Abstract
Docetaxel is primarily metabolized by CYP3A4 and susceptible to alterations in clearance by CYP3A4 inhibition and induction. Imatinib is a CYP3A4 inhibitor. A phase I study of docetaxel and imatinib in metastatic breast cancer (MBC) was conducted to test the hypothesis that imatinib decreased docetaxel clearance. Docetaxel was administered weekly × 3 with daily imatinib, repeated every 28 days; during cycle 1, imatinib was started on day 8. Docetaxel and imatinib pharmacokinetics, and hepatic CYP3A4 activity (erythromycin breath test) were evaluated during cycles 1 and 2. Toxicity and efficacy were assessed. Twelve patients were enrolled to three docetaxel/imatinib dose levels: 20 mg/m2/600 mg (DL1), 25 mg/m2/600 mg (DL2), and 25 mg/m2/400 mg (DL2a). Median number of prior chemotherapy regimens was 2 (range, 0–8). Toxicities were primarily observed at DL2; dose-limiting toxicities were Grade 3 transaminase elevations and diarrhea, and 5 patients had grade 2 nausea. Two patients had partial responses (7 months); two stable disease (2 and 4 months); five had progressive disease. Despite a 42% decrease in CYP3A4 activity after 3 weeks of imatinib co-administration, docetaxel clearance was unchanged. Mean ± standard deviation steady-state imatinib trough concentration (2.6 ± 1.2 μg/ml) was approximately 2.6-fold higher than previously observed in other cancer populations, and likely contributed to the poor tolerability of the combination in MBC. In conclusion, imatinib inhibited CYP3A4 but did not affect docetaxel clearance. Clinically, further investigation of this combination in MBC is not warranted due to excessive toxicities. However, these unexpected pharmacokinetic findings support further investigation of mechanisms underlying docetaxel elimination pathways.
Keywords: Docetaxel, Imatinib, Pharmacokinetics, CYP3A4 inhibitor, Breast cancer
Introduction
Docetaxel is an antineoplastic agent that acts as a microtubule stabilizer, leading to mitotic block in proliferating cells [1]. Chemotherapy regimens incorporating docetaxel have demonstrated a survival benefit over non taxane-containing regimens, alone or in combination with biologic therapies, in both the adjuvant and metastatic breast cancer settings [2–6]. However, new agents and combination regimens are needed to improve the efficacy of current therapies and patient outcomes. Targeted agents, such as small molecule tyrosine kinase inhibitors, may help reverse chemotherapy resistance and enhance response to standard chemotherapy in patients with breast cancer. Promising preclinical and clinical data led to the initial investigation of imatinib in patients with advanced disease [7–9].
Imatinib is a small molecule inhibitor that targets the ABL, c-KIT, and platelet-derived growth factor receptor (PDGFR) protein tyrosine kinases and numerous other kinases [10, 11]. PDGFR expression is present in breast cancer tissue and surrounding stromal cells [12], and inhibition of PDGFR signaling has been shown to decrease interstitial fluid pressure and enhance delivery of chemotherapy to tumors [13, 14]. High expression of oncogenic c-KIT has also been identified in the “basal”-like breast cancer subtype [15]. In preclinical breast cancer models, imatinib inhibited cell growth and invasiveness [7], and sensitized cells to chemotherapy [8].
The impact of a novel therapeutic agent such as i-matinib on docetaxel pharmacokinetics, which could ultimately affect safety of this and other drug combinations, has not been examined. Previously, a drug interaction study in cancer patients demonstrated that docetaxel clearance is reduced by approximately 50% in the presence of the CYP3A4 inhibitor ketoconazole [16]. The clinical relevance of this drug interaction was demonstrated previously; a reduction in docetaxel clearance by as little as 25% was associated with a significant increase in the odds of developing febrile neutropenia [17]. Imatinib has been shown to inhibit CYP3A4 in vivo using the erythromycin breath test as a phenotypic probe [18]. We conducted a phase I study with docetaxel and imatinib in patients with advanced breast cancer to test the hypothesis that imatinib decreases docetaxel clearance through CYP3A4 inhibition. We also evaluated the safety profile, determined the maximum tolerated dose (MTD), and attempted to identify a recommended phase II dose (RP2D) of the combination.
Patients and methods
Eligibility
Women (≥18 or older) with a histologically confirmed locally advanced (stage 3b/c) or metastatic (stage 4) adenocarcinoma of the breast were eligible. Additional eligibility criteria included ECOG performance status (PS) of 0–1; measurable or evaluable disease; at least 4 weeks since prior chemotherapy or 2 weeks since prior radiation therapy; and adequate hematologic, hepatic and renal function. Prior therapy with a taxane in the adjuvant or metastatic setting was allowed.
Patients were excluded if they had untreated brain metastases, another active malignancy, serious concurrent medical conditions or pregnancy. Concomitant use of warfarin (a CYP450 substrate) or strong inhibitors/inducers of CYP3A4 were not permitted. The clinical protocol was approved by the Johns Hopkins Institutional Review Board and all subjects provided written informed consent prior to study drug administration.
