Treg cell differentiation.
Results
Spontaneous Arthritis Developed in NOX2-Deficient Mice.
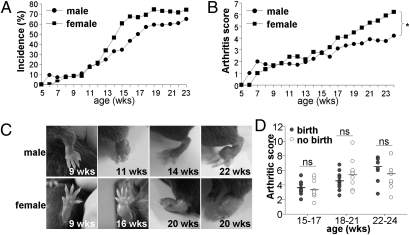
To clarify the intrinsic functions and cellular mechanisms of NOX2 in inflammation, we performed a longitudinal experiment using NOX2-deficient mice (KO). We observed that KO mice spontaneously developed arthritis at as early as 6 or 7 wk of age, and the incidence increased to 60–70% by 15–18 wk of age (Fig. 1A). The prevalence and incidence of arthritis increased with age and were more prominent in female than in male mice >13 wk of age (Fig. 1A). Furthermore, the increase in arthritis severity was proportional to age, and significantly higher in females (Fig. 1B). Swelling was observed in paws, digits, ankles, knees, and shoulder joints, and was also more severe in the aged female group (Fig. 1C). Additional analysis revealed that birth experience was not critically associated with arthritic severity in females (Fig. 1D). These results strongly indicate that NOX2 KO mice spontaneously develop arthritis upon aging, with a female predominance.
Fig. 1.
Aging-dependent arthritis development in NOX2 KO mice. All mice were examined in a blind manner. (A) Arthritis incidence was calculated in male (n = 73) and female (n = 63) mice. (B) Arthritis severity was scored in arthritic KO mice (n = 25 males, n = 34 females). *P < 0.05. (C) Pictures of swollen paws in KO mice. (D) Arthritis severity was determined in two female groups (n = 10): those with birth experience (birth) and those with no experience (no birth). ns, not significant.
Bone Abnormality and Inflammatory Arthritis Were Profound in NOX2 KO Mice.
Because bone abnormalities and reduction of trabecular bone mineral density (BMD) are very common in arthritis patients (25), we investigated the tomography of bone and BMD using a high-resolution in vivo microcomputed tomography (micro-CT) scanner. Scanned images confirmed bone erosion and destruction in the arthritic lesions of NOX2 KO mice (Fig. 2A). Histological analysis of knee joints verified the disordered arrangement and diminution of chondrocytes, granuloma formation, and infiltrating leukocytes in KO mice (Fig. 2B). In addition, trabecular BMD was significantly attenuated in KO mice (Fig. 2C), whereas the bone erosion marker RANKL (receptor activator of nuclear factor kappa B ligand) was substantially increased in NOX2-deficient bone marrow (Fig. 2D). Furthermore, NOX2 deficiency enhanced the expression of tartrate-resistant acid phosphatase (TRAP), matrix metalloproteinases-9 (MMP-9), and proinflammatory cytokines IL-1β and TNF-α (Fig. 2 E and F) in joint cells. Subsequent analysis of serum Ig levels demonstrated that IgE, IgA, and specific autoantibodies against CII IgG1 and CII IgG2a were significantly elevated in KO mice (Fig. 2 G and H). These results suggest that in NOX2 KO mice, arthritis was accompanied with substantial bone deterioration and increased expression of inflammatory arthritis mediators.
Fig. 2.
Characterization of inflammatory arthritis in KO mice. All mice were analyzed at 15–18 wk of age. (A) WT and KO mice (n = 6) were anesthetized and scanned with the eXplore Locus in vivo micro-CT scanner. Representative images of paw and knee joint are presented. (B) Knee joint was sectioned and stained with H&E (n = 5). BM, bone marrow; C, chondrocyte. (Scale bars: Upper, 100 μm; Lower, 50 μm.) (C) BMD was calculated using the micro-CT scanner, and mean ± SEM for six mice was determined. (D) Bone marrow cells were stained with anti-RANKL Ab, followed by flow cytometry (n = 4). (E and F) Reverse transcription and quantitative real time-PCR determined the expression level of TRAP and MMP-9 (E) and proinflammatory cytokines IL-1β and TNF-α (F) in total joint cells. Data are expressed as mean ± SEM for four mice. (G) Whole blood was collected by an eye bleeding, and the resulting serum was subjected to ELISA to measure IgE and IgA. (H) ELISA plate precoated with CII collagen was incubated with serum and subsequently incubated with anti-mouse Ig subtypes. Color changes with substrate were determined, and data were obtained from at least three independent experiments (n = 6). *P < 0.05, **P < 0.005, ***P < 0.0005.
