Abstract
Brain dopamine has long been implicated in cognitive control processes, including working memory. However, the precise role of dopamine in cognition is not well understood, partly because there is large variability in the response to dopaminergic drugs both across different behaviors and across different individuals. We review evidence from a series of studies with experimental animals, healthy humans and patients with Parkinson’s disease, which highlight two important factors that contribute to this large variability. First, the existence of an optimum dopamine level for cognitive function implicates the need to take into account baseline levels of dopamine when isolating dopamine’s effects. Second, cognitive control is a multi-factorial phenomenon, requiring a dynamic balance between cognitive stability and cognitive flexibility. These distinct components might implicate the prefrontal cortex and the striatum respectively. Manipulating dopamine will thus have paradoxical consequences for distinct cognitive control processes depending on distinct basal or optimal levels of dopamine in different brain regions.
Keywords: Working memory, cognitive control, prefrontal cortex, striatum, dopamine, fMRI
Introduction
The neurotransmitter dopamine (DA) is well known to play an important role in complex cognitive functions such as working memory and cognitive control. These somewhat ill-defined terms generally refer to the functionally opposing computations of (i) ‘on-line’ stabilization of task-relevant representations and (ii) flexible updating of those representations in response to novel information (1–3). Such working memory and cognitive control functions are critically important for a wide range of cognitive abilities such as reasoning, language comprehension, planning, and spatial processing and have been associated most commonly with the prefrontal cortex (PFC) (4–7).
The PFC contains a large number of DA receptors (8–10) and is highly sensitive to its dopaminergic environment, which is not surprising given diffuse ascending inputs from midbrain dopaminergic neurons (11). The anatomical distribution of brainstem DA projections provides a logical basis for proposing a role for DA in working memory and cognitive control (for reviews, see (12–15). The mesocortical and mesolimbic dopaminergic systems originate in the ventral tegmental area of the midbrain and project to the PFC, anterior cingulate cortex, anterior temporal structures such as the amygdala, hippocampus and entorhinal cortex and the basal forebrain (16). Although DA in medial temporal structures also plays a role in modulating human cognition, in particular long-term memory (17), we here focus on the role of DA in fronto-striatal processing, including working memory and cognitive control, not least because there is a clear anterior/posterior gradient in the brain for the concentration of DA where it is highest in the PFC (18). Thus, the anatomical distribution of the dopaminergic system suggests that it should have a greater influence on anterior than on posterior brain structures.
Consistent with this anatomy, a landmark study in 1979 by Brozoski and colleagues revealed that DA depletion in the PFC of monkeys caused severe impairment on a now classic test of working memory, the delayed response task (19). This working memory impairment was as severe as that in monkeys with complete ablations of the PFC, and was not observed in monkeys in which other neurotransmitters, such as serotonin, were depleted. Furthermore, DA receptor agonists administered to these same monkeys reversed their working memory impairment (19, 20). Subsequent work with both animals and humans substantiated the necessity of DA for working memory (21–25) as well as other cognitive functions such as future planning and cognitive flexibility (26–28). For example, administration of DA receptor agonists like bromocriptine and pergolide to healthy young volunteers (24, 29–33) improved performance on working memory tasks. In these studies, drug effects were functionally selective, as they did not alter other abilities such as sensorimotor function. In keeping with these findings, administration of the D2 receptor antagonist sulpiride, which blocks DA receptor stimulation, impaired performance on several tasks sensitive to PFC function (34). Again, these effects could not be accounted for by nonspecific changes, such as generalized sedative or motoric influences of the drug.
However, recent progress has revealed that the relationship between brain DA and task performance is highly complex. The effects of dopaminergic drugs often seem paradoxical, as both improvements as well as impairments are observed. These paradoxical effects are observed across different individuals who perform the same task or within the same individual across different tasks (35–37). Elucidating the factors that determine this large variability in drug effects and characterizing the nature of the complex relationship between task performance and DA is the focus of the present review.
The importance of answering this question stems from two facts. First, DA is of fundamental importance to the etiology of a wide variety of neurobehavioral disorders such as Parkinson’s disease (PD), attention deficit hyperactivity disorder, schizophrenia and drug addiction. Deficits in working memory and cognitive control are core to these disorders, which are associated with cognitive inflexibility, impulsivity and/or compulsivity. Moreover, DA drugs are used widely in the treatment of a variety of brain disorders, and to a lesser extent in the treatment of psychostimulant addiction. While it should be important to understand the cognitive effects of any drug that is commonly prescribed, it is especially important in the case of drugs used to treat disorders with cognitive deficits. In addition, even acute and/or mild stress and fatigue can lead the mind to be inflexible or unfocused. Accumulating evidence from research with monkeys has revealed that the catecholamines (DA and NA) also play an important role in these normal states (12). Thus, a better understanding of DA function will advance not only the treatment development for, and understanding of the abnormal mind but also that of the usually adaptive, but at times inflexible, unfocused healthy mind. The high concentration of DA receptors in the PFC and strongly connected structures gives us a leverage point for studying normal and disordered brain function. Different dopaminergic agents provide different selectivity profiles, based on their actions on different DA receptors, and it will be valuable to understand both the behavioral and neural effects of these drugs.
Second, the questions we raise are motivated at a theoretical level by basic questions about the neurobiological basis of higher cognitive function. How does DA contribute to higher cognitive functions like working memory and cognitive control? Which brain areas mediate these contributions? Which cognitive functions are served by dopaminergic pathways and the cortical regions they innervate?
Here we review two methodological approaches to studying the effects of DA on human cognition – administration of DA receptor agents to healthy subjects, and controlled withdrawal of dopaminergic medication in patients with PD.
Individual differences in dopamine action
Findings from psychopharmacological studies with human volunteers indicate that the effects of dopaminergic drug administration depend on baseline levels of performance (29, 32, 38–41). For example, we first observed in 1997 that the effects of bromocriptine on PFC function are not the same for all subjects, but interact with the subject's baseline working memory abilities (29). The drug improved cognition in subjects with lower baseline working memory abilities in the ‘un-drugged’ state, while worsening cognition in those with higher baseline working memory capacity. Since reporting this initial finding, a series of studies have replicated this observation that administration of dopaminergic drugs to humans can have diametrically opposite effects on cognition depending on working memory capacity (often measured with the listening span test (42, 43). These effects have been observed on tasks of set shifting (29, 44, 45), working memory updating (40, 44) and working memory retrieval (38). Thus, it appears that effects of dopaminergic drugs on cognitive function can, at least partly, be predicted from the initial state of the individual (Figure 1). An important clinical implication is that, whilst low levels of performance due to psychopathology are likely to be remedied by drug therapy with agonists, conversely, the same drugs may worsen already-optimized performance.
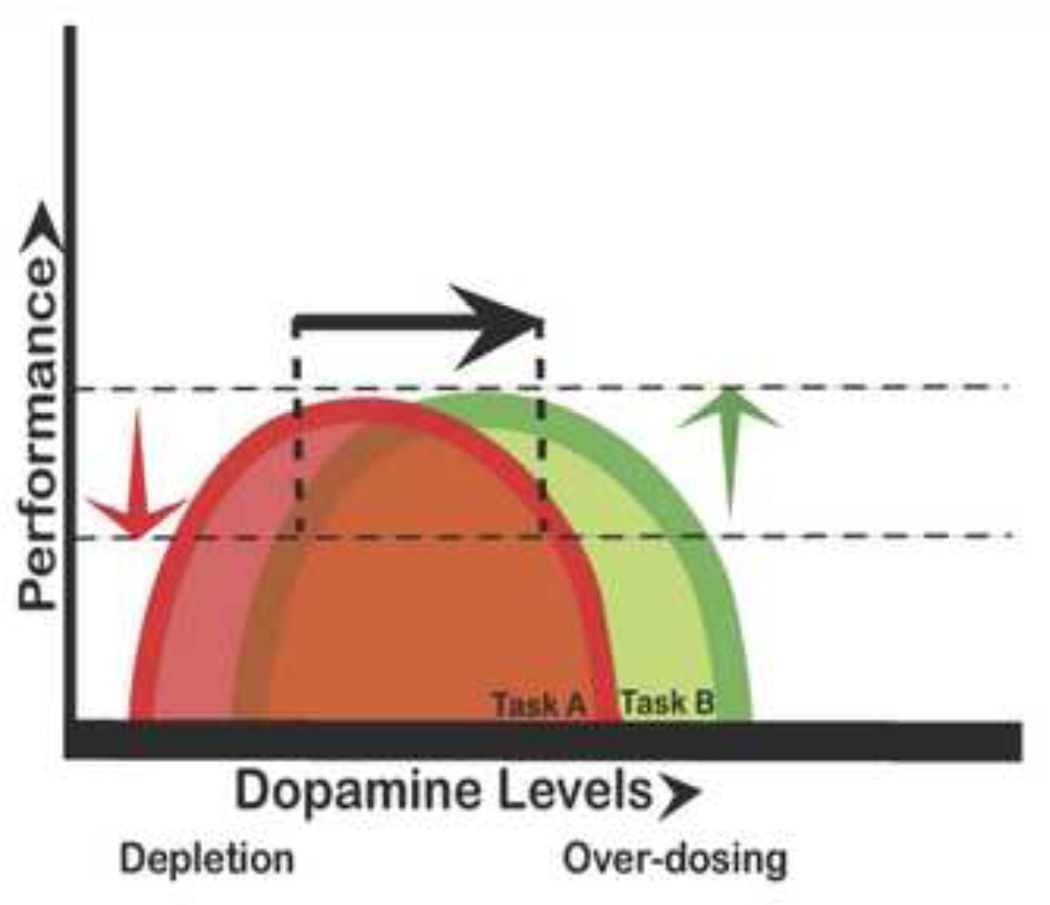
Figure 1.
