Abstract
Background
Alterations in the composition of gut microbiota - known as dysbiosis - has been proposed to contribute to the development of obesity, thereby supporting the potential interest of nutrients targeting the gut with beneficial effect for host adiposity. We test the ability of a specific concentrate of water-extractable high molecular weight arabinoxylans (AX) from wheat to modulate both the gut microbiota and lipid metabolism in high-fat (HF) diet-induced obese mice.
Methodology/Principal Findings
Mice were fed either a control diet (CT) or a HF diet, or a HF diet supplemented with AX (10% w/w) during 4 weeks. AX supplementation restored the number of bacteria that were decreased upon HF feeding, i.e. Bacteroides-Prevotella spp. and Roseburia spp. Importantly, AX treatment markedly increased caecal bifidobacteria content, in particular Bifidobacterium animalis lactis. This effect was accompanied by improvement of gut barrier function and by a lower circulating inflammatory marker. Interestingly, rumenic acid (C18:2 c9,t11) was increased in white adipose tissue due to AX treatment, suggesting the influence of gut bacterial metabolism on host tissue. In parallel, AX treatment decreased adipocyte size and HF diet-induced expression of genes mediating differentiation, fatty acid uptake, fatty acid oxidation and inflammation, and decreased a key lipogenic enzyme activity in the subcutaneous adipose tissue. Furthermore, AX treatment significantly decreased HF-induced adiposity, body weight gain, serum and hepatic cholesterol accumulation and insulin resistance. Correlation analysis reveals that Roseburia spp. and Bacteroides/Prevotella levels inversely correlate with these host metabolic parameters.
Conclusions/Significance
Supplementation of a concentrate of water-extractable high molecular weight AX in the diet counteracted HF-induced gut dysbiosis together with an improvement of obesity and lipid-lowering effects. We postulate that hypocholesterolemic, anti-inflammatory and anti-obesity effects are related to changes in gut microbiota. These data support a role for wheat AX as interesting nutrients with prebiotic properties related to obesity prevention.
Introduction
Recent studies demonstrated that diet-induced obesity was linked to changes in the gut microbial ecology, resulting in an increased capacity of the distal gut microbiota to promote host adiposity [1], [2]. We have previously shown that inulin-type fructans, non-digestible carbohydrates obtained from chicory root, restore the drop of bifidobacteria numbers occurring in the caeco-colon of high fat/carbohydrate-free diet-fed mice and thereby improves metabolic alterations associated with obesity, including dyslipidemia, impaired gut permeability, endotoxemia, inflammation and diabetes [3]–[6]. Inulin-type fructans are typically studied as they were the first compounds to respond to the prebiotic concept, defined as the selective stimulation of growth and/or activity of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host [7]. Other non-digestible/fermented carbohydrates, which are gradually fermented throughout the colon and which can be applied in different food matrices, may be valuable alternative substrates to test for their health effects related to their influence on gut microbiota composition.
AX are the most important non-digestible carbohydrates present in wheat. They represent 50% of dietary fibers and are mostly present in the bran and aleurone fractions [8]. AX are selectively degraded in the colon by intestinal bacteria possessing AX-degrading enzymes such as xylanases and arabinofuranosidases and represent a new class of potential prebiotics [9]–[11]. Existing in different forms, ranging from soluble to insoluble fibers and high-molecular weight to enzymatically modified short-chain fractions, the physiological effects of AX are largely unknown [12]. However, several studies indicate that they behave like fermentable fibers in the colon, with different fermentation profiles depending on the physicochemical properties and degree of polymerization, and with potential impact on lipid and glucose metabolism [8], [9], [13]–[16]. Molecular weight has been shown to play an important role, with highest activity for high-molecular weight AX [17]. Accordingly, the purpose of this study was to examine the effects of an AX concentrate, containing long-chain water-extractable AX, on the gut microbiota and lipid metabolism with focus on the expression of genes relevant to energy homeostasis and fat storage in a mice model of HF diet-induced obesity. We have chosen high molecular weight AX concentrate because previous in vitro studies have described an efficient fermentation of the AX, leading to stimulation of specific stimulation of certain bifidobacterial species and specific fermentation profiles (high propionate production) using Simulator of Human Intestinal Microbial Ecosystem (SHIME) [18], [19].