Disorders of the Immune System Were Substantial in NOX2-Deficient Mice.
We next explored the development of immune cells in NOX2 KO mice. NOX2-deficient bone marrow was pale, with few or no erythrocytes, compared with that in WT mice. Myeloid cells expressing CD11b and Ly6C occupied up to 90% of pale bone marrow cell populations (Fig. 3A). In addition, lymphomegaly and splenomegaly were remarkable in KO mice, as evidenced by three- to fourfold increases in total cell number (Fig. 3B). Whereas T cells were decreased in the lymph nodes of KO mice compared with those of WT mice, the numbers of other cells, including B220+ B cells, F4/80+ macrophages, and CD11c+ cells were increased. In particular, CD11b+Gr-1+ myeloid cells were strikingly expanded in KO mice (Fig. 3C). The percentages of CD4+ and CD8+ T cells were consistently diminished in KO spleen; however, NOX2-deficient T cells expressed higher levels of activation marker CD69 (Fig. 3D), suggesting an aberrant function of T cells in KO mice. Consistent with the increased number of myeloid precursors in bone marrow, CD11b+ myeloid cells—particularly, CD11b+Gr-1+ myeloid cells—were strikingly increased in NOX2-deficient spleen, and expressed high levels of activation markers CD86 and CD80 (Fig. 3E). Upon LPS stimulation, CD11b+ myeloid cells produced the most TNF-α and IL-6 in NOX2 KO mice (Fig. 3F). Though the total amount of TNF-α was higher in NOX2 KO mice than in WT mice, total IL-6 level was comparable between WT and KO spleen upon LPS stimulation. Our results further suggest that NOX2 may be important for the modulation of development and activation of CD11b+Gr-1+ myeloid cells.
Fig. 3.
Aberrant immune cell development in NOX2 deficiency. All mice were analyzed at 15–18 wk of age. (A) Hind limbs were collected from WT and KO mice (n = 5). Bone marrow cells were isolated from femur and tibia and stained with anti-CD11b or anti-Ly6C. (B) Comparison of lymph node and spleen between WT and KO mice. Total cell number was determined as mean ± SEM for five mice. *P < 0.05, ***P < 0.0005. (C–E) Single-cell suspensions of spleen were stained with surface marker Abs as indicated, and then subjected to flow cytometry analysis. (F) Spleen cells (n = 4) were stimulated in vitro with LPS (0.1 μg/mL) for 6 h and incubated with anti–TNF-α or anti–IL-6 for intracellular cytokine staining.
IFN-γ and IL-17 Production by CD4+ T Cells Were Enhanced in NOX2 KO Mice.
To assess the expression levels of IFN-γ and IL-17 associated with arthritis development (26), total lymph node cells from WT and KO mice were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 4 h. IFN-γ and IL-17 were produced at higher levels in NOX2-deficient cells than in WT cells, and were substantially produced by CD4+ T cells (Fig. 4A). Furthermore, RORγt expression and tyrosine phosphorylation of STAT3 were greatly increased in the spleen cells of KO mice (Fig. 4B), indicating that NOX2 deficiency promoted Th17 cell development. We next evaluated cytokines produced by CD11b+ myeloid cells isolated from the spleens of WT and KO mice. Although IL-6 expression was unchanged between WT and KO CD11b+ cells, IL-1β and TNF-α were significantly increased in KO cells (Fig. 4C). Addition of NOX2-deficient CD11b+ cells to CD4+ T cells also substantially enhanced IFN-γ and IL-17 production compared with WT CD11b+ cells (Fig. 4D). Both WT and KO CD11b+ cells had no effect on CD4+ T-cell proliferation in response to T-cell receptor (TCR) stimulation (Fig. 4E). These findings suggest that NOX2 deficiency may alter cytokine production by CD11b+ myeloid cells and thus affect development of Th1 and Th17 cells.
Fig. 4.