The relationship between cognitive performance and DA levels follows an ‘Inverted U-shaped’ function, where both too little and too much DA impairs performance. How likely it is that a drug will cause beneficial or detrimental effects depends partly on basal dopamine levels. A single ∩ curve is insufficient to predict performance: Some tasks benefit from increasing dopamine (green), while performance on other tasks is disrupted by increasing dopamine (red). The black arrow represents the dopamine-enhancing effect of a hypothetical drug, leading to a beneficial effect on task A (red), but a detrimental effect on task B (green). Reproduced with permission from Cools and Robbins [13].
The insight that drug effects can be baseline-dependent stems from as early as the 1950s, when Wilder (46) first observed that (the intensity and direction of) drug effects on blood pressure and pulse rate depend on the pre-experimental level of the function tested (‘Law of Initial Value’). Discoveries that methamphetamine in pigeons reduced high rates of responding but increased low rates of responding led to the notion that drug effects on motor activity can also be predicted partly from the initial state of the system (47, 48). More recent evidence from work with experimental animals concurs with the above reviewed evidence from work with healthy volunteers, indicating that similar baseline-dependency exists for the effects of dopaminergic drugs on cognitive functions (e.g. (49, 50). For example, it was demonstrated that infusion of a DA receptor agonist enhanced performance on an attention task in rats with poor performance in the ‘un-drugged’ state, but not in rats with good performance. Conversely, infusion of a DA receptor antagonist impaired performance only in rats with high (but not low) baseline performance levels (49).
Variability in basal dopamine levels in the PFC of non-human animals
What might be the origin of these performance-dependent effects of dopaminergic drug administration? Accumulating evidence from research with mice, rats and monkeys indicates that it likely reflects variability in baseline levels of DA, specifically in the PFC (51–55). For instance, Phillips and colleagues have shown in rats that poor performance on a difficult (working) memory task (with a long delay) was accompanied by low DA levels in the PFC, while good performance on an easy task (with a shorter delay) was accompanied by high DA levels in the PFC (56). Interestingly, performance on the difficult task was improved by administration of a DA D1 receptor agonist, whereas good performance on the easy task was impaired (50) (see also (57)). Similar results have been found in monkeys. In fact, baseline-dependent effects of DA were first observed by Arnsten, Goldman-Rakic and colleagues in monkeys performing working memory tasks. For example, in 1994, Arnsten and colleagues (20) demonstrated that administration of a D1 receptor antagonist impaired performance of young monkeys but not aged monkeys with presumed DA depletion. In contrast, a D1 receptor agonist improved performance in aged monkeys but not in young monkeys. Furthermore, stress-induced working memory deficits were ameliorated by pretreatment with DA receptor antagonists. This finding suggests that excessive DA release in the PFC during stress led to the observed working memory deficits (52). Indeed, a number of animal studies have now shown that either too little (23, 58) or too much (53, 59) D1 receptor activity in the PFC impairs performance on working memory tasks. There are some subtle differences in the nature of these deficits, with random responding resulting from too little, but perseverative or overly persistent responding resulting from too much D1 receptor stimulation (50, 53, 60).
The central role of the PFC in DA function is also supported by data collected at the cellular level. Using a technique of iontophoretic application of drugs onto single neurons in awake behaving monkeys, Williams and Goldman-Rakic (61) demonstrated that the effect of a D1 receptor antagonist on delay period activity was dose-dependent and highly selective for PFC neurons with memory fields for particular locations. Cellular mechanisms underlying these effects of DA receptor stimulation have been proposed, based on in vitro recordings, to include: (i) increased impact of the NMDA (N-methyl-D-aspartate) component of excitatory synaptic input onto PFC neurons, thought to be essential for the maintenance of current PFC activity (62), (ii) reduced calcium currents, that convey information from dendrites to cell bodies of pyramidal PFC neurons (63) and (iii) increased excitability of inhibitory GABA-ergic inter-neurons, thereby hypothetically attenuating the strength of further excitatory input (64). These cellular mechanisms may lead to an increased signal-to-noise ratio, and a ‘quelling’ of activity in all but the most strongly active cell assemblies. This would result in a single strengthened working memory representation resistant to subsequent inputs. With supra-optimal receptor stimulation, this ‘quelling’ of activity would take on excessive forms, thus leading to a blocking of all new input to the PFC, corresponding with perseverative responding and severe working memory impairment. These ideas are quite similar to a more abstract proposal from approximately 20 years ago, according to which DA induced changes in the gain of neuronal input/output functions (65).
Recent in vitro recordings have suggested a mechanism by which DA might induce changes in the gain of neuronal input/output in an ‘Inverted-U’ shaped manner (94). This study revealed DA effects using patch-clamp recordings in co-cultures of the PFC and the VTA (94). Administration of exogenous DA to these cultures altered PFC activity in a DA concentration-dependent fashion. Thus, in VTA-containing cultures (which possessed a tonic DA level and where stimulation of the VTA evoked DA transients within the PFC), high DA concentrations reduced spontaneous network activity (i.e. up-states) and diminished excitatory synaptic inputs (EPSPs) evoked during the down-state. Conversely, low DA concentrations had no effect on spontaneous network activity in these VTA-containing cultures, but selectively increased the efficiency of a train of EPSPs to evoke spikes during the up-state. Critically, when background DA was eliminated, spontaneous network activity (i.e. up-states) could be enhanced by low concentrations of DA. These findings highlight the importance of considering how DA can modulate the input and output of individual neurons, but also the effects of these neurons embedded in an active network.
The biophysical mechanisms underlying the supra-optimal effect of DA receptor stimulation are not known. According to one hypothesis, excitatory NMDA effects might dominate at lower stimulation levels, while inhibitory GABAA effects might dominate at higher DA activation levels (66). A consequent abolition of calcium currents with supra-optimal levels of DA receptor stimulation (63) might lead to perseveration, for example due to a lack of new input to the PFC necessary to update currently active representations.
The hypothesis that excessive D1 receptor stimulation in the PFC blocks new input concurs with neurophysiological data from monkeys (67). In this study monkeys engaged in an oculomotor delayed response task, while spatially tuned delay-related activity was measured in terms of the difference between firing to preferred and non-preferred directions. An inverted-U shaped response to D1 receptor stimulation was observed in terms of spatial tuning, but this was a consequence of suppression of delay-related activity in both low and high doses. It was only after the high dose that firing was suppressed in both preferred as well as in non-preferred directions. Thus, whereas low levels of D1 receptor stimulation improved working memory tuning by suppressing only task-irrelevant representations, high levels of D1 receptor stimulation impaired working memory tuning by suppressing delay-related firing for both relevant as well as irrelevant representations. Like the human studies, this study revealed that these effects depended on the baseline state of the unit, in this case the neuron: Iontophoresis of a low dose D1 receptor agonist on weakly tuned cells unmasked spatial activity by suppressing only noisy task-irrelevant activity, whereas spatial tuning was less improved or even worsened in strongly tuned cells. Iontophoretic pharmacology revealed that these suppressive effects of D1 receptor stimulation depended on the second-messenger cyclic adenosine monophosphate (AMP) signaling pathway. It might be noted that this in vivo study did not find any evidence for the excitatory actions of D1 receptors stimulation on PFC activity, reported by in vitro studies (see above). One possibility is that these excitatory actions of the D1 receptor, detected in vitro, are already fully engaged by endogenous DA in vivo. Thus the apparent discrepancy between the in vitro slice studies and the in vivo recordings from cognitively-engaged monkeys is that the pyramidal cell recurrent excitation is absent in the former, while predominating in the latter. In any case, the finding that DA-induced improvements of spatial tuning are accompanied by suppressive effects on PFC activity concurs with the general observation from human functional imaging studies that working memory improvement after DA-enhancing drug administration is accompanied by reductions in PFC activity (40, 68–70).
Baseline-dependent mechanisms of dopamine action in humans
So far we have seen that dopaminergic drug effects in non-human animals vary as a function baseline levels of DA in the PFC. Is there evidence for similar baseline-dependency of dopaminergic drug effects in humans? One source of such evidence comes from studies of drug effects that take into account genetic differences between individuals (see for review (71)).