Materials and Methods
Ethics Statement
The animal experiments were approved by the local ethic committee; the agreement of the animal experiments performed in this study was given by the ethical committee for animal care of the Health Sector of the Université catholique de Louvain, under the supervision of prof. P. Gianello P et JP Dehoux under the specific number ULC/MD/2007/003. Housing conditions were as specified by the Belgian Law of 14 November, 1993 on the protection of laboratory animals (agreement n° LA 1230314).
Animals and diet intervention
Twenty four male C57bl6/J mice (9 weeks old at the beginning of the experiment, Charles River Laboratories, France) were housed in groups of 4 per cage in a controlled environment (12-hour daylight cycle) with free access to food and water. After one week of acclimatisation, the mice were divided into 3 groups (n = 8/group): a control group (CT), fed with a control diet (AO4, SAFE, Villemoison-sur-Orge, France), a group fed a HF diet and a group fed the same HF diet, supplemented with AX (90% HF (w/w)+10%AX; HF-AX group). The full composition of both the HF diet (D12492, Research Diets) and the A04 standard diet was given in the Table S1. The energy content of the HF diet consisted of fat for 60%, carbohydrate for 20% and protein for 20%. BioActor (Ghent, Belgium) supplied AX (Naxus®, batch 07NX-001) with a purity of 62%, degree of substitution of 0.7, and a varying degree of polymerization, with an average above 60; the composition of the batch used for the study was 67% non-starch polysaccharides (62% AX), 18% protein, 0.5% lipids, 3.8% ash. Food intake was recorded taking into account spillage twice a week during 4 weeks. The total caloric intake was obtained by multiplying total food intake (g) for 4 mice per cage (n = 2) by the caloric value of the diets, i.e. 3.10 Kcal/g, 5.24 Kcal/g and 4.83 Kcal/g for CT, HF and HF-AX, respectively. The caloric value of the HF-AX diet was calculated taking in to account that this diet was composed of 90% HF diet and 10% AX. After 4 weeks and a 6-hour period of fasting, mice were anesthetised (ketamine/xylazine i.p., 100 and 10 mg/kg, respectively) and blood samples were harvested for further analysis. Liver, three types of white adipose tissues (visceral, epididymal and subcutaneous), vastus lateralis muscle, jejunum and caecum were carefully dissected and weighted before immersion in liquid nitrogen before storage at −80°C.
Oral glucose tolerance test (OGTT)
After 3 weeks of treatment, an oral glucose tolerance test was performed on 6 h fasted-mice. Glucose was administered orally (3 g/kg body weight, 66% glucose solution) and blood glucose levels were determined using a glucose meter (Roche Diagnostics) on 3.5 µl of blood collected from the tip of the tail vein both before (−30 min and 0 min) and after glucose administration (15, 30, 60, 90, 120 min). Twenty microliters of blood were sampled 30 min before and 15 min after the glucose load to assess plasma insulin concentrations.
Microbial analysis of the caecal contents
At the end of the experiment, the total caecum content was collected and weighed before storage at −80°C. For analysis of the microbial content, metagenomic DNA was extracted from the caecal content of all mice, using the QIAamp DNA stool mini kit (Qiagen, Venlo, The Netherlands) according to the manufacturer's instructions. Denaturing Gradient Gel Electrophoresis (DGGE) on total bacteria, bifidobacteria, lactobacilli and Bacteroides-Prevotella spp. was performed to study the qualitative effect of the treatment on the structure and composition of the intestinal microbial community [20]. DGGE with a 45–60% denaturing gradient was used to separate the polymerase chain reaction (PCR) products obtained with a nested approach for the 16S rRNA genes of bifidobacteria (primers BIF164f-BIF662r), lactobacilli (SGLAB0159f-SGLAB0667r) and the Bacteroides-Prevotella cluster (FD1-RBacPre) [21]. The first PCR round was followed by a second amplification with primers 338F-GC and 518R. The latter primers were also used to amplify the 16S rDNA of all bacteria on total extracted DNA. The DGGE patterns obtained were subsequently analyzed using the Bionumerics software version 5.10 (Applied Maths, Sint-Martens-Latem, Belgium). In brief, the calculation of the similarities was based on the Pearson (product–moment) correlation coefficient. Clustering analysis was performed using the unweighted pair group method with arithmetic mean clustering algorithm (UPGMA) to calculate the dendrograms of each DGGE gel and a combination of all gels. The latter was performed on a created composite dataset. The composite dataset of the 3 DGGE patterns was also used to perform principal component analysis (PCA). PCA ordinations were calculated using the Pearson product-moment correlation coefficient. Within each character set, this coefficient subtracts each character from the average value, and divides it by the variance of the character set.