Increased IFN-γ and IL-17 production in NOX2-deficient cells. WT and KO mice were analyzed at 12–18 wk of age. (A) Total lymph node cells were stimulated with PMA and ionomycin for 6 h, and subjected to measurement of IFN-γ and IL-17 by ELISA (n = 5) and intracellular cytokine staining by gating CD4+ T cells. nd, not detected. **P < 0.005, ***P < 0.0005. (B) The expression of NOX2, pSTAT3, STAT3, RORγt, and β-actin in spleen was determined by immunoblotting. (C) CD11b+ cells were isolated, stimulated with LPS (0.1 μg/mL) for 6 h (n = 6), and harvested for total RNA preparation. Relative transcript levels of IL-6, IL-1β, and TNF-α were determined. (D) WT and KO CD4+ T cells were activated with anti-CD3 and anti-CD28 for 72 h along with CD11b+ cells that were stimulated with LPS for 6 h. Culture supernatants were analyzed by ELISA to determine IFN-γ and IL-17 levels. (E) CD4+ T cells were labeled with CFSE and cultured in the presence of LPS-stimulated CD11b+ cells. After 72 h, cells were analyzed using a FACSCalibur flow cytometer. CD4+ and CD11b+ cells in C, D, and E were isolated from spleen. **P < 0.005.
Nox2 Deficiency Compelled Effector Th Cell Development and Preferentially Affected Th17 Cell Differentiation.
Because IFN-γ and IL-17 production were enhanced in NOX2-deficient cells, we further analyzed T-cell–autonomous functions of NOX2 and performed in vitro Th cell differentiation from naïve CD4+ T cells. Development of Th1, Th17, and Th2 cells was significantly accelerated in KO cells, as evidenced by increased levels of IFN-γ from Th1 cells, IL-17 from Th17 cells, and IL-5, IL-4, and IL-13 from Th2 cells (Fig. 5A). After stimulation with anti-TCR for 30 h, the levels of lineage-specific transcription factors T-bet and GATA-3 were not changed in WT and KO CD4+ T cells. However, RORγt level was increased in KO cells (Fig. 5B), suggesting that NOX deficiency could modulate Th17 cell development in a T-cell–intrinsic manner. Interestingly, FoxP3 expression was somewhat decreased in NOX2-deficient cells (Fig. 5B). Consistent with Th17 elevation by NOX2 deficiency, treatment of Th17 cells with diphenyleneiodonium (DPI), a general inhibitor of flavoenzymes such as NOX, reduced ROS levels and increased IL-17 expression (Fig. 5 C and D). Moreover, expression of RANKL was increased fourfold in developing CD4+ T cells of KO mice (Fig. 5E), coinciding with the higher expression of RANKL + Th17 cells in inflammatory arthritis (27). These results indicate that ROS may directly modulate Th17 cell development in a T-cell–intrinsic manner.
Fig. 5.
Promoter Th17 cell development in the absence of NOX2. (A) Naïve CD4+ T cells were isolated and stimulated with plate-bound anti-CD3 and anti-CD28. Cells were additionally cultured under Th1-, Th17-, or Th2-skewing conditions for 5 d. After restimulation with anti-CD3 for 24 h, signature cytokines IFN-γ and IL-17, and Th2 cytokines IL-4, IL-5, and IL-13, were determined in Th1, Th17, and Th2 cells, respectively. (B) CD4+ T cells were harvested by stimulation with anti-CD3 and anti-CD28 for 30 h. Relative expression levels of T-bet, GATA-3, RORγt, and FoxP3 were determined by quantitative real-time PCR. (C–E) Th17 cells were generated and treated with vehicle (–) or 10 μM DPI (+) for 4 h. Cells were stained with CM-dichlorodihydrofluorescein diacetate (DCFDA) (C) or harvested for measuring the relative expression of IL-17 mRNA (D). (E) CD4+ T cells were activated for 3 d and analyzed with anti-RANKL Ab. Three independent experiments were conducted for all data in A–D. One representative result is presented in E. All mice were killed at 12–18 wk of age. *P < 0.05, ***P < 0.0005.
NOX2 Deficiency Eliminated Natural and Inducible Treg Cells.