One of the best studied polymorphisms is the Val158Met polymorphism in the COMT gene. Catechol-O-methyltransferase (COMT) is an enzyme that breaks down DA released into the synaptic gap between two neurons and is thought to have a much greater influence on DA levels in the PFC than in the striatum (72–74). This regional specificity derives from the observation that DA transporters are less abundant in the PFC than in the striatum, so that DA metabolism in the PFC would depend more readily on enzymatic degradation by COMT than on transport and reuptake by the DAT. Relatively little COMT activity would imply more DA in the synapse (and more action at DA receptors on the receiving neuron), whereas relatively greater COMT activity would imply less DA in the synapse. In the general population there are two common variants of the gene determining COMT levels. Individuals with the Val-allele have relatively high COMT activity, and presumably low baseline DA; conversely individuals with the Met-allele have relatively low COMT activity and presumably high baseline DA. Consistent with these assumptions are findings that individuals with the Met-allele (high DA) perform significantly better on tasks requiring cognitive control and working memory than those with the Val variant (75–77). For example, Egan et al (2001) have shown that the high DA Met subjects make fewer errors on the Wisconsin Card Sorting Test (WCST), a task traditionally associated with the PFC (78), than do low DA Val subjects. However, more recent studies have shown that, as is the case with dopaminergic drugs, there is no overall effect of the COMT polymorphism on cognition (79). Rather effects depend on the particular task demands under study (71, 80) and associated neural system, with computations associated specifically with the PFC, such as the ability to stabilize task-relevant representations and to protect them from intervening information, being particularly sensitive (81, 82). Given such functional and regional specificity, it is not surprising that inconsistent results are revealed by studies of complex neuropsychological task (e.g. WCST) performance, which depends on a multitude of processes and brain regions (79). By contrast to this inconsistency, differences in PFC activity during cognitive control and working memory tasks between Val and Met participants seem relatively robust. Specifically, the high DA Met individuals often show lower levels of PFC activity, suggestive of more efficient processing, than do the low DA Val individuals (70, 83).
Critically, it is this PFC activity of Val individuals during cognitive control and working memory tasks that is attenuated with dextroamphetamine, a stimulant that increases DA levels (70), or tolcapone, a COMT inhibitor, to more closely resemble that of their Met peers (84, 85). Conversely, in line with the ‘Inverted-U’ shaped function hypothesis, exactly the same drugs increased PFC activity in Met participants. Similar contrasting effects of dopaminergic drug administration have been observed as a function of the Taq1A polymorphism (86), which has been reported to be associated with altered DA receptor density (87), presumably via indirect linkage with DRD2 polymorphisms (88). Accordingly, these genetic studies suggest that, as in the case of nonhuman animals, dopaminergic drug effects in humans also depend on baseline levels of DA.
These genetic studies, however, do not provide direct evidence for the hypothesis that performance-dependent effects of dopaminergic drugs reflect baseline levels of DA. Thus there is no definitive evidence that Val-carriers have lower baseline levels of DA in the PFC, and the functionality of the Taq1A polymorphism is controversial. Direct measurements of DA transmission in humans can be made only using neurochemical positron emission tomography (PET), although most applications of this method are limited to visualizing striatal DA, not being optimized for detecting DA levels in the PFC. We have recently employed this technique with the radiotracer 6-[18F]fluoro-L-m-tyrosine (FMT) to quantify individual differences in the degree to which DA is synthesized in the terminals of midbrain DA neurons. In this study, subjects with high-and low working memory capacity underwent an FMT PET scan (Figure 2). Intriguingly, subjects with low working memory capacity had significantly lower DA synthesis capacity in the striatum than did subjects with high working memory capacity (89). This finding was seen also in older individuals (90), in whom striatal dopamine synthesis capacity predicted not only working memory capacity, but also PFC activity during working memory performance. Therefore these data provided the first direct evidence in support of the hypothesis that the dependency of dopaminergic drug effects on baseline working memory capacity reflects differential baseline levels of DA function. Even more direct evidence for this hypothesis came from the finding that the effects of the DA receptor agonist bromocriptine could also be predicted from baseline levels of dopamine synthesis capacity in the striatum (91). Specifically, we found that healthy young individuals with low DA synthesis (and working memory) capacity benefited from bromocriptine, while subjects with high DA synthesis (and working memory) capacity were impaired by the same drug. Thus, individual variability in dopaminergic drug effects on human cognition reflected individual variability in basal levels of DA.
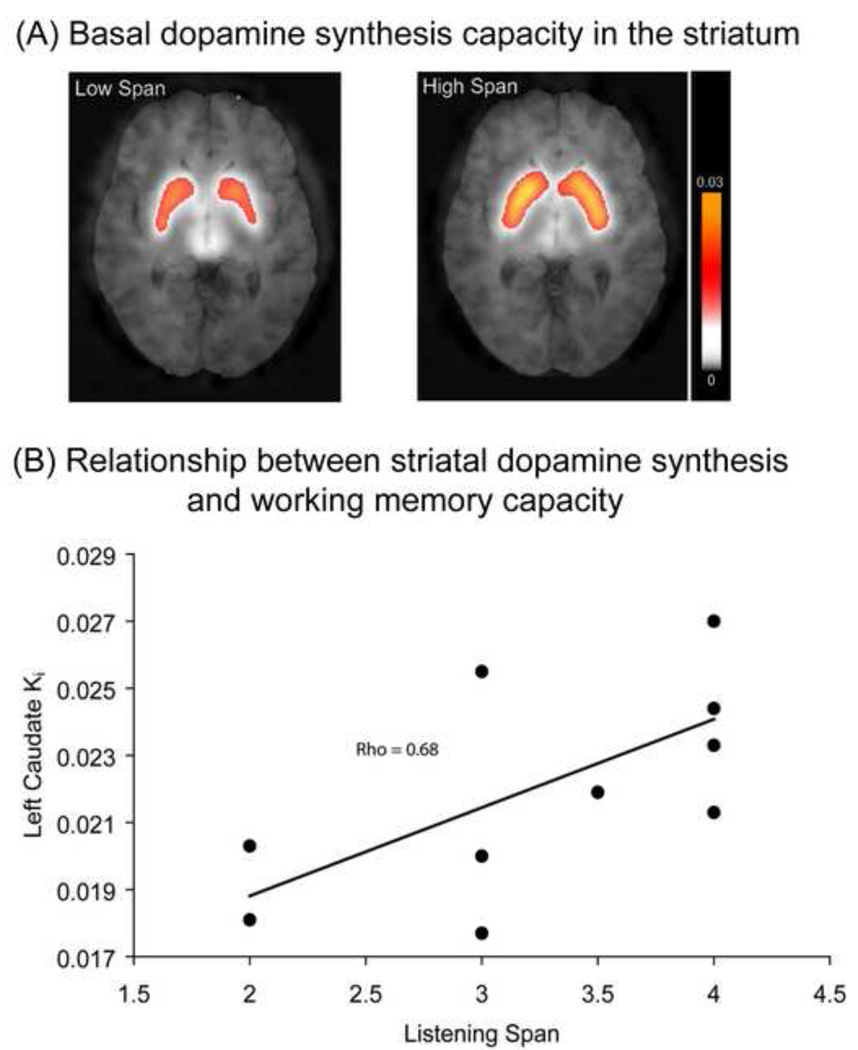
Figure 2.
(a) The mean raw axial (MR co-registered) whole-brain FMT PET Ki images from the high-span group (left panel) and from the low-span group (right panel) overlaid on a normalized MR image. Data represent Ki-values. Right is right according to neurological conventions; (b) Correlation between working memory capacity (on the x-axis: listening span) and dopamine synthesis capacity in the striatum (on the y-axis: Ki values from the left caudate nucleus). Adapted with permission from Figure 1 and 2 from Cools et al [89].
These observations from human studies are remarkably consistent with those reported in experimental animals, reviewed above. Indeed the literature converges across species, except for one noteworthy aspect: While animal work has highlighted the role of basal DA levels specifically in the PFC, human PET work has revealed an important role for basal dopamine in a different brain region, i.e. the striatum. A critical role for the striatum in DA function is not surprising, given that dopaminergic projections are strongest, and receptors most abundant, in the striatum, as well as given existence of strong anatomical connections between the PFC and the striatum in so-called fronto-striatal circuits (92).
Although it remains possible that PET measurements of striatal DA transmission are an index of DA levels in PFC (which cannot be easily detected with PET imaging), the human PET work does raise an alternative hypothesis. Specifically the baseline-dependent effects of DA on PFC function may reflect modulation of fronto-striatal connectivity, which varies as a function of basal DA levels in the striatum rather than in the PFC. To test this hypothesis, we recently investigated the effect of bromocriptine administration to healthy individuals on functional interactions between the PFC and striatum (93). Re-analyzing an original dataset from Gibbs & D’Esposito (38), we found that during the engagement of working memory retrieval processes, bromocriptine increased fronto-striatal connectivity in individuals with low working memory capacity, corresponding with performance improvement. In contrast, individuals with high working memory capacity exhibited a decrease in fronto-striatal connectivity after bromocriptine administration, corresponding with worsened performance. Such effects of bromocriptine may reflect direct action at the level of the striatum, or alternatively, they may reflect stimulation of D2 receptors on layer V cells in the PFC, which project to the striatum.
The difference between animal and human studies in terms of emphasis on basal levels of DA in the PFC versus the striatum might reflect one or more of multiple factors. First the only method available and used so far for studying human DA transmission directly is PET, which is optimized for detecting signals in the striatum. Signals in the PFC are generally weak for most applications. Second, human studies have primarily investigated effects of DA drugs that have the greatest affinity for the D2 receptor family, partly due to the lack of D1-selective drugs available for human research. D2 receptors are more abundant in the striatum than in the PFC (95–98). Finally, human studies have employed cognitive paradigms that require different cognitive operations from those employed in animal studies. For example, demands for the flexible updating of current goal representations (which may be more dependent on striatal function, see below) have generally been higher in human cognitive paradigms than in animal paradigms, the latter often focusing on the delay period of working memory tests (but see (99, 100)). The nature of the required cognitive operation may determine the degree to which DA levels in the striatum or in the PFC are predictive of drug effects.