Quantitative PCR (Q-PCR) was performed to study the quantitative effect of the treatment on the composition of the intestinal microbial community. The Q-PCR for total bacteria (using primers PRBA338f and P518r) and specific for bifidobacteria were performed as reported by Possemiers et al. [22]. The Q-PCR for Roseburia spp. was performed as described before [23], using the primers Ros-F1 and Ros-R1, and the Power SYBR Green PCR Master kit (Applied Biosystems, Foster City). The Q-PCR for Bacteroides-Prevotella spp. was performed as described by Rinttilä et al. [24], using the Q-PCR Core kit for SYBR Green I (Eurogentec, Seraing, Belgium) and primers Bacter140f and Bacter140r. The Q-PCR for Bifidobacterium animalis lactis was performed as described by Ventura et al. [25] using the primers (Bflact2 and Bflact5). All Q-PCR were performed with an ABI PRISM SDS 7000 Sequence Detection System (Applied Biosystems, Nieuwerkerk a/d Ijssel, the Netherlands).
Blood parameters
Plasma insulin concentrations were determined using an ELISA kit (Mercodia, Uppsala, Sweden). The insulin resistance index was calculated by multiplying the area under the curve for glucose, and the area under the curve for insulin, calculated from −30 min until 15 min after glucose challenge [5], [26]. Concentrations of IL-6 and MCP-1 were determined in 15 µl of plasma using a multiplex immunoassay kit (Bio-Plex Cytokine Assay, Bio-Rad, Belgium) and measured using Luminex® technology (Bioplex®, Bio-Rad, Belgium). Adiponectin concentrations were determined using an ELISA kit designed to measure full-length mouse adiponectin levels in serum (Quantikine® Mouse adiponectin, R&DSystems).
Plasma triglycerides, cholesterol and non esterified fatty acid concentrations were measured using kits coupling enzymatic reaction and spectrophotometric detection of reaction end-products (Diasys Diagnostic and Systems, Holzheim, Germany). High density lipoprotein cholesterol (HDL-cholesterol) concentration was measured enzymatically after very low density lipoprotein (VLDL), chylomicrons and low density lipoprotein cholesterol (LDL-cholesterol) antibodies precipitation (Diasys Diagnostic and Systems, Holzheim, Germany). LDL was estimated by the Friedewald formula [27].
Lipid analysis in the liver
Triglycerides and cholesterol were measured in the liver tissue after extraction with chloroform–methanol as described by Neyrinck et al. [28].
Fatty acid synthase (FAS) activity
Homogenate of subcutaneous adipose tissue was performed in phosphate buffer (100 mg tissue/500 µl buffer). Cytosolic fractions were obtained after 2 successive centrifugations at 4°C (1000 g-15 min and 20000 g 30 min) The procedure described by Linn was used for the measurement of FAS activity in cytosolic fractions [29]. Protein was determined by the Bradford method.
Adipose tissue morphometry
Number of adipocytes per microscopic field (density) was estimated on paraffin-embedded hematoxylin-stained eosin- counterstained sections of subcutaneous adipose tissue using the image analyzer software (Motic Image Plus 2.0 ML), as previously described [30].
Fatty acid profile analysis in adipose tissue
Fatty acid analysis, including conjugated linoleic acid (c9, t11 CLA), in subcutaneous adipose tissue was performed according to the method described by Dewulf et al [30].
Expression of selected genes in tissues
Total RNA was isolated using the TriPure isolation reagent kit (Roche Diagnostics Belgium, Vilvoorde). cDNA was prepared by reverse transcription of 1 µg total RNA using the Kit Reverse transcription System (Promega, Leiden, The Netherlands). Real-time PCRs were performed with the StepOnePlus™ real time PCR system and software (Applied Biosystems, Den Ijssel, The Netherlands) using Mesa Fast qPCR™ (Eurogentec, Seraing, Belgium) for detection according to the manufacturer's instructions. RPL19 RNA was chosen as housekeeping gene. Primer sequences for the targeted mouse genes are available on request (audrey.neyrinck@uclouvain.be). All samples were run in duplicate in a single 96-well reaction plate and data were analysed according to the 2-ΔCT method [31]. The identity and purity of the amplified product was checked through analysis of the melting curve carried out at the end of amplification.