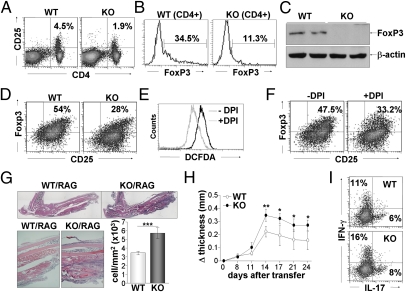
All effector Th subsets were enlarged in KO mice, and FoxP3 level was significantly decreased in NOX2-null CD4+ T cells, prompting us to investigate the effects of ROS on Treg cell development. Peripheral CD4+CD25+ natural Treg cells and FoxP3 expression were substantially decreased in KO mice (Fig. 6 A and B). FoxP3 protein level was also diminished in spleen cells of KO mice (Fig. 6C). We additionally induced Treg cell development from CD4+CD25− T cells in vitro. CD25+FoxP3+ Treg cells were produced at a rate of 54% in WT, whereas NOX2-deficient cells generated 28% Treg cells (Fig. 6D), suggesting that NOX2 is involved in FoxP3+ Treg cell development. To ensure the ROS function in Treg cell development, Treg cells were treated with DPI. DPI treatment significantly lowered the ROS level and reduced development of FoxP3+ Treg cells (Fig. 6 E and F). These results indicate the critical role of ROS in controlling the development of FoxP3+ Treg cells in vitro and in vivo.
Fig. 6.
Attenuation of FoxP3+ Treg cells by NOX2 deficiency. (A–C) Total spleen cells obtained from WT and KO mice were stained with anti-CD4, anti-CD25, and anti-Foxp3 according to the manufacturer's protocol. CD4- and CD25-expressing cells are presented in dot plots (A) and FoxP3-expressing CD4+ cells are expressed in histogram plots (B). (C) The level of FoxP3 in spleen cells was determined by immunoblotting assay. (D) Inducible Treg cells were generated from naïve CD4+ T cells of WT and KO mice in the presence of anti-IFN-γ, anti-IL-4, IL-2, and TGF-β and stained with anti-CD25 and anti-Foxp3. (E and F) Inducible Treg cells were treated with vehicle (–) or DPI (+) for 4 h and incubated with CM-DCFDA dye (E) or anti-CD25 and anti-Foxp3 (F). (G and H) CD4+ T cells were isolated from WT and KO mice at 16 wk of age and transferred into RAG KO mice (n = 6), followed by injection with CII. Mice were analyzed 4 wk after adoptive transfer. (G) Paws were evaluated by histological staining, and infiltrating immune cells were quantitatively analyzed using the Tissue FAXS program. ***P < 0.0005. (H) Paw thickness was measured, and the difference was calculated in a time-dependent manner. *P < 0.05. (I) Cells were harvested from lymph node of reconstituted RAG KO mice, stimulated with PMA and ionomycin for 4 h, and then subjected to staining for IFN-γ and IL-17 production.
To confirm the critical role of CD4+ T cells in arthritis development, we carried out the adoptive transfer of CD4+ T cells into RAG KO mice, followed by collagen injection. WT CD4+ T cells moderately induced inflammation in the foot, whereas KO CD4+ T cells significantly augmented inflammatory arthritis in RAG KO mice. Immune cell infiltration was significantly greater in NOX2 deficiency compared with control (Fig. 6G). Furthermore, increases in paw thickness were more substantial in the RAG KO mice injected with NOX2-deficient CD4+ T cells (Fig. 6H). Collectively, NOX2-deficient CD4+ T cells may be involved in the development of inflammatory arthritis in vivo.
Discussion
Our findings show that NOX2 KO mice spontaneously develop arthritis upon aging, with a female preponderance, which is phenotypically similar to human arthritis. NOX2 deficiency compelled the development of CD11b+ myeloid cells and augmented expression of inflammatory cytokines and RANKL, resulting in bone erosion and destruction, and joint inflammation. In addition, the lack of NOX2 affected both FoxP3 and RORγt expression in CD4+ T cells, thus increasing Th17 cells and diminishing Treg cell development in a ROS-dependent and T-cell–intrinsic manner.