Distinct roles for striatal and PFC dopamine
Traditionally, cognitive effects of DA are ascribed to modulation of the PFC. However, recent theories as well as empirical data have highlighted a complementary role for DA in the striatum in working memory and cognitive control (101–106). Critically, studies in animals investigating the role of D1 receptors in the PFC have most commonly focused on the delay period of delayed response tasks, which requires the stabilization of an earlier presented stimulus across a short delay (19, 22, 58, 107). According to recent ideas, the functional role of DA in the striatum might be qualitatively different from that of DA in the PFC, extending beyond the persistent stabilization of information. Specifically, striatal DA might rather be more important for the ability to flexibly update those goal representations when new information becomes available, for example, as measured during the encoding and probe period of the delayed response task. Empirical and computational work has indeed suggested that such updating during the encoding and probe periods of the delayed response task is not modulated by D1 receptor stimulation in the PFC, but rather by D2 receptor stimulation (66, 107, 108). Consistent with the observation that there are relatively few D2 receptors in the PFC, recent computational work has emphasized the role of DA in the striatum in such updating of current goal representations (101, 109). The suggestion that the (DA in the) striatum is well suited to serve the gating mechanism that updates goal representations in the PFC concords with a rapidly growing body of data from functional neuroimaging and animal studies on working memory (105, 110–113). Furthermore, it also concurs with empirical data from human imaging and animal studies showing (effects of DA D2 receptor manipulation on) striatal involvement during set shifting (15, 104, 114–119). For example, we have recently shown, using dynamic causal modelling of fMRI data that activity in the striatum may regulate attentional set shifting by modulating (or ‘gating’) connectivity between the PFC and task-relevant representations in posterior cortex (114). Furthermore, genetic over-expression of striatal D2 receptors (115) and abnormal increases in D2 receptor activity in the rodent striatum causes a set shifting impairment (116), which might underlie attenuated striatal BOLD responses in humans who are treated with DA-enhancing drugs (117, 119).
An interesting observation is that updating and stabilization can be conceptualized as representing functionally opponent processes. If we update too readily, then we are likely to get distracted, rendering our behavior unstable. Conversely, if our representations are overly persistent or stable, then there is a danger of inflexibility and unresponsiveness to new information. A pure form of reciprocity would imply that we need only a single mechanism that can be adjusted dynamically depending on task demands. However, we often need to be both flexible and persistent at the same time, at least at the global level. Thus while we should be flexible in response to task-relevant changes, we should be simultaneously stable as long as the changes are irrelevant. To resolve this apparent paradox it is more plausible to postulate two separate mechanisms that nevertheless work together. The need for two separate mechanisms is also illustrated by the observation that various disorders, such as ADHD, are accompanied by a combination of inflexible as well as unstable behaviour and distractibility. Empirical data support the hypothesis that these two separate mechanisms might be subserved by DA in the striatum and the PFC respectively. For example Roberts and colleagues (21, 99, 100, 113) injected the neurotoxin 6-OHDA into the striatum or PFC of non-human primates and revealed that, whilst DA lesions in the PFC led to improved flexibility (attentional set shifting) (99), DA lesions in the striatum actually impaired flexibility (attentional set shifting) (113). Subsequent work showed that this modulation of flexibility during attentional set shifting may have resulted from effects on performance during the preceding set-maintenance stages of the task (100). Specifically, that study revealed that DA lesions in the PFC led to enhanced distractibility (poor attentional set maintenance), while DA lesions in the striatum actually reduced distractibility (enhanced attentional set maintenance). Thus the contrasting effects on set maintenance may well underlie the contrasting changes measured in the subsequent attentional set shifting stages of the task. These opposing effects of striatal and frontal DA lesions underline the possible competition between the PFC and the striatum (120) and suggest that a dynamic balance between stabilization and flexible updating may depend on precisely balanced DA transmission within the PFC and the striatum respectively. These ideas concur with observations that DA (D1) receptor stimulation in the PFC promotes the stabilization of representations by increasing distractor-resistance (108) and by sculpting or sharpening PFC networks (67, 121). Conversely, DA in the striatum might promote cognitive flexibility, by allowing the updating of newly relevant representations (2, 102, 122). The functional opponency between stability and flexibility maps well onto the neurochemical reciprocity between DA in the PFC and the striatum: Increases and decreases in PFC DA lead to decreases and increases in striatal DA respectively (77, 120, 123). One implication of this model is that stability and flexibility trade off in the healthy brain, where DA levels interact dynamically. Thus optimal DA levels in the PFC might be good for stability but bad for flexibility, whereas optimal DA levels in the striatum might be good for flexibility but bad for stability. Note that, according to this model, supra-optimal levels of DA in the PFC would potentiate stabilization to its extreme, thus inducing perseveration, whereas supra-optimal levels of DA in the striatum would potentiate flexible updating to its extreme, thus inducing distractibility.
This working hypothesis is reminiscent of the dual-state theory put forward recently by Durstewitz and Seamans (60, 66), which is grounded in in vitro neurophysiology and biophysically realistic computational modelling work. According to this theory, PFC networks can be either in a D1-dominated state, which is characterized by a high energy barrier favouring robust stabilization of representations, or in a D2-dominated state, which is characterized by a low energy barrier favouring fast flexible switching between representations. Consistent with this proposal are findings that D2 receptor agonists act in opposite ways to D1 receptor agonists, at least in vitro, on NDMA and GABA currents, neuronal excitability as well as on cyclic AMP production (66) with D2 receptor stimulation inducing reduction in NMDA currents and GABAergic inhibition. To cite Durstewitz and Seamans (66): in the D2-dominated state, “the valleys of the energy landscape become so flat and nearby that noise may easily push the system from one state into the other.” According to the theory, this D2-state corresponds with a state that facilitates flexible updating of goal representations in response to new inputs, and contrasts, or even competes with a D1-state that facilitates stabilization, eventually eliciting perseveration due to excessive blockade of new input. Although it is clear that D1 and D2 receptor action can be synergistic as well as antagonistic, this hypothesis is corroborated by findings in humans that the DA D2-receptor antagonist sulpiride (shown to modulate brain activity in the striatum (124)) impaired performance on task switching, but, by contrast, improved performance on a delayed response task that required the stabilization of representations in the face of task-irrelevant distraction (35). In rodents, blockade of D2 receptors in the PFC impaired set shifting, while leaving unaltered performance on working memory tasks (125). In monkeys D2 receptor stimulation in the PFC had no effect on delay-related firing, but increased response-related firing during the probe period of the delayed response task (107). Together these data strengthen the hypothesis that D2 receptor stimulation in the PFC might subserve a different, perhaps opponent subcomponent process than does D1 receptor stimulation (see also (125)). This alternative receptor-based theory is not necessarily inconsistent with our working hypothesis according to which DA in the striatum and the PFC subserve the distinct roles of updating and stabilization respectively. Indeed D2 receptors are more abundant in the striatum than in the PFC, which contains fewer D2 than D1 receptors (95, 96, 126, 127). Furthermore D2 receptors are synthesized by layer V PFC neurons that project to striatum.
As stated above, one implication of this model is that different functions require distinct levels of DA in different brain regions: High DA levels in the PFC might be good for stabilization, but bad for flexible updating. Conversely, high DA levels in the striatum might be good for flexible updating, but bad for stabilization. Based on this hypothesis, we might expect that natural genetic variations affecting DA primarily in the PFC but not in the striatum (e.g. between those of us with the Val and Met versions of the COMT gene) confer both behavioral costs as well as behavioral benefits (see also (71)). Indeed, Nolan et al. (128) observed that individuals who were homozygous for the Met polymorphism, were better than Val individuals at sticking to task, but worse when flexible updating was needed. Two recent studies of flexible updating (task-switching and reversal learning) (129, 130) have confirmed that individuals with the Met allele, who have repeatedly been shown to perform better on working memory tasks that require cognitive stabilization, also exhibit impaired performance on tasks of flexible updating, compared with Val individuals. These data further strengthen the working hypothesis that increases in DA might have paradoxical consequences for distinct cognitive functions, reflecting functional specificity of DA’s effects in the PFC.
Consistent with this notion that distinct brain regions might mediate different effects of DA are findings from a recent study which demonstrated that DA receptor stimulation modulated the PFC or the striatum depending on task demands (45). In this pharmacological fMRI study, young healthy individuals were scanned on two occasions, once after administration of bromocriptine and once after placebo while performing a working memory task. In this task, subjects had to encode, maintain and retrieve visual stimuli. Four such stimuli (two faces and two scenes) were presented during the encoding period, which was followed by a delay period during which subjects had to maintain the relevant stimuli in memory. Following this initial delay period, another stimulus was presented, which subjects were instructed to ignore. This distractor was either a scrambled image (the non-distractor) or a novel face or scene (the congruent distractor). It was followed by a second delay, after which subjects were probed to respond with the right or left finger depending on whether the probe stimulus matched one of the two task-relevant encoding stimuli (Figure 3a). Critically, subjects were instructed on each trial to attend to either the faces or the scenes. If the fixation cross was blue they had to memorize the faces; if it was green, then they had to memorize the scenes. The blue face trials and the green scene trials were randomized within blocks, enabling the measurement of the flexible switching of attention between faces and scenes. The critical measure of flexible updating, which was predicted to depend on striatal function, was the switch-cost, which was calculated by subtracting performance (error rates and reaction times measured at probe) on non-switch trials from that on switch trials. The critical measure of stabilization, which was predicted to depend on PFC function, was the distractor-cost, calculated by subtracting performance (measured at probe) after scrambled non-distractors from that after congruent distractors. We had two predictions. First, bromocriptine would modulate PFC activity during cognitive stabilization (as a function of distractor-type), but striatal activity during flexible updating (as a function of attention switching). Second, we predicted that effects of bromocriptine would depend on baseline levels of DA. Bromocriptine would remedy function of brain regions with low baseline levels of DA, while detrimentally overdosing function of brain regions with already optimized baseline levels of DA.