Drug dosage and administration
Commercially available docetaxel was used for the study and imatinib was supplied by Novartis Oncology. Doce-taxel was stored, prepared and handled per standard instructions for the commercially available product [19, 20]. Imatinib was provided as 100 mg capsules packaged in bottles. Patients were instructed to swallow tablets in a sitting position with a large (250 ml) glass of liquid and not to take with grapefruit juice.
Docetaxel was administered intravenously with dexamethasone pre-medication on days 1, 8, and 15 repeated every 28 days (one cycle). Imatinib was commenced on cycle 1 day 8 once the first set of extensive docetaxel pharmacokinetic sampling was complete. The starting dose for docetaxel was 20 mg/m2 weekly and imatinib 600 mg daily oral continuously. The starting dose of imatinib was subsequently reduced to 400 mg daily per study amendment due to excessive nausea (Table 1). Patients documented administration with a study drug diary and pill counts were performed. Concomitant use of colony stimulation factors was permitted on a case by case basis. Missed doses were not substituted. Treatment was continued until unacceptable toxicity, disease progression, or withdrawal of consent.
Table 1.
Dose levels explored
| Dose level | Number of patients (evaluable) | Imatinib oral daily continuously (1st dose: Cycle 1 Day 8) (mg) | Docetaxel intravenous days 1, 8, and 15 (mg/m2) |
|---|---|---|---|
| Dose level 1 (Starting Cohort) | 4 (3) | 600 | 20 |
| Dose level 2 | 6 (4) | 600 | 25 |
| Dose level 2a | 2 (2) | 400 | 25 |
Dose modification
Toxicity was assessed using the National Cancer Institute/Division of Cancer Treatment Common Toxicity Criteria (NCI CTC) version 3. Dose-limiting toxicity (DLT) was defined for cycle 1 and included treatment-related febrile neutropenia, grade 4 neutropenia if lasting <5 days, grade 4 thrombocytopenia of any duration, or ≥grade 3 non-hematologic toxicities.
Study design
Three to six patients were enrolled at each dose level (Table 1). Cohorts were expanded if one out of three patients experienced DLT. If no more than two out of six patients experienced DLT, the next cohort was treated at the next dose level. The MTD was reached if more than two out of six patients experienced DLT. The largest dose level at which less than three out of six patients experienced DLT was to be expanded to up to 12 patients to confirm the appropriateness of this dose level for future studies. Patients who were inevaluable for toxicity assessment in cycle 1 due to removal from the study were replaced to achieve a total of three evaluable patients per cohort.
Pretreatment and follow up studies
Baseline evaluations included routine history and physical examination, complete blood counts, serum chemistries and radiologic evaluations. Laboratory tests were repeated weekly during the first cycle and then before treatment and before each cycle thereafter. Clinical evaluations occurred prior to each cycle. Response of measurable lesions was assessed using Response Evaluation Criteria in Solid Tumors after cycles 2 and 4 and then every 3 cycles [21]. Patients were evaluable for response if they received two full cycles of therapy. Patients were followed for toxicity assessment for 30 days after going off study.
CYP3A4 activity
CYP3A4 activity was assessed using the erythromycin breath test (ERMBT) as previously described [22]. The test was administered within 48 h of commencing cycle 1 (without imatinib administration) and on cycle 1 day 8 (with imatinib administration). A breath sample was obtained at 20 min after administration of 14C-labeled erythromycin and shipped to Metabolic Solutions (Nashua, NH) for measurement of breath carbon dioxide. The data were reported as the flux of 14CO2. The parameter of interest was the percentage of 14C exhaled per minute at 20 min after administration of the radiolabeled erythromycin (C20 min [% dose/min]).
Pharmacokinetic sampling and analysis
Docetaxel pharmacokinetics were evaluated during cycles 1 (without imatinib) and 2 (with imatinib administration) at the following time points: immediately before docetaxel treatment, 30 min into the infusion, at 59 min (just before the end of the docetaxel infusion), and post infusion at 30 min, 1, 2, 4, and 8 h; on days 2 and 3; and weekly trough levels on days 8, 15, and 22 (cycle 1). Docetaxel concentrations in plasma were quantitated using a validated analytic assay consisting of high-performance liquid chromatography (HPLC) with mass spectrometric detection [23]. The lower limit of quantitation for docetaxel was 0.0004 μg/ml. Imatinib pharmacokinetics were evaluated at the following time points during cycles 1 and 2: before the initiation of treatment, and pre-treatment the morning of days 8, 15, and 22. In addition, serial pharmacokinetic studies were performed on cycle 2 day 1 after imatinib administration at the times listed above for docetaxel. Imatinib concentrations in plasma were quantitated using a validated analytic assay based on reversed-phase HPLC with UV detection [24]. The lower limit of quantitation for imatinib was 0.2 μg/ml.