Statistical analysis
Results are presented as mean ± SEM. Statistical analysis was performed by ANOVA followed by post hoc Tuckey's multiple comparison test (GraphPad Software, San Diego, CA, USA); p<0.05 was considered as statistically significant. Correlations between parameters were assessed by Pearson's correlation test; correlations were considered significant as follows: *p<0.01, **p<0.001, ***p<0.0001 with the absolute value of Pearson r>0.5.
Results
Supplementation with arabinoxylan modified the gut microbiota composition
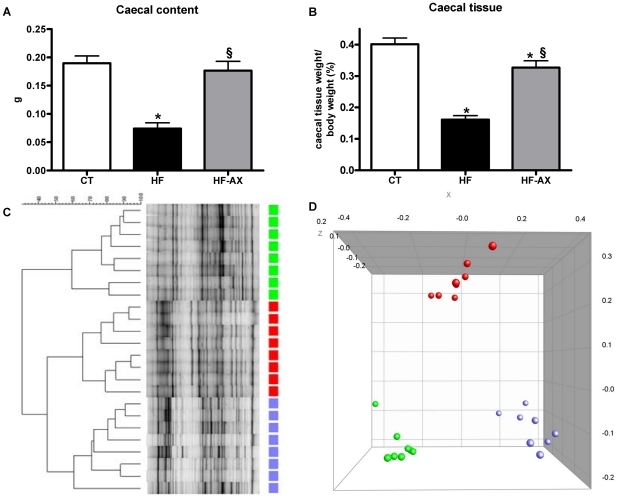
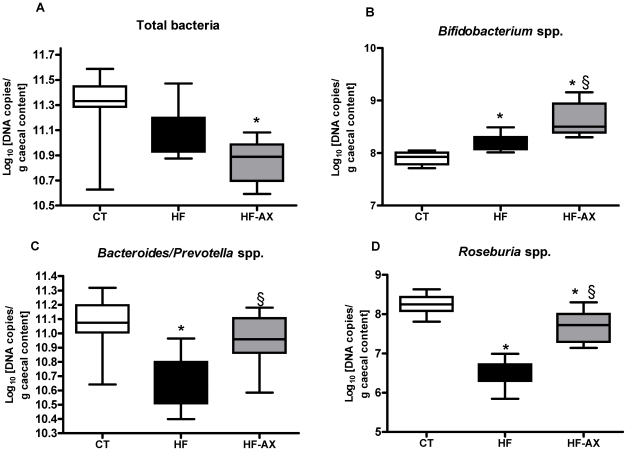
HF feeding decreased both the caecal content weight and tissue weight as compared to the control condition (Figures 1A and 1B). Clustering of the DGGE fingerprints for total bacteria (Figure 1C) indicated a separate cluster for the CT and HF groups. PCA analysis of combined DGGE fingerprints of total bacteria, bifidobacteria, lactobacilli and Bacteroides-Prevotella spp. shows distinct clusters between the HF and CT groups (Figure 1D). This was further confirmed following Q-PCR analyses of different bacterial groups (Figure 2 and Figure S1). HF diet induced a drop in Roseburia spp. and Bacteroides-Prevotella spp. numbers. Conversely, the number of bifidobacteria per gram of caecum content was significantly higher in the HF group, as compared to control mice (Figure 2B).
Figure 1. Caecum weight, DGGE fingerprints and PCA analysis in the caecal content.
Caecal content (A) and caecal tissue (B) weight. Denaturing gradient gel electrophoresis (DGGE) fingerprint patterns of the caecal microbial community; the DGGE profiles were constructed using primers for total bacteria (C). Principal Coordinate Analysis (PCA) was used to explore the similarity within a composite data set consisting of DGGE fingerprints of total bacteria, bifidobacteria, lactobacilli and the Bacteroides-Prevotella spp. cluster (D). Mice were fed a standard (CT, green symbols), a high fat diet (HF, red symbols) or a high fat diet supplemented with 10% arabinoxylan (HF-AX, blue symbols) for 4 weeks. *p<0.05 versus CT and §p<0.05 versus HF (ANOVA).