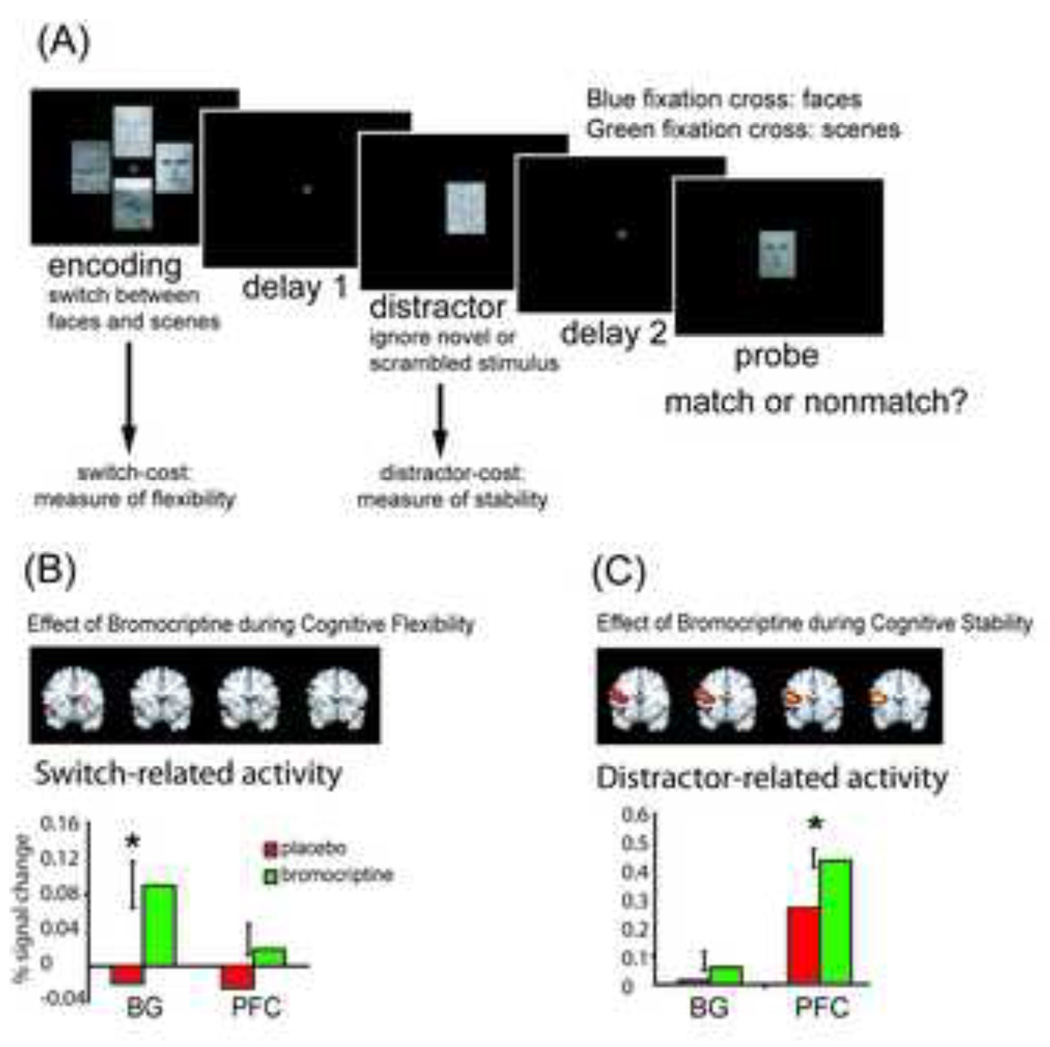
Figure 3.
The effects of dopamine receptor stimulation depend on task demands and neural site of modulation. (a) A delayed match-to-sample (DMS) task was used that provide a measure of flexible updating (cognitive switching during encoding) as well as a measure of stabilization (distractor-resistence during the delay). Subjects memorized faces or scenes depending on the color of the fixation cross. Subjects occasionally switched between encoding faces and scenes. A distractor was presented during a delay. Subjects were instructed to ignore this distractor. (b) Top panel: Effects of bromocriptine on striatal activity during updating as a function of group (the group × drug interaction effect, whole-brain contrast values (> 25) are overlaid on 4 coronal slices [slice numbers displayed on top] from the Montreal Neurological Institute high-resolution single subject MR image; Abbreviations: L = left; R = right) (note that the effect in the dorsomedial frontal cortex did not reach significance after correction for multiple comparisons); Bottom panel: Effects of bromocriptine on updating-related activity in the striatum and left PFC in low-span subjects only. (c) Top panel: Effects of bromocriptine on frontal activity during stabilization as a function of group (the group × drug interaction effect, all contrast values > 25 shown); Bottom panel: Effects of bromocriptine on distractor-related activity in the striatum and left PFC in low-span subjects only. Reproduced and adapted with permission from Cools et al [45].
To investigate individual differences in baseline levels of DA, we assessed drug effects separately in two groups of subjects that differed in their baseline working memory capacity, as measured with the listening span test. The results were consistent with our hypotheses: Bromocriptine modulated distinct brain regions, the striatum and the lateral PFC, during flexible updating (switching) and stabilization (distractor-resistance) respectively (Figure 3b). Critically, these effects depended on individual differences in working memory capacity. Specifically, bromocriptine improved flexible updating in the low-span subjects but impaired flexible updating in the high-span subjects. In the low-span subjects, bromocriptine significantly potentiated striatal activity during updating yet non-significantly attenuated striatal activity during updating in the high-span subjects. Thus, a drug-induced improvement in updating was accompanied by a drug-induced potentiation of striatal activity in the low-span subjects. Conversely, a drug-induced (non-significant) impairment in updating was accompanied by a drug-induced (non-significant) attenuation of striatal activity in the high-span subjects. We also assessed updating-related activity in the lateral PFC. As predicted, the lateral PFC was not modulated by bromocriptine during updating. Importantly, lateral PFC activity was modulated by bromocriptine during the delay period of the task when distracting stimuli were present, i.e. during stabilization. Specifically, PFC activity was potentiated by bromocriptine in the low-span subjects, while remaining unaltered in the high-span subjects. Similar effects were not observed in the striatum. Together, these data concur with the hypothesis that flexible updating and stabilization are mediated by dopaminergic modulation of the striatum and the PFC respectively. Furthermore, the effects of bromocriptine were not only regionally specific as a function of task demands, but also baseline-dependent, as illustrated by the opposite effects in high- and low-span subjects.
It might be noted that this functional and regional specificity of dopaminergic drug effects might also account for some apparent discrepancy between effects of the relatively specific D2 receptor agonist bromocriptine and the mixed DA D1/D2 receptor agonist pergolide. For example, we have previously revealed that the relationship between baseline working memory capacity and pergolide in young healthy volunteers (32, 131) is in fact opposite to the one described above for bromocriptine. Thus, whilst low-span subjects benefited more from bromocriptine than did high-span subjects, we have also reported and replicated that the effect of a single dose of pergolide in young healthy was more beneficial for subjects with greater working memory capacities (32, 131). This apparent discrepancy between the effects of pergolide and bromocriptine may well be due to differential selectivity of the drugs for D1 and D2 receptors respectively, the resulting differential (frontal versus striatal) site of modulation and the differential performance measure. Thus the effects of bromocriptine, and their dependency on span, in the 1997 and 2007 studies were observed on tasks requiring some form of flexible updating, implicating the striatum, and not for the delayed response tasks. Conversely, the effects of pergolide, and their effects on span, in the 2003 and 2006 studies were restricted to the delayed response tasks, presumably implicating primarily the PFC, and not extending to the task-switching paradigm (32, 131). Based on known neurochemical reciprocity and our observation that the listening span correlates positively with DA synthesis capacity in the striatum, we might hypothesize that the relationship between listening span and drug effects is positive for PFC function (i.e. stabilization), but negative for striatal function (i.e. flexible updating).
Parkinson’s disease studies strengthen the link between DA and cognition
A different approach towards assessing DA’s influence on cognitive function in humans is by testing patients with Parkinson’s disease (PD). PD is a progressive neurodegenerative movement disorder and characterized by a spatio-temporal progression of nigrostriatal and mesocortical DA depletion. In addition to deficits in motor control, PD is also accompanied by significant cognitive impairments even in the early stages of the disease. Central nervous system levels of DA can be manipulated over short periods of time by withdrawing the normal regimen of DA replacement drugs (i.e. levodopa), because the half-life of these drugs is relatively short. Effects can be easily monitored by observing deterioration in the patient’s motor status.