Numerous animal models of arthritis have been developed by the use of transgenes, gene deletion, or various stimuli, and exhibit common arthritis features of joint inflammation, production of autoantibodies against cartilage components, and bone abnormalities. Although no single animal model of arthritis fully represents the human arthritis disease, experimental arthritis models have contributed to understanding the mechanisms of arthritis, in part. The increased expression of cytokines IL-1β, IL-6, and TNF-α, production of autoantibodies, and persistent activation of T cells play key roles in the initiation of arthritis and pathogenesis of destructive arthritis (28). Given the prominent production of IL-17 by Th17 cells, IL-17 and Th17 cells have recently come into the focus of arthritis research as potential therapeutic targets (29). In addition to Th17 cells, Treg cells are believed to be beneficial for controlling arthritis pathogenesis because Treg cells are deficient in arthritis patients (30). Based on our findings that NOX2-deficient mice developed extensive cartilage and bone destruction and granulomatous synovial inflammation with aging, the NOX2 KO system is strongly suggested to act as a spontaneous arthritis model close to human disease, which may promote pathway analysis in arthritis and provide beneficial roles in therapeutic targeting.
Interestingly, NOX2 deficiency increased the number of CD11b+ myeloid cells in bone marrow, draining the lymph node and spleen. These CD11b+ myeloid cells were ascertained as Gr-1+ neutrophils or granulocytes, not macrophages. CD11b+Gr-1+ myeloid cells have been identified as myeloid-derived suppressor cells (MDSCs), causing T-cell dysfunction and antigen-specific T-cell tolerance in tumor (31). MDSCs are increased in a tumor-bearing model, in which they induce the activation of tumor-specific Treg cells and thereby inhibit antitumor immune response (32). Accordingly, expansion of MDSCs and inactivation of Treg cells are remarkable in experimental autoimmune encephalomyelitis (33) and inflammatory bowel disease (34). However, our results show that immunosuppressive MDSCs were increased in NOX2 KO mice, whereas Treg and Th17 cells were respectively diminished and promoted. Moreover, total IL-6 levels produced by CD11b+ myeloid cells were comparable between WT and KO mice. These findings suggest that NOX2 deficiency may have distinct roles in the differentiation of CD4+ T cells and MDSCs, noting prominent T-cell functions in arthritis development in the absence of NOX2.
Our results demonstrate that NOX2 deficiency or ROS depletion significantly promoted development of effector Th subsets Th1, Th2, and Th17 cells, and suppressed development of natural and inducible Treg cells, thereby resulting in destruction of joint and bone. These results imply that the basal level of ROS generated in the process of aging is essential for immune homeostasis by controlling Th17/Treg balance. This conclusion is consistent with a previous report that increased ROS generation in GPx1- or PrxIII-deficient T cells attenuated Th17 cell differentiation and its related immune response (35). It is likely that disruption of the precise fine-tuning between generation and elimination of ROS may cause inflammation in multiple tissues in a T-cell-dependent mechanism. Our study here demonstrates that CD4+ T cells were intrinsically biased toward Th17 development in NOX2 deficiency, influencing spontaneous arthritis development in NOX2 KO mice upon aging. Based on the findings of the present study, future investigations are necessary to demonstrate the precise molecular functions of several ROS-generating and -scavenging molecules involved in cytokine production in T cells and T-cell–mediated immune responses.
Materials and Methods
Materials.
All cytokines and anti-cytokine Abs were purchased from BD Pharmingen, eBioscience Inc., and R&D Systems. LPS was obtained from Sigma-Aldrich Corp.
Mice.
WT and NOX2 KO mice (10) were purchased from the Jackson Laboratory and maintained under specific pathogen-free conditions at Ewha Womans University. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) and were approved by the IACUC committee at Ewha Womans University.
Measurement of Arthritis Incidence and Severity.
Mice were examined every other day, and an arthritic severity score was assigned to the mice in a blind manner. Arthritis severity was calculated using a macroscopic scoring system: one point was given for swelling of one digit, mild redness, or slight swelling of ankle or wrist; two points for swelling of more than two digits or severe swelling of ankle or wrist; three points for severe swelling of digits, ankle, wrist, knee and elbow; and four points for maximal inflammation, including severe swelling of digits, ankle, wrist, knee, elbow, hips, or shoulder joint, adding up to a maximal score of 16 points per mouse.
Micro-CT Analysis.
In vivo micro-CT using an eXplore Locus scanner (GE Healthcare Life Sciences) was conducted on every mouse. Scanning was performed by optimized micro-CT use protocol. The X-ray tube voltage was set to 80 kV; anode current, 450 μA; number of views, 400; exposure time, 400 ms; scan technique, short scan; detector bin mode, 2 × 2; and effective pixel size, 0.046 mm. After scanning, 3D images were reconstituted from images of the regions of interest and maximum intensity projection. Trabecular BMD was also evaluated by micro-CT.