Individual docetaxel and imatinib plasma concentration–time data were analyzed by noncompartmental methods using WinNonlin version 5.3 (Pharsight, Inc.) [25]. Average minimum docetaxel concentrations were calculated as an average of the pre-treatment concentrations from days 8, 15, and 22 during both cycles. Since docetaxel disposition is linear within the range of infusion durations and doses applied, the observed exposure parameters (i.e., AUC, Cmax, and C168h) in each patient was normalized to a dose of 25 mg/m2 without applying further correction (normalized parameter = observed parameter × [25/actual dose])[20]. Steady-state minimum imatinib concentrations (Css,min) were calculated as an average of the pre-treatment concentrations from cycle 1 day 15 through cycle 2 day 22. Imatinib Css,min was also dose normalized to a dose of 600 mg/day due to linear pharmacokinetics over the dose range studied [26].
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, efficacy, and safety data. Pharmacokinetic parameters were summarized by descriptive statistics using dose-normalized parameters for dose-dependent parameters and actual values for dose-independent parameters. For docetaxel pharmacokinetic parameters and CYP3A activity, a Wilcoxon matched pairs signed-rank test was used to compare parameters between cycles 1 and 2.
Results
Patient characteristics
Fifteen patients with metastatic breast cancer were consented between February 2004 and May 2006. Twelve were eligible and initiated treatment on study and patient characteristics are summarized in Table 2. A total of 33 cycles of therapy were administered and three patients did not complete cycle 1. The median number of cycles was 2 (range 1–7). Four patients received starting doses of i-matinib 600 mg and docetaxel 20 mg/m2 (dose level 1), six patients received imatinib 600 mg and docetaxel 25 mg/m2 (dose level 2) and two patients received imatinib 400 mg and docetaxel 25 mg/m2 (dose level 2a) (Table 1).
Table 2.
Patient characteristics
| Characteristics | Number of patients (n = 12) |
|---|---|
| Age, years | |
| Median | 57 |
| Range | 28–73 |
| Race | |
| Caucasian | 10 |
| Black | 1 |
| Asian | 1 |
| ECOG Performance status | |
| 0 | 6 |
| 1 | 6 |
| Location of disease | |
| Visceral | 10 |
| Non-visceral | 2 |
| Hormone-receptor (ER, PR, or both) positive/HER-2 neg | 5 |
| HER2-pos (ER-pos; ER-neg) | 4 (3; 1) |
| Triple-negative (ER,PR,HER-2 negative) | 3 |
| Number of prior regimens for metastatic disease | |
| 1 | 2a |
| 2 | 3 |
| 3 | 3 |
| ≥4 | 1 |
| Median no. of regimens for metastatic disease (range) | 2 (0–8) |
| No. of prior taxane regimens | |
| Any setting | 10 |
| Metastatic setting | 7 |
| Median number of prior taxane regimens | |
| Any setting (range) | 1 (0–2) |
| Metastatic setting (range) | 0 (0–2) |
ER estrogen receptor, PR progesterone receptor, ECOG Eastern Cooperative Oncology Group
1 patient underwent chemo-embolization to liver twice also
Sequence of dose levels studied and DLTs
Among four patients enrolled in dose level 1, no DLT occurred and no dose modifications were required in three evaluable patients. A fourth patient developed disease-related deep venous thrombosis, began anticoagulation and discontinued protocol therapy while still in cycle 1. In dose level 2, a total of 6 patients were treated. The first DLT at this dose level was a patient with grade 3 transaminase elevation who received three additional cycles following dose reduction of docetaxel. During expansion of dose level 2, a second patient experienced dose-limiting grade 3 diarrhea, but continued therapy after imatinib dose reduction with disease progression after cycle 2. One patient in this cohort came off study during cycle 1 due to progressive disease. Two of the remaining subjects developed grade 2 nausea beginning with cycle 1, which was associated with the administration of imatinib. Although not a DLT, this toxicity was not alleviated by taking the study drug with a full stomach or splitting the daily dose in two and both patients opted to go off study after one and four cycles, respectively. Consequently, the starting dose of imatinib was reduced from 600 to 400 mg once daily and a new dose level 2a was created. Two patients were treated at dose level 2a with no occurrence of DLT and no dose modification was required. However, further enrolment was not pursued as mentioned in the “Discussion” section.
Safety and tolerability
Potential treatment-related toxicities of all grades and for all cycles are listed in Table 3. Grade 3 drug-related toxicities were infrequent and were observed only at dose level 2. Grade 3 neutropenia occurred in one patient treated at dose level 2 during cycle 2 requiring docetaxel to be held for one dose. Treatment was resumed at full dose and the patient completed a total of 7 cycles of therapy with no further neutropenia. The most frequent non-hematologic adverse events were nausea (91%), fatigue (67%), alopecia (58%), and dyspepsia (58%). One patient experienced grade 3 dyspepsia (cycle 3) and grade 3 fatigue (cycle 4), and subsequently received a reduced dose of imatinib. Five patients experienced grade 2 nausea at dose level 2.
Table 3.