Many studies have obtained findings using this method by testing PD patients on tasks thought to be sensitive to PFC dysfunction (132). Superficially, the deficits seen in PD patients resemble those observed in patients with PFC lesions, particularly when they are off their normal dopaminergic medication. For example, impairments are seen on the Tower of London planning task, spatial working memory tests and a test of attentional set-shifting (37, 68, 133–140). The deficits on these tests of working memory and cognitive control contrasted with their intact performance on tests thought to implicate the medial temporal lobe, such as those of long-term memory (134, 141). This characteristic pattern of performance suggested that the cognitive deficits seen in mild PD patients resemble that seen with patients with frontal lobe lesions (135, 142, 143).
However, further work with more sophisticated cognitive paradigms has demonstrated that, in fact, there are important differences between the cognitive sequelae of frontal lesions and those of PD (144, 145). Moreover, functional imaging studies with PD patients have revealed abnormal task-related signals not only in the PFC, but also in the striatum (119, 146–148). This is not surprising, because in the early stages of the disease, DA depletion is relatively restricted to the dorsal striatum (i.e. the putamen and the dorsal caudate nucleus). It progresses to limbic and cortical structures such as the nucleus accumbens and the PFC only in later stages of the disease (149–151). In fact, in clinically very mildly affected patients, DA function may even be up-regulated in the PFC, as measured in vivo by [(18)F]dopa PET studies (152, 153). This up-regulation may reflect compensatory processes and is consistent with a reciprocal relationship between frontal and striatal DA as shown in rats and monkeys (99, 120). Because of this spatio-temporal progression of DA depletion, mild PD provides a good model for understanding the regionally selective and baseline-dependent role of DA in distinct brain regions, e.g. the striatum versus the PFC.
In particular, PD might be predicted to be accompanied by an inflexible state, due to low striatal DA levels, that is however also abnormally stable, due to high frontal DA levels. Evidence for the first part of this hypothesis, namely impairments in flexible updating, is overwhelming. Set-shifting difficulties have been shown on a variety of task ranging from WCST-like discrimination learning tasks, to more rapid task-switching paradigms (154–157). For example, using the latter paradigm, we have shown that mild PD patients exhibited significantly enhanced switch costs, compared with matched control subjects (136, 158). Moreover, the deficit in switching between task-sets was alleviated by administration of dopaminergic medication (37, 137). Notably, several studies have revealed that these beneficial effects occur in the context of detrimental effects of the same medication in the same patients on other cognitive tasks (37, 137), and therefore, cannot be accounted for by global effects on motor symptoms, arousal and/or motivation.
To test the second part of our hypothesis, i.e. that mild PD patients exhibit paradoxically enhanced cognitive stabilization, we investigated PD patients, once on and once off their normal dopaminergic medication on the delayed response task described above (Figure 3a) (145). As expected, medication withdrawal significantly worsened their movement symptoms. Intriguingly, there was also a significant difference between patients OFF their medication and controls in terms of the distractor-cost. Specifically, patients OFF medication exhibited paradoxically reduced distractor-costs, i.e. enhanced stabilization, compared with controls, who responded more slowly after a congruent distractor than after a scrambled non-distractor. Thus when they were OFF their medication patients were less distracted by the congruent distractor during the delay than were controls. This pattern of performance of the patients in their OFF state was particularly striking given their significantly increased motor symptoms. Furthermore the reduced distractor-cost was normalized when the same patients were tested ON their normal dopaminergic medication, so that the distractor-cost of the patients no longer differed from that of controls when they were ON medication.
These data confirm that the DA-depleted state of PD is accompanied by changes in cognitive control. However, PD seems to confer either deficits or benefits depending on the precise task demands under study. Whereas they suffer enhanced switch-costs (i.e. impaired flexible updating), they also show reduced distractibility (i.e. enhanced stabilization). Based on their anatomical pattern of DA depletion, the fMRI data reviewed above (Figure 3) and the resemblance of the performance pattern to that seen in monkeys with striatal DA lesions (100), we hypothesize that the combination of poor flexibility and good stability in PD patients OFF medication reflects depletion of striatal DA and up-regulation of DA in the PFC respectively. An intriguing possibility is that the restoration of switch- and distractor-costs by dopaminergic medication reflects a normalization of the balance between frontal and striatal DA.
Summary
In summary, DA plays a critical role in cognitive control, which is a multi-factorial phenomenon that requires a dynamic balance between flexible updating and cognitive stabilization. Understanding the precise effects of DA on these subcomponent processes, is not straightforward, partly because the relationship between DA and performance is non-linear and inverted-U shaped, with both excessive as well as insufficient levels impairing performance. In addition effects of DA depend on the brain region that is targeted, with modulation of one and the same brain region having paradoxical consequences for different subcomponent processes. Specifically, we have put forward a working hypothesis that DA might act at the striatum and the PFC to facilitate flexible updating and cognitive stabilization respectively. Although this hypothesis likely reflects an oversimplified view of dopamine’s complex effects on working memory and cognitive control (with different forms of flexible updating implicating distinct neural and neurochemical systems), we believe that it provides a plausible starting point for further empirical work.
A DA U-shaped function: empirical observation or neural mechanism?
It should be noted that the observation that the relationship between DA and cognitive function is non-linear and inverted-U shaped, with both excessive as well as insufficient levels impairing performance, is an empirical one, and provides a descriptive rather than a mechanistic account of DA’s action. We believe it is necessary to highlight this observation and advocate the taking into account of individual differences in baseline DA levels, either by proxy via working memory capacity or genetic variation, or preferably via direct measurement of DA transmission using PET. It is only when individual differences are taken into account that we can begin to address the mechanisms of DA’s action on cognition.
What mechanisms might underlie these inverted-U shaped actions of DA on cognition? Seamans, Yang and Durstewitz (60, 66) as well as Arnsten (159) have already provided excellent reviews of the cellular mechanisms of D1 (and D2) receptor action within the PFC in relation to DA’s effects on working memory and cognitive control. Yet, as reviewed above, there is no direct evidence that effects on human working memory also depend on basal DA levels in the PFC. Instead, the literature has highlighted a role for baseline DA levels in the striatum. The cellular mechanisms of action of DA in the PFC are quite different from those in the striatum, with a greater number of D2 receptors, more localized effects and faster kinetics (60). Accordingly, the proposal that, for example, an abolition of calcium currents (63) or a suppression of PFC activity (67) by excessive D1 receptor stimulation underlies impaired PFC function cannot necessarily also explain the detrimental overdose effects of D2 receptor stimulation on striatal function. Unlike D1 receptors, D2 receptors can also be found on the presynaptic element of the neuron releasing the transmitter, where they serve as a self-regulatory autoreceptor. It is not unlikely that self-regulatory mechanisms play a role in the striatum, where D2 receptors are more abundant than in the PFC. Thus excessive dopamine D2 receptor stimulation might activate these presynaptic autoreceptors and lead to paradoxical inhibition of firing, synthesis or release of DA, thus impairing performance that depends on postsynaptic DA transmission. It is well possible that such presynaptic autoreceptors are more sensitive (and postsynaptic receptors less sensitive) to increases in DA in those individuals with already optimized levels of DA, precisely to ensure homeostasis. Conversely, sensitivity of presynaptic autoreceptors might be reduced, and sensitivity of postsynaptic receptors enhanced in individuals with insufficient levels of DA. Such a homeostatic arrangement could explain the common finding that different individuals might respond to a drug challenge in opposite ways, despite exhibiting similar performance under placebo.
Other systems
In future studies, a deeper understanding of DA’s interaction with other neurotransmitters, as well as neurohormonal systems will be necessary.
For example, regarding hormone-neurotransmitter interactions, strong evidence indicates that estrogen enhances DA activity by increasing DA synthesis, release and turnover as well as by modifying basal firing rates of DA neurons via membrane estrogen receptors (160). In a recent human fMRI study, female subjects were pre-selected for COMT genotype and scanned twice performing a working memory task when their estrogen levels were at their peak and trough during their menstrual cycle. We found that estrogen levels modulated PFC activity in a manner consistent with the DA effects we have described previously (161). That is, Val genotype individuals in a low estrogen state (lowest DA group) showed the greatest PFC activity, followed by Val individuals in a high estrogen state, Met genotype individuals in a low estrogen state, and finally, Met individuals in a high estrogen state (highest DA group). Thus, higher DA levels (e.g. high estrogen, Met genotype or both) were associated with lower PFC activation, while lower DA levels (e.g. low estrogen, Val genotype or both) were associated with greater PFC activation, in keeping with the effects of DA on neural efficiency observed previously. Importantly, these neural effects were accompanied by significant differences in behavioral performance within individuals at different points in time. For example, Val genotype individuals performed more poorly on the working memory task when they were in a low estrogen state and improved in a high estrogen state, and Met genotype individuals performed better in a low estrogen state and worsened in a high estrogen state. These findings provide further support for DA’s inverted U-shaped effects on neural function and cognition.