Histological Analysis.
Hind paws obtained from WT and KO mice were fixed in 10% formalin at 4 °C for 5 d and then washed in running tap water for 1 h. Hind limbs were dissected, decalcified in 10% EDTA in 0.1 M Tris (pH 7.4) buffer, and embedded in paraffin. Tissue sections were cut at 10-μm thickness and stained with H&E solution. Images were collected using an Eclipse E200 microscope (Nikon). The amounts of infiltrating immune cells were quantitatively analyzed using the TissueFAXS software (TissueGnostics).
ELISA.
The 96-well EIA/RIA plates (Corning Inc.) were coated overnight with purified Abs against cytokines (IL-4, IL-5, IL-6, IL-13, IL-17, or IFNγ), Ig (IgA or IgE), or CII collagen in PBS at 4 °C. Plates were blocked with 10% FBS, incubated with either cell supernatants or serum, and subsequently incubated with biotin-labeled Abs, followed by addition of alkaline phosphatase-conjugated streptavidin. The plates were washed, and the absorbance was measured at 405 nm by an ELISA plate reader (Molecular Devices).
Isolation of CD4+ T Cells and in Vitro Differentiation.
Single-cell suspensions were prepared from lymph nodes and incubated with either anti-CD4 microbeads (Miltenyi Biotech) or antibody mixture solution in naïve CD4+ T-cell isolation kit (R&D Systems) according to the manufacturer's instructions. CD4+ T cells were stimulated with plate-bound anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL) Abs and cultured in the presence of recombinant human IL-2 (10 U/mL; R&D Systems). Cells were additionally treated with cytokines and anti-cytokine Abs for Th cell commitment as previously described (35). Treg cells were generated in the presence of anti-IFNγ (5 μg/mL), anti-IL-4 (5 μg/mL), and TGF-β (5 ng/mL; R&D Systems) for 3 d and restimulated with PMA (100 ng/mL, Sigma-Aldrich Corp.) and ionomycin (1 μg/mL; Calbiochem).
Surface Marker and Cytokine Staining and ROS Measurement.
Cells were fixed in 4% paraformaldehyde and incubated with fluorescence-conjugated Abs against surface markers. For intracellular cytokine and FoxP3 staining, cells were suspended in permeabilization buffer (2% FBS and 0.05% saponin in PBS) before staining with anti-cytokine Abs (anti-IFNγ, anti-IL-17, anti-IL-6, or anti–TNF-α) or mouse monoclonal anti-FoxP3 Ab (BD Pharmingen). To measure ROS level, cells were incubated with 500 μM CM-DCFDA (Invitrogen) at 37 °C for 20 min in the dark. Cells were then analyzed using a FACSCalibur flow cytometer and CellQuest software (BD Pharmingen).
Reverse Transcription and Quantitative Real-Time PCR.
Total RNA was prepared and subjected to reverse transcription by SuperScript II reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed with an ABI Prism 7000 Sequence Detection System (Applied Biosystems) using specific primer sets: TRAP, 5′-tcctggctcaaaaagcagtt-3′, 5′-acatagcccacaccgttctc-3′; MMP-9, 5′-ggaactcacacgacatcttcca-3′, 5′-gaaactcacacgccagaagaattt-3′; IL-1β, 5′-ggacagaatatcaaccaacaagtgata-3′, 5′-gtgtgccgtctttcattacacag-3′; TNF-α, 5′-catcttctcaaaattcgagtgacaa-3′, 5′-tgggagtagacaaggtacaaccc-3′; IL-6, 5′-gaggataccactcccaacagacc-3′, 5′-aagtgcatcatcgttgttcataca-3′; T-bet, 5′-gccagggaaccgcttatatg-3′, 5′-gacgatcatctgggtcacattgt-3′; GATA3, 5′-agtccgcatctcttcaccttcc-3′, 5′-ggcactctttctcatcttgccg-3′; RORγt, 5′-ccgctgagagggcttcac-3′, 5′-tgcaggagtaggccacattac-3′; FoxP3, 5′-ggcccttctccaggacaga-3′, 5′-gctgatcatggctgggttgt-3′; IL-17, 5′-caggacgcgcaaacatga-3′, 5′-gcaacagcatcagagacacagat-3′; and β-actin, 5′-agagggaaatcgtgcgtgac-3′, 5′-caatagtgatgacctggccgt-3′. Relative expression level was calculated after normalization to the level of β-actin.