Treatment-related side effects per dose level
| Toxicity | Events | Dose level
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 3)
|
2 (n = 6)
|
2a (n = 2)
|
||||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | ||
| Neutropenia | 1 | 1 | ||||||||
| Anemia | 6 | 2 | 1 | 2 | 1 | |||||
| Fever | 1 | 1 | ||||||||
| Fatigue | 8 | 2 | 4 | 1 | 1 | |||||
| Pain musculoskeletal | 5 | 2 | 2 | 1 | ||||||
| Neuropathy sensory | 2 | 2 | ||||||||
| Nausea | 11 | 3 | 1 | 5 | 2 | |||||
| Vomiting | 6 | 2 | 1 | 2 | 1 | |||||
| Diarrhea | 6 | 1 | 2 | 1* | 1 | 1 | ||||
| Dyspepsia | 7 | 2 | 2 | 1 | 1 | 1 | ||||
| Anorexia | 5 | 1 | 3 | 1 | ||||||
| Taste alteration | 5 | 1 | 1 | 1 | 1 | 1 | ||||
| Flatulence | 2 | 2 | ||||||||
| ALT elevation | 1 | 1* | ||||||||
| AST elevation | 1 | 1 | ||||||||
| Dry skin | 2 | 1 | 1 | |||||||
| Rash | 6 | 3 | 1 | 1 | 1 | |||||
| Nasal reactions | 2 | 1 | 1 | |||||||
| Epiphora | 5 | 1 | 4 | |||||||
| Dry eye | 6 | 1 | 1 | 1 | 2 | 1 | ||||
| Alopecia | 7 | 2 | 1 | 4 | ||||||
| Nail changes | 2 | 1 | 1 | |||||||
| Mucositis | 1 | 1 | ||||||||
| Edema limb | 3 | 1 | 2 | |||||||
| Facial edema | 5 | 2 | 3 | |||||||
| Hot flashes | 1 | 1 | ||||||||
| Flushing | 2 | 1 | 1 | |||||||
| Dehydration | 1 | 1 | ||||||||
| Hypokalemia | 1 | 1 | ||||||||
Note: Number of worst grade adverse events possibly, probably, or definitely attributed to docetaxel or imatinib during study drug administration. Toxicities are graded per the NCI CTCAE version 3 criteria. G1 Grade 1, G2 Grade 2, G3 Grade 3. Dose-limiting toxicities are indicated by *
Response evaluation
Nine patients were evaluable for response. Two patients had a partial response at first assessment and showed disease progression after 7 cycles of treatment. Both had received a prior taxane in the adjuvant (paclitaxel) or neoadjuvant (docetaxel) setting. Two patients had stable disease, but study treatment was stopped after 2 and 4 cycles due to progressive disease and persistent nausea, respectively. The other five patients had progressive disease as best response and stopped study therapy.
CYP3A activity and docetaxel and imatinib pharmacokinetics
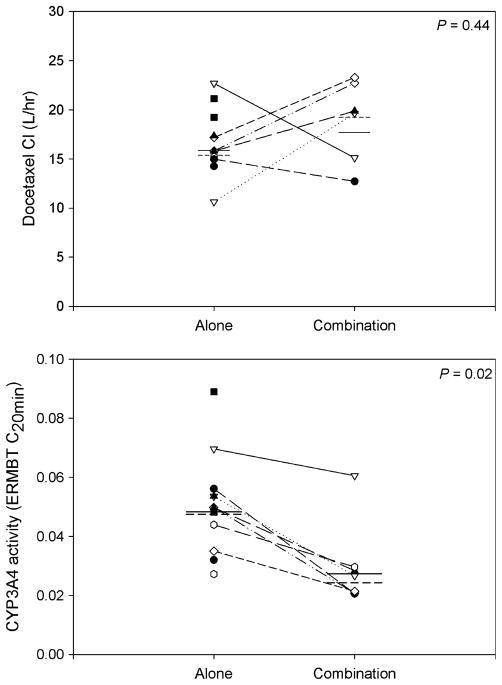
All 12 patients completed the pharmacokinetic and ER-MBT studies during the first cycle and 7 completed studies during the second cycle (Table 4). Five patients did not receive docetaxel nor undergo CYP3A4 testing during cycle 2. CYP3A4 activity decreased by 42% after imatinib administration (Fig. 1). Mean ± standard deviation ERMBT C20 min parameter values were 0.051 ± 0.017% dose/min before cycle 1 and 0.030 ± 0.014% dose/min before cycle 2 (P = 0.016). However, the change in CYP3A4 activity did not correlate with alteration in docetaxel pharmacokinetic parameters. Docetaxel clearance was similar during cycles 1 and 2 with mean values of 16.3 ± 3.59 and 18.1 ± 4.35 l/h, respectively (P = 0.44). No differences were observed between cycles 1 and 2 for other docetaxel pharmacokinetic parameters (P < 0.05). Imatinib pharmacokinetic parameters are summarized in Table 4. Mean ± SD imatinib steady-state trough concentration was 2.6 ± 1.2 μg/ml. These trough concentrations fall within the higher range (4th quartile) of those observed in a large population of patients with chronic myelogenous leukemia or gastrointestinal tumor receiving imatinib 400 or 600 mg daily; trough concentrations in the 4th quartile were associated with an increased incidence of adverse events including nausea [27, 28].
Table 4.
CYP3A4 activity and docetaxel and imatinib pharmacokinetics
| Parameter | Cycle
|
P | |
|---|---|---|---|
| 1 | 2 | ||
| Mean ± SD (n)* | Mean ± SD (n)* | ||
| ERMBT C20min % dose/minute | 0.051 ± 0.017 (12) | 0.030 ± 0.014(7) | 0.016 |
| Docetaxel | |||
| Normalized (μg/ml) | 2.6 ± 0.7 (12) | 2.2 ± 0.3(7) | 0.08 |
| Normalized (μg h/ml) | 2.9 ± 0.7 (12) | 2.8 ± 0.6(7) | 0.45 |
| Normalized average (μg/ml) | 0.0015 ± 0.0006(12) | 0.0017 ± 0.0005(7) | 0.088 |
| CL (l/h) | 16.3 ± 3.59(12) | 18.1 ± 4.35(7) | 0.44 |
| T1/2 (h) | 45.1 ± 20.0(12) | 60.2 ± 31.0(7) | 0.23 |
| Imatinib | |||
| Normalized (μg/m) | – | 6.5 ± 2.8(7) | – |
| Tmax (h) | – | 5.0 [2.1–7.2](7) | – |
| Normalized (μg/m h) | – | 98.6 ± 41.7(7) | – |
| CL/F (l/h) | – | 6.8 ± 2.2(7) | – |
| Normalized (μg/ml) | – | 2.6 ± 1.2(12) | – |
Data is presented as the mean ± SD (n), except Tmax which is presented as the median [range] (n)
SD standard deviation, ERMBT erythromycin breath test, C20min flux of 14CO2 at 20 min after administration of the ERMBT, Cmax maximum plasma concentration, AUCinf area under the concentration–time curve from time zero to infinity, CL clearance, T1/2 half-life, AUCτ area under the concentration–time curve during the dosing interval, CL/F apparent oral clearance, Cmin,ss minimum plasma concentration at steady-state
Data is normalized to a dose of 25 mg/m2
Data is normalized to a dose of 600 mg/day
Fig. 1.
Docetaxel clearance and CYP3A4 activity, as measured by the erythromycin breath test (ERMBT) when docetaxel was administered alone or in combination with imatinib. Each circle represents an observation while lines and dashes are the mean and median values, respectively
Discussion
The taxanes paclitaxel and docetaxel have emerged as a fundamental component of chemotherapy regimens for many women with breast cancer. The administration of docetaxel as a single agent or in combination with other chemotherapeutic or biologic agents resulted in a significant improvement in overall survival over non taxane-containing regimens in the adjuvant and metastatic settings [2–6]. Docetaxel itself is also approved for the treatment of patients with gastric, head and neck, non-small cell lung, and hormone-refractory prostate cancer. Therefore, efforts to improve the efficacy of current therapies and overcome drug resistance led to combination studies of docetaxel with novel therapeutic agents in early phase clinical trials. However, careful pharmacokinetic and toxicity studies must be performed before embarking on larger efficacy studies testing new combinations, as potentially deleterious pharmacokinetic/pharmacodynamic interactions may occur between agents resulting in diminished efficacy and/or unacceptable toxicity.
Drug interaction studies have shown that docetaxel pharmacokinetics is sensitive to inhibition of CYP3A4, the liver enzyme responsible for the metabolism and elimination of docetaxel. For example, in patients with advanced cancer, administration of the potent CYP3A4 inhibitor ketoconazole inhibited docetaxel clearance by half [16]. As imatinib has been shown to inhibit CYP3A4 in vitro and in vivo, [18] and to decrease the clearance of the CYP3A4 substrate simvastatin by 70% in vivo [29], we anticipated that daily administration of imatinib would also decrease the clearance of docetaxel. Indeed, our data confirm those of Gurney and colleagues [18] that the daily administration of imatinib results in a 42% decrease in mean CYP3A activity as assessed by the ERMBT, an assay that has been shown to be a reliable drug elimination phenotyping probe for single agent docetaxel [30–32]. However, we observed no change in the mean docetaxel clearance value. This suggests that, in the continued presence of CYP3A4 inhibition by imatinib, docetaxel may be cleared by other elimination pathways despite CYP3A4 being the primary metabolic pathway. These other pathways may include the CYP2C8 enzyme, efflux transporters, or renal elimination, and might explain why we observed no effective change in clearance of docetaxel despite CYP3A4 inhibition as a result of daily continuous imatinib.
Alternatively, a possible explanation for our unexpected pharmacokinetic findings may relate to the limitations of liver phenotypic probes in general and the limitations of the ERMBT for drug interaction studies. Before being metabolized by CYP3A4, many compounds must first be taken up into the liver by a process involving carrier-mediated transport. It is possible that erythromycin and docetaxel may be transported into the liver by non-overlapping mechanisms. This is supported by recent in vitro data indicating that both erythromycin and docetaxel are substrates for liver OATP1B3, but docetaxel is also a substrate for liver OAT2 [33, 34]. Imatinib has previously been shown to be a substrate for OATP1B3 but not OAT2 [35, 36]. If imatinib differentially inhibits OATP1B3, docetaxel could still be transported into the liver by OAT2 and subsequently metabolized by CYP3A4. This has been observed for other drugs. As an example, altered CYP3A4 activity in vivo as assessed by the ERMBT has been associated with inhibition of most of the OATPs by rifampicin or inhibition of the efflux transporter P-glycoprotein by lansoprazole [37]. While the ERMBT is the most commonly used phenotypic probe for CYP3A4 activity, the choice of a phenotyping test to assess liver metabolism of a drug or to evaluate the effect of a drug on liver CYP3A4 activity should consider the transporters potentially involved in drug disposition.
Excessive gastrointestinal (GI) toxicity was experienced by our patients, causing many to discontinue study therapy most frequently at the higher dose of 600 mg of imatinib. GI toxicity (and resulting dose reductions) was also observed with the combination of daily imatinib 600 mg and weekly docetaxel in hormone-refractory prostate cancer [9]. Those authors postulated that a drug interaction might explain their observation, but their study did not include any pharmacokinetic component. The increased incidence of GI toxicity may be due, in part, to the higher imatinib steady-state trough concentrations observed in our metastatic breast cancer patient population compared to previous reports in patients with chronic myelogenous leukemia or gastrointestinal stromal tumor receiving similar doses of imatinib [27, 28]. Our study was not designed to determine the effect of a weekly infusion of docetaxel on imatinib clearance, as there was no supporting preclinical data to suggest a possible interaction. The association between systemic inflammation that can occur with malignancy and inhibition of drug metabolism and transport mechanisms is well described, and it is possible that patients with metastatic cancer have intrinsically reduced imatinib clearance [38]. It is also possible that the observed GI toxicity was due to administration of both drugs in combination in the absence of a pharmacokinetic interaction.
This excessive GI toxicity in addition to more recent data comparing various taxane schedules in early stage breast cancer [39, 40] suggested that weekly docetaxel may not be an optimum taxane schedule for further development, and led us to close this trial despite evidence of clinical activity in patients previously exposed to taxanes. Along these lines, while preclinical [41] and early clinical evidence from phase I trials investigating the combination of imatinib and a taxane in prostate cancer [9] was promising, subsequent studies did not confirm these findings and questioned the value of adding PDGFR inhibitors to taxane chemotherapy in this setting [42, 43]. Studies in breast cancer patients have also yielded disappointing results. Imatinib monotherapy has no antitumor activity in metastatic breast cancer patients selected [44] or not [45] based on PDGFR expression. Phase II studies combining imatinib with chemotherapy also did not observe a benefit over single agent capecitabine [46] or docetaxel [47]. None of these trials included a pharmacokinetic component, and patients in the latter study also required imatinib dose reduction.
In summary, we did not identify a recommended phase II dose for combining docetaxel with imatinib, and do not recommend further studies of this combination in breast cancer. However, our unexpected pharmacokinetic findings warrant further investigation of docetaxel elimination pathways. Factors beyond CYP3A interference affect docetaxel metabolism, and future studies must account for other enzymatic interactions and use improved CYP3A probes. More fundamentally, our study highlights the critical need to incorporate detailed pharmacokinetic studies in addition to toxicity assessments when testing novel agents in combination with standard chemotherapy drugs.
Acknowledgments
This research was supported by funding from an Avon/NCI Progress for Patients Awards Program (3P40 CA006973-41S) and by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH P30 CA069773). We are grateful to Novartis for providing the investigational supply of imatinib mesylate used in this trial. We also would like to thank Ping He for her technical support and Susan Davidson for her quality assurance of the data.
Footnotes
Conflict of interest No authors have any conflict of interest to declare.
Contributor Information
Roisin M. Connolly, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Michelle A. Rudek, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Elizabeth Garrett-Mayer, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA.
Stacie C. Jeter, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Michele G. Donehower, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Laurie A. Wright, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Ming Zhao, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA.
John H. Fetting, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Leisha A. Emens, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Vered Stearns, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA.
Nancy E. Davidson, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Sharyn D. Baker, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
Antonio C. Wolff, Email: awolff@jhmi.edu, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans Street, CRB1-189, Baltimore, MD 21231-1000, USA
References
- 1.Lavelle F, Bissery MC, Combeau C, Riou JF, Vrignaud P, Andre S. Preclinical evaluation of docetaxel (Taxotere) Semin Oncol. 1995;22:3–16. [PubMed] [Google Scholar]
- 2.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, Guevin R, Howell A, Fornander T, Hainsworth J, Coleman R, Vinholes J, Modiano M, Pinter T, Tang SC, Colwell B, Prady C, Provencher L, Walde D, Rodriguez-Lescure A, Hugh J, Loret C, Rupin M, Blitz S, Jacobs P, Murawsky M, Riva A, Vogel C. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Holmes FA, O’Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, Pippen JE, Bordelon JH, Kirby RL, Sandbach J, Hyman WJ, Richards DA, Mennel RG, Boehm KA, Meyer WG, Asmar L, Mackey D, Riedel S, Muss H, Savin MA. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 4.Nabholtz JM, Senn HJ, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, Vandenberg TA, Rapoport B, Rosso R, Trillet-Lenoir V, Drbal J, Molino A, Nortier JW, Richel DJ, Nagykalnai T, Siedlecki P, Wilking N, Genot JY, Hupperets PS, Pannuti F, Skarlos D, Tomiak EM, Murawsky M, Alakl M, Aapro M, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol. 1999;17:1413–1424. doi: 10.1200/JCO.1999.17.5.1413. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed online 15 Jan 2011]; http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory.
- 6.Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, Aranda I, Rodriguez-Lescure A, Grosse R, Calvo L, Barnadas A, Isla D, Martinez del Prado P, Ruiz Borrego M, Zaluski J, Arcusa A, Munoz M, Lopez Vega JM, Mel JR, Munarriz B, Llorca C, Jara C, Alba E, Florian J, Li J, Lopez Garcia-Asenjo JA, Saez A, Rios MJ, Almenar S, Peiro G, Lluch A. Adjuvant doce-taxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 7.Roussidis AE, Mitropoulou TN, Theocharis AD, Kiamouris C, Papadopoulos S, Kletsas D, Karamanos NK. STI571 as a potent inhibitor of growth and invasiveness of human epithelial breast cancer cells. Anticancer Res. 2004;24:1445–1447. [PubMed] [Google Scholar]
- 8.Sims JT, Ganguly S, Fiore LS, Holler CJ, Park ES, Plattner R. STI571 sensitizes breast cancer cells to 5-fluorouracil, cisplatin and camptothecin in a cell type-specific manner. Biochem Pharmacol. 2009;78:249–260. doi: 10.1016/j.bcp.2009.04.007. S0006-2952(09)00292-510.1016/j.bcp.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew P, Thall PF, Jones D, Perez C, Bucana C, Troncoso P, Kim SJ, Fidler IJ, Logothetis C. Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase I trial in androgen-independent prostate cancer. J Clin Oncol. 2004;22:3323–3329. doi: 10.1200/JCO.2004.10.11622/16/3323. [DOI] [PubMed] [Google Scholar]
- 10.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. 353/2/17210. 1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 11.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 12.Bhardwaj B, Klassen J, Cossette N, Sterns E, Tuck A, Deeley R, Sengupta S, Elliott B. Localization of platelet-derived growth factor beta receptor expression in the periepithelial stroma of human breast carcinoma. Clin Cancer Res. 1996;2:773–782. [PubMed] [Google Scholar]
- 13.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- 14.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, Rubin K. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–2934. [PubMed] [Google Scholar]
- 15.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engels FK, Ten Tije AJ, Baker SD, Lee CK, Loos WJ, Vulto AG, Verweij J, Sparreboom A. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004;75:448–454. doi: 10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 18.Gurney H, Wong M, Balleine RL, Rivory LP, McLachlan AJ, Hoskins JM, Wilcken N, Clarke CL, Mann GJ, Collins M, Delforce SE, Lynch K, Schran H. Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin Pharmacol Ther. 2007;82:33–40. doi: 10.1038/sj.clpt.6100201. [DOI] [PubMed] [Google Scholar]
- 19. [Accessed online 15 Jan 2011]; http://products.sanofi-aventis.us/Taxotere/taxotere.html#S15.
- 20.Baker SD, Zhao M, Lee CK, Verweij J, Zabelina Y, Brahmer JR, Wolff AC, Sparreboom A, Carducci MA. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2004;10:1976–1983. doi: 10.1158/1078-0432.ccr-0842-03. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, Schenk PW, Charles KA, Clarke SJ, Carducci MA, McGuire WP, Dawkins F, Gelderblom H, Verweij J, Sparreboom A. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res. 2004;10:8341–8350. doi: 10.1158/1078-0432.CCR-04-1371. 10/24/834110.1158/1078-0432.CCR-04-1371. [DOI] [PubMed] [Google Scholar]
- 23.Baker SD, Zhao M, He P, Carducci MA, Verweij J, Sparreboom A. Simultaneous analysis of docetaxel and the formulation vehicle polysorbate 80 in human plasma by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2004;324:276–284. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 24.van Erp NP, Gelderblom H, Karlsson MO, Li J, Zhao M, Ouwerkerk J, Nortier JW, Guchelaar HJ, Baker SD, Sparreboom A. Influence of CYP3A4 inhibition on the steady-state pharmacokinetics of imatinib. Clin Cancer Res. 2007;13:7394–7400. doi: 10.1158/1078-0432.CCR-07-0346. [DOI] [PubMed] [Google Scholar]
- 25.Gibaldi M, Perrier D. Noncompartmental analysis based on statistical moment theory. In: Gibaldi M, Perrier D, editors. Pharmacokinetics. Marcel Dekker; New York, NY: 1982. pp. 409–417. [Google Scholar]
- 26.Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44:879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 27.Larson RA, Druker BJ, Guilhot F, O’Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 28.Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141–3147. doi: 10.1200/JCO.2008.20.4818. JCO.2008.20.481810.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien SG, Meinhardt P, Bond E, Beck J, Peng B, Dutreix C, Mehring G, Milosavljev S, Huber C, Capdeville R, Fischer T. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome p450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer. 2003;89:1855–1859. doi: 10.1038/sj.bjc.6601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooker AC, Ten Tije AJ, Carducci MA, Weber J, Garrett-Mayer E, Gelderblom H, McGuire WP, Verweij J, Karlsson MO, Baker SD. Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther. 2008;84:111–118. doi: 10.1038/sj.clpt.6100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH. The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res. 2000;6:1255–1258. [PubMed] [Google Scholar]
- 32.Slaviero KA, Clarke SJ, McLachlan AJ, Blair EY, Rivory LP. Population pharmacokinetics of weekly docetaxel in patients with advanced cancer. Br J Clin Pharmacol. 2004;57:44–53. doi: 10.1046/j.1365-2125.2003.01956.x. 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, Franke RM, Hu S, Schuetz EG, Lamba V, Messersmith WA, Wolff AC, Carducci MA, Sparreboom A. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–163. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franke RM, Baker SD, Mathijssen RH, Schuetz EG, Sparreboom A. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin Pharmacol Ther. 2008;84:704–709. doi: 10.1038/clpt.2008.94. [DOI] [PubMed] [Google Scholar]
- 35.Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, Burger H, Baker SD, Sparreboom A. Interaction of i-matinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 36.Franke RM, Sparreboom A. Inhibition of imatinib transport by uremic toxins during renal failure. J Clin Oncol. 2008;26:4226–4227. doi: 10.1200/JCO.2008.18.4390 author reply 4227–4228. [DOI] [PubMed] [Google Scholar]
- 37.Frassetto LA, Poon S, Tsourounis C, Valera C, Benet LZ. Effects of uptake and efflux transporter inhibition on erythromycin breath test results. Clin Pharmacol Ther. 2007;81:828–832. doi: 10.1038/sj.clpt.6100148. [DOI] [PubMed] [Google Scholar]
- 38.Schmith VD, Foss JF. Effects of inflammation on pharmacokinetics/pharmacodynamics: increasing recognition of its contribution to variability in response. Clin Pharmacol Ther. 2008;83:809–811. doi: 10.1038/clpt.2008.62. [DOI] [PubMed] [Google Scholar]
- 39.Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 40.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, Wood WC, Davidson NE. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uehara H, Kim SJ, Karashima T, Shepherd DL, Fan D, Tsan R, Killion JJ, Logothetis C, Mathew P, Fidler IJ. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 42.Mathew P, Thall PF, Bucana CD, Oh WK, Morris MJ, Jones DM, Johnson MM, Wen S, Pagliaro LC, Tannir NM, Tu SM, Meluch AA, Smith L, Cohen L, Kim SJ, Troncoso P, Fidler IJ, Logothetis CJ. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432. CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 43.Mathew P, Pisters LL, Wood CG, Papadopoulos JN, Williams DL, Thall PF, Wen S, Horne E, Oborn CJ, Langley R, Fidler IJ, Pettaway CA. Neoadjuvant platelet derived growth factor receptor inhibitor therapy combined with docetaxel and androgen ablation for high risk localized prostate cancer. J Urol. 2009;181:81–87. doi: 10.1016/j.juro.2008.09.006 discussion 87. [DOI] [PubMed] [Google Scholar]
- 44.Cristofanilli M, Morandi P, Krishnamurthy S, Reuben JM, Lee BN, Francis D, Booser DJ, Green MC, Arun BK, Pusztai L, Lopez A, Islam R, Valero V, Hortobagyi GN. Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-Kit or PDGFR-beta: clinical activity and biological correlations. Ann Oncol. 2008;19:1713–1719. doi: 10.1093/annonc/mdn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modi S, Seidman AD, Dickler M, Moasser M, D’Andrea G, Moynahan ME, Menell J, Panageas KS, Tan LK, Norton L, Hudis CA. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2005;90:157–163. doi: 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]
- 46.Chew HK, Barlow WE, Albain K, Lew D, Gown A, Hayes DF, Gralow J, Hortobagyi GN, Livingston R. A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: Southwest Oncology Group Study 0338. Clin Breast Cancer. 2008;8:511–515. doi: 10.3816/CBC.2008.n.062. 62183P0858797V3N10.3816/CBC.2008.n.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yardley DA, Burris HA, III, Markus T, Spigel DR, Greco FA, Mainwaring M, Waterhouse DM, Webb CD, Hainsworth JD. Phase II trial of docetaxal plus imatinib mesylate in the treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2009;9:237–242. doi: 10.3816/CBC.2009.n.040. [DOI] [PubMed] [Google Scholar]