Extensive research indicates that working memory performance depends not only on DA transmission. Noradrenaline (NA), acetylcholine (162) and glutamate (163) are also critical, the latter two possibly via modulation of attention and expectancy respectively. In the case of NA, for example, Arnsten and colleagues (121) have shown that the ability of a network of neurons to maintain firing over a delay period is weakened by cAMP-potassium channel signaling, and strengthened by noradrenergic alpha-2 receptor stimulation, which inhibits cAMP-potassium channel signaling (as well as by other molecular events that depolarize the spine (eg nicotinic alpha-7 receptor stimulation)). Furthermore, Aston-Jones and Cohen (164) have invoked constructs similar to the ‘inverted-U-shaped’ function for NA function and like DA, NA enhances the signal-to-noise ratio of target systems. This is relevant for understanding effects of DA in PD, which also affects the noradrenergic system, and where L-dopa enhances NA transmission as well as DA transmission. Nevertheless, while the different neurotransmitter systems clearly interact to orchestrate integrated behavior, comparison of relatively specific neurochemical manipulations on common cognitive paradigms has revealed differential implication in distinct cognitive functions (28, 121, 165, 166). Precisely how and why these systems are different should be the primary aim of future work. Such future work will benefit from adoption of a cognitive mechanistic approach, by which issues of cognitive control and working memory are placed on a common footing with other forms of behavioral control, e.g. reinforcement learning (167). This is particularly pertinent given the implication of striatal DA in both cognitive control and working memory as well as reinforcement learning, and will help to further define the computational nature of the flexibility-stability paradox.
Conclusion
This review highlights the complex nature of the relationship between dopamine and cognitive control and summarizes the research that begins to elucidate the factors that contribute to this complex relationship. In particular we emphasize two factors. First, distinct optimum levels of dopamine exist for different cognitive functions. Second, cognitive control is a multi-factorial phenomenon, requiring a dynamic balance between cognitive stability and cognitive flexibility. Current research is beginning to suggest that these distinct components might implicate the prefrontal cortex and the striatum respectively. Accordingly, high levels of dopamine receptor stimulation in the PFC might be good for cognitive stability but bad for cognitive flexibility, whereas high levels of dopamine in the striatum might be good for cognitive flexibility but bad for cognitive stability. Manipulation of DA will thus have paradoxical cognitive consequences depending on the type of task component under study, the brain region that is implicated, and the baseline levels of DA in that brain region.
Acknowledgements
The work was supported by NIH grants MH63901 (to MD), NS40813 (to MD), DA02060 (to MD and RC) and AG027984 (to MD) and by a VIDI grant of The Netherlands Organisation for Scientific Research (to RC) and a fellowship of the dutch Brain foundation to support dementia research (to RC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Bibliography
- 1.Cohen J, Braver T, Brown J. Computational perspectives in dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 2.Bilder R, Volavka K, Lachman H, Grace A. The Catechol-O-Methyltransferase Polymorphism: Relations to the Tonic-Phasic Dopamine Hypothesis and Neuropsychiatric Phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 3.D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster J. The prefrontal cortex. New York: Raven Press; 1989. [Google Scholar]
- 5.Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Chao L, Knight R. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. NeuroReport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Shallice T. From Neuropsychology to Mental Structure. Cambridge, England: Cambridge University Press; 1988. [Google Scholar]
- 8.Goldman-Rakic . Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. In: Uylings HBM, Eden CGV, DeBruin JPC, Corner MA, Feenstra MGP, editors. Progress in Brain Research. Elsevier Sciene Publishers; 1990. pp. 325–336. [DOI] [PubMed] [Google Scholar]
- 9.Goldman-Rakic P. Dopamine-mediated mechanisms of the prefrontal cortex. Semin Neurosci. 1992;4:109–118. [Google Scholar]
- 10.Goldman-Rakic P. Cellular Basis of Working Memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 11.Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Experimental Brain Research. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 12.Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–446. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- 13.Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- 14.Cools R, D'Esposito M. Dopaminergic modulation of flexible control in humans. In: Bjorklund A, Dunnett SB, Iversen LL, Iversen SD, editors. Dopamine Handbook. Oxford: Oxford University Press; 2009. [Google Scholar]
- 15.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 16.Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- 17.Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Brown RM, Crane AM, Goldman PS. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vitro synthesis rates. Brain Research. 1979;168:133–150. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- 19.Brozoski TJ, Brown R, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in the prefrontal cortex of rhesus monkeys. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 20.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 21.Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. Journal of Cognitive Neuroscience. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- 22.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 23.Sawaguchi T, Matsumura M, Kubota K. Effects of dopamine antagonists on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- 24.Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory in humans by a D2 dopamine receptor agonist. Journal of Cognitive Neuroscience. 1992;4:58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- 25.Simon H. Dopaminergic A10 neurons and the frontal system. J Physiol. 1981;77:81–95. [PubMed] [Google Scholar]
- 26.Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 27.Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology. 1999;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 28.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological Psychology. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Kimberg DY, D'Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 30.Luciana M, Collins P. Dopaminergic modulation of working memory for spatial but not object cues in normal volunteers. J Cogn Neurosci. 1997;9:330–347. doi: 10.1162/jocn.1997.9.3.330. [DOI] [PubMed] [Google Scholar]
- 31.Mehta MA, Swainson R, Ogilvie AD, Sahakian BJ, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D2 agonist bromocriptine in human volunteers. Psychopharmacology. 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- 32.Kimberg DY, D'Esposito M. Cognitive effects of the dopamine receptor agonist pergolide. Neuropsychologia. 2003;41:1020–1027. doi: 10.1016/s0028-3932(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 33.Muller U, von Cramon DY, Pollman S. D1- versus D2-Receptor Modulation of Visuospatial Working Memory in Humans. Journal of Neuroscience. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson's disease. Psychopharmacology (Berl) 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- 35.Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- 36.Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 37.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs SE, D'Esposito M. Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cogn Affect Behav Neurosci. 2005;5:212–221. doi: 10.3758/cabn.5.2.212. [DOI] [PubMed] [Google Scholar]
- 39.Mattay VS, Callicot JH, Bertolino A, Heaton I, Frank JA, Coppola R, et al. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- 40.Mehta M, Calloway P, Sahakian B. Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. J Psychopharm. 2000;14:299–302. doi: 10.1177/026988110001400314. [DOI] [PubMed] [Google Scholar]
- 41.Kimberg D, Aguirre G, Lease J, D'Esposito M. Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Human Brain Mapping. 2001;12:246–257. doi: 10.1002/1097-0193(200104)12:4<246::AID-HBM1019>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daneman M, Carpenter P. Individual differences in working memory and reading. J Verbal Learning Verbal Behav. 1980;19:450–466. [Google Scholar]
- 43.Salthouse T, Babcock R. Decomposing Adult Age Differences in Working Memory. Dev Psychol. 1991;27:762–766. [Google Scholar]
- 44.Frank MJ, O'Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- 45.Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilder J. Paradoxic reactions to treatment. NY State J Med. 1957;57:3348–3352. [PubMed] [Google Scholar]
- 47.Dews P. Rate-dependency hypothesis. Science. 1977;198:1182–1183. doi: 10.1126/science.563103. [DOI] [PubMed] [Google Scholar]
- 48.Dews PB. Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmac Exp Ther. 1958:122. [PubMed] [Google Scholar]
- 49.Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins T. Enhanced and Impaired Attentional Performance After Infusion of D1 Dopaminergic Receptor Agents into Rat Prefrontal Cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Floresco S, Phillips A. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;2001:4. [PubMed] [Google Scholar]
- 51.Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: Immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- 52.Arnsten AF, Goldman-Rakic PS. Noise Stress Impairs Prefrontal Cortical Cognitive Function in Monkeys. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 53.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal Stimulation of D1 Dopamine Receptors in the Rodent Prefrontal Cortex Impairs Spatial Working Memory Performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai JX, Arnsten AF. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmac Exp Ther. 1997;282:1–7. [PubMed] [Google Scholar]
- 55.Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci Res. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- 56.Phillips A, Ahn S, Floresco S. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;14:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- 58.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increasing dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proceedings of the National Academy of Sciences USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 62.Seamans J, Durstewitz D, Christie B, Stevens C, Sejnowski T. Dopamine D1/D5 receptor modulation of excitatory synaptic input to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V-VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seamans J, Gorelova N, Durstewitz D, Yang C. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Servan-Schreiber D, Printz H, Cohen J. A Network Model of Catecholamine Effects: Gain, Signal-to-Noise Ratio, and Behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- 66.Durstewitz D, Seamans J. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatr. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Vijayraghavan S, Wang M, Birnbaum S, Williams G, Arnsten A. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:176–184. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 68.Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 69.Mattay VS, Tessitore A, Callicott JH, Bertonlino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;58:630–635. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 70.Mattay V, Goldberg T, Fera F, Hariri A, Tessitore A, Egan M, et al. Catechol O-methyltransferase Val158 -met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frank MJ, Fossella JA. Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology. 2011;36:133–152. doi: 10.1038/npp.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- 73.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tunbridge E, Bannerman D, Sharp T, Harrison P. Catechol-O-Methyltransferase Inhibition Improves Set-Shifting Performance and Elevates Stimulated Dopamine Release in the Rat Prefrontal Cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and Neurochemical Modulation of Prefrontal Cognitive Functions in Children. Arch Gen Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- 77.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 78.Milner B. Effects of different brain lesions on card sorting: The role of the frontal lobes. Archives of Neurology. 1963:90–100. [Google Scholar]