Figure 2. Bacterial quantification per gram of caecal content.
Caecal bacterial content of total bacteria (A), Bifidobacterium spp. (B), Bacteroides-Prevotella spp. (C), Roseburia spp. (D). Bacterial quantities are expressed as Log10 (bacterial cells/ g caecal content wet weight). Mice were fed a standard (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks. *p<0.05 versus CT and §p<0.05 versus HF (ANOVA).
AX supplementation induced caecal enlargement (Figures 1A and 1B) and a profound shift in the microbial community in comparison with the HF group, as shown by the distinct clustering in the DGGE profile and PCA analysis (Figures 1C and 1D). AX supplementation restored the HF diet-induced microbial community changes as shown by the significant increase in Bacteroides–Prevotella spp. and Roseburia spp. (Figures 2C and 2D). Moreover, AX induced a specific increase in bifidobacteria (Figure 2B), in particular bifidobacterium animalis ssp lactis (expressed as Log10 [DNA copies/g caecal content]: 5,67±0,07, 6,03±0,07*, and 6,37±0,06*§ for CT, HF and HF-AX, respectively; ANOVA, * p<0.05 versus CT and §p<0.05 versus HF).
Supplementation with arabinoxylan modified the expression of markers related to gut barrier function
The mRNA levels of both ZO-1 and occludin, which are tight-junction proteins, were measured in the jejunum, as well as the mRNA level of proglucagon, which is the precursor of the intestinotrophic peptide GLP-2, known to reduce gut permeability [4]. HF feeding did not modify the expression of these genes (Figure S2). Interestingly, AX supplementation significantly increased the mRNA levels for both tight-junction proteins and proglucagon expression, as compared to the HF and/or CT groups.
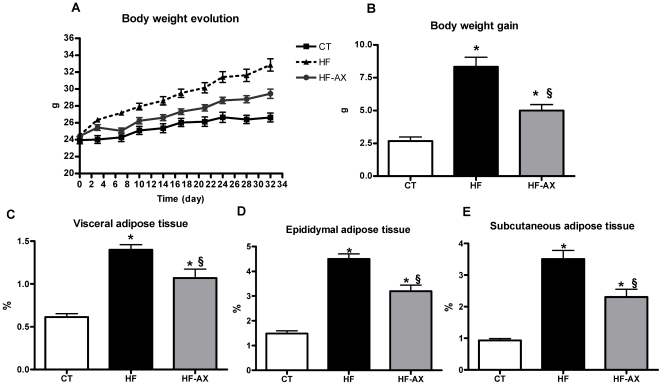
Supplementation with arabinoxylan decreased body weight gain and fat mass development
The mice fed with HF-AX did not gain weight as rapidly as HF-fed mice (Fig. 3A). In fact, AX supplementation decreased body weight gain by about 40% as compared to HF (fig. 3B). Moreover, AX treatment induced a lower fat mass development as shown by the weight of epididymal, subcutaneous and visceral adipose tissues (Figures 3C, 3D and 3E). This effect could not be explained by changes in energy intake since the total calorie intake (for 4 mice, n = 2) was not different between the HF and HF-AX groups (1579±30 kcal and 1539±15 kcal, respectively).
Figure 3. Body weight and fat mass.
Body weight evolution (A), body weight gain (B), visceral (C), epididymal (D), and subcutaneous (E) adipose tissue weight (% versus body weight) of mice fed a standard (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks. *p<0.05 versus CT and §p<0.05 versus HF (ANOVA).
Supplementation with arabinoxylan improved insulinoresistance index and cholesterol levels
HF feeding induced fasting hyperglycemia and hyperinsulinemia, and increased the insulin resistance index upon OGTT, as compared to mice fed normal chow diet (Table 1). Surprisingly, adiponectin level was not modified after HF treatment. HF diet feeding induced hepatic triglyceride accumulation (by about 28%) and increased serum total, LDL- and HDL-cholesterol levels without affecting serum triglycerides and non-esterified fatty acids (Table 1). The HF-induced hypercholesterolemia was associated to a