Carboxyfluorescein Succinimidyl Ester (CFSE) T-Cell Proliferation Assay.
CD4+ T cells (1 x107 cells) were labeled by incubation in 5 μM CFSE and stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of CD11b+ cells. CD11b+ cells were isolated from lymph nodes and spleen of WT and KO mice using anti-CD11b microbeads. Cells were harvested after 48 h, and cell division numbers were determined by flow cytometry analysis.
Adoptive Transfer of T Cells into RAG KO Mice and Induction of Arthritis.
CD4+ T cells (1.5 × 106 cells) were isolated from spleen of WT and KO mice and transferred to recombination activating gene (RAG)-deficient mice. RAG KO mice (n = 6) were subsequently injected with CII (2 mg/mL; Chondrex Inc.) emulsified in CFA (Chondrex Inc.) (17, 18). All mice were killed 4 wk after adoptive transfer, and joint inflammation was examined.
Statistical Analysis.
All experiments were performed in triplicate, and data were expressed as the mean ± SEM. Data were analyzed by one-way ANOVA and unpaired Student's t test. P values <0.05 were considered statistically significant.
Acknowledgments
We thank Drs. T. S. Chang, Y. S. Bae, M. R. Cho, and Y. Yun for NOX2 KO and RAG KO mice and helpful discussions. This work was supported by Health Care Medical Technology R&D Project 2008-1396-1-1 (funded by the Korea Health Industry Development Institute) and National Core Research Center Program 2011-0006244 (funded by the Ministry of Education, Science, and Technology).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 2.Phillips DC, Dias HK, Kitas GD, Griffiths HR. Aberrant reactive oxygen and nitrogen species generation in rheumatoid arthritis (RA): Causes and consequences for immune function, cell survival, and therapeutic intervention. Antioxid Redox Signal. 2010;12:743–785. doi: 10.1089/ars.2009.2607. [DOI] [PubMed] [Google Scholar]
- 3.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 4.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 5.Schrenzel J, et al. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–737. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 6.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 7.Segal AW. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987;326:88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- 8.Volpp BD, Nauseef WM, Clark RA. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988;242:1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollock JD, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 11.van de Loo FA, et al. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. Am J Pathol. 2003;163:1525–1537. doi: 10.1016/S0002-9440(10)63509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hultqvist M, et al. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George-Chandy A, et al. Th17 development and autoimmune arthritis in the absence of reactive oxygen species. Eur J Immunol. 2008;38:1118–1126. doi: 10.1002/eji.200737348. [DOI] [PubMed] [Google Scholar]
- 14.Nakano Y, et al. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 16.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci USA. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hultqvist M, Bäcklund J, Bauer K, Gelderman KA, Holmdahl R. Lack of reactive oxygen species breaks T cell tolerance to collagen type II and allows development of arthritis in mice. J Immunol. 2007;179:1431–1437. doi: 10.4049/jimmunol.179.3.1431. [DOI] [PubMed] [Google Scholar]
- 19.Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364–369. doi: 10.1016/s1568-9972(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 20.De Ravin SS, et al. Chronic granulomatous disease as a risk factor for autoimmune disease. J Allergy Clin Immunol. 2008;122:1097–1103. doi: 10.1016/j.jaci.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Korn T, Kuchroo VK. Th17: The third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marson A, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 25.Arimitsu S, et al. A three-dimensional quantitative analysis of carpal deformity in rheumatoid wrists. J Bone Joint Surg Br. 2007;89:490–494. doi: 10.1302/0301-620X.89B4.18476. [DOI] [PubMed] [Google Scholar]
- 26.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Berg WB. Lessons from animal models of arthritis over the past decade. Arthritis Res Ther. 2009;11:250. doi: 10.1186/ar2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toh ML, et al. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS ONE. 2010;5:e13416. doi: 10.1371/journal.pone.0013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boissier MC, et al. Regulatory T cells (Treg) in rheumatoid arthritis. Joint Bone Spine. 2009;76:10–14. doi: 10.1016/j.jbspin.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 33.Zhu B, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 34.Haile LA, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Won HY, et al. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal. 2010;13:575–587. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]