- 79.Barnett J, Jones P, Robbins T, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatr. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- 80.Ullsperger M. Genetic association studies of performance monitoring and learning from feedback: the role of dopamine and serotonin. Neurosci Biobehav Rev. 2010;34:649–659. doi: 10.1016/j.neubiorev.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 81.Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci U S A. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 84.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32:1011–1020. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 85.Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biological Psychiatry. 2009;66:997–1004. doi: 10.1016/j.biopsych.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Cohen MX, Krohn-Grimberghe A, Elger CE, Weber B. Dopamine gene predicts the brain's response to dopaminergic drug. Eur J Neurosci. 2007;26:3652–3660. doi: 10.1111/j.1460-9568.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 87.Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cools R, Gibbs S, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cools R, Frank M, Gibbs S, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 93.Wallace D, Vytlacil J, Gibbs S, Nomura E, D'Esposito M. Annual meeting of the Organization for Human Brain Mapping. San Francisco: 2009. The effect of dopamine on functional connectivity, working memory and individual differences. [Google Scholar]
- 94.Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4:e6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 96.Camps M, Cortes R, Gueye B, Probst A, Palacios J. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neurosci. 1989;28 doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- 97.Goldman-Rakic P, Lidwo M, Smiley J, Williams M. The anatomy of dopamine in monkey and human prefrontal cortex. Journal of Neural Transmission. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- 98.Sedvall G, Farde L, Wiesel FA. Quantitative determination of D2 dopamine receptor characteristics in healthy human subjects and psychiatric patients. Life Sci. 1987;41:813–816. doi: 10.1016/0024-3205(87)90169-x. [DOI] [PubMed] [Google Scholar]
- 99.Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine Lesions of the Prefrontal Cortex in Monkeys Enhance Performance on an Analog of the Wisconsin Card Sort Test: Possible Interactions with Subcortical Dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crofts HS, Dalley JW, Van Denderen JCM, Everitt BJ, Robbins TW, Roberts AC. Differential Effects of 6-OHDA Lesions of the Frontal Cortex and Caudate Nucleus on the Ability to Acquire an Attentional Set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 101.Braver TS, Cohen JD. On the Control of Control: The Role of Dopamine in Regulating Prefrontal Function and Working Memory. In: Monsell S, Driver J, editors. Control of Cognitive Processes Attention and Performance XVIII; Cambridge, Massachusetts. London, England: The MIT Press; 2000. pp. 713–737. [Google Scholar]
- 102.Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 103.Lewis S, Dove A, Robbins T, Barker R, Owen A. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- 104.Leber A, Turk-Browne N, Chun M. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 2008;105:13592–13597. doi: 10.1073/pnas.0805423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 106.Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 108.Durstewitz D, Seamans J, Sejnowski T. Dopamine-Mediated Stabilization of Delay-Period Activity in a Network Model of Prefrontal Cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- 109.Hazy TE, Frank MJ, O'Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 110.Dodds CM, Clark L, Dove A, Regenthal R, Baumann F, Bullmore E, et al. The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology (Berl) 2009;207:35–45. doi: 10.1007/s00213-009-1634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- 112.Marklund P, Larsson A, Elgh E, Linder J, Riklund KA, Forsgren L, et al. Temporal dynamics of basal ganglia under-recruitment in Parkinson's disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132:336–346. doi: 10.1093/brain/awn309. [DOI] [PubMed] [Google Scholar]
- 113.Collins P, Wilkinson LS, Everitt BJ, Robbins TW, Roberts AC. The Effect of Dopamine Depletion From the Caudate Nucleus of the Common Marmoset (Callithrix jacchus) on Tests of Prefrontal Cognitive Function. Behav Neurosci. 2000;114:3–17. doi: 10.1037//0735-7044.114.1.3. [DOI] [PubMed] [Google Scholar]
- 114.van Schouwenburg MR, den Ouden HE, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kellendonk C, Simpson E, Polan H, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 116.Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- 117.Dodds CM, Muller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cools R, Lewis S, Clark L, Barker R, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology. 2007;32:180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]
- 120.Pycock CJ, Kerwin RW, Carter CJ. Effect of Lesion of Cortical Dopamine Terminals on Sub-Cortical Dopamine-Receptors in Rats. Nature. 1980;286:74–77. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- 121.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- 123.Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mehta M, McGowan S, Lawrence A, Aitken M, Montgomery A, Grasby P. Systemic sulpiride modulates striatal blood flow: relationships to spatial working memory and planning. NeuroImage. 2003;20:1982–1994. doi: 10.1016/j.neuroimage.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 125.Floresco S, Magyar O, Ghods-Sharifi S, Vexelman C, Tse M. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 126.Camps M, Cortés R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: Autoradiographic distribution of D1 sites. Neuroscience. 1989;28:275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- 127.Camps M, Kelly P, Palacios J. Autoradiographic localization of dopamine D1 and D2 receptors in the brain of several mammalian species. J Neural Transm Gen Sect. 1990;80:105–127. doi: 10.1007/BF01257077. [DOI] [PubMed] [Google Scholar]
- 128.Nolan K, Bilder R, Lachman H, Volavka K. Catechol O-Methyltransferase Val158Met Polymorphism in Schizophrenia: Differential Effects of Val and Met Alleles on Cognitive Stability and Flexibility. Am J Psychiatr. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- 129.Krugel LK, Biele G, Mohr PN, Li SC, Heekeren HR. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci U S A. 2009;106:17951–17956. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48:2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 131.Gibbs SE, D'Esposito M. A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience. 2006;139:359–371. doi: 10.1016/j.neuroscience.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 132.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 133.Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-Dopa withdrawal in Parkinson's disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology. 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- 134.Owen AM, James M, Leigh JM, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 135.Owen AM, Sahakian BJ, Hodges JR, Summers BA, Polkey CE, Robbins TW. Dopamine-dependent frontostriatal planning deficits in early Parkinson's disease. Neuropsychology. 1995;9:126–140. [Google Scholar]
- 136.Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- 137.Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 138.Gotham AM, Brown RG, Marsden CD. 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- 139.Cooper JA, Sagar HJ, Doherty M, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholingergic therapies on cognitive and motor function in Parkinson's disease. Brain. 1992;115:1701–1725. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- 140.Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J. Working memory in medicated patients with Parkinson's disease: the central executive seems to work. J Neurol Neurosurg Psychiatry. 1996;60:313–317. doi: 10.1136/jnnp.60.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sahakian B, Morris R, Evenden J, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- 142.Brown R, Marsden C. 'Subcortical dementia': The neuropsychological evidence. Neurosci. 1988;25:363–387. doi: 10.1016/0306-4522(88)90246-1. [DOI] [PubMed] [Google Scholar]
- 143.Dubois B, Pillon B, Malapani C, Deweer B, Verin M, Partiaud A, et al. Subcortical dementia and Parkinson's disease: what are the cognitive functions of the basal ganglia? In: Boller F, Grafman J, editors. Handbook of neuropsychology. New York: Elsevier; 1995. pp. 195–240. [Google Scholar]
- 144.Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1179. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- 145.Cools R, Miyakawa A, Sheridan M, D'Esposito M. Enhanced frontal function in Parkinson's disease. Brain. 2010;133:225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain. 1998;121:949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- 147.Lewis S, Dove A, Robbins T, Barker R, Owen A. Cognitive Impairments in Early Parkinson's Disease Are Accompanied by Reductions in Activity in Frontostriatal Neural Circuitry. J Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monchi I, Petrides M, Doyon J, Postuma R, Worsley K, Dagher A. Neural Bases of Set-Shifting Deficits in Parkinson's Disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 150.Agid Y, Ruberg M, Javoy-Agid, Hirsch E, Raisman-Vozari R, Vyas S, Faucheux B, et al. Are Dopaminergic Neurons Selectively Vulnerable to Parkinson's Disease? Adv Neurol. 1993;60:148–164. [PubMed] [Google Scholar]
- 151.Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks D. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- 152.Rakshi J, Uema T, Ito K, Bailey D, Morrish P, Ashburner J, et al. Frontal, midbrain and striatal dopamergic function in early and advanced Parkinson's disease. A 3D [(18)F]dopa-PET study. Brain. 1999;122:1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- 153.Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, et al. Increased frontal [(18)F]fluorodopa uptake in early Parkinson's disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- 154.Bowen FP, Kamienny RS, Burns MM, Yahr MD. Parkinsonism: Effects of levodopa treatment on concept formation. Neurology. 1975;25:701–704. doi: 10.1212/wnl.25.8.701. [DOI] [PubMed] [Google Scholar]
- 155.Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson's disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- 156.Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson's disease. The cortical focus of neostriatal outflow. Brain. 1986;109:845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- 157.Cools AR, Van Den Bercken JHL, Horstink MWI, Van Spaendonck KPM, Berger HJC. Cognitive and motor shifting aptitude disorder in Parkinson's disease. J Neurol Neurosurg Psychiatr. 1984;47:443–453. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hayes AE, Davidson MC, Keele SW. Towards a functional analysis of the basal ganglia. J Cogn Neurosci. 1998;10:178–198. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- 159.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of "representational knowledge": a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17 Suppl 1:i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 160.Becker JB. Oestrogen effects on dopaminergic function in striatum. Novartis Foundation symposium. 2000;230:134–145. discussion 145–154. [PubMed] [Google Scholar]
- 161.Jacobs E, D'Esposito M. Annual meeting of the Society for Neuroscience. Chicago: 2009. Estrogen shapes dopamine-dependent cognitive processes in prefrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corlett PR, Honey GD, Krystal JH, Fletcher PC. Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology. 2011;36:294–315. doi: 10.1038/npp.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 165.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 166.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 167.O'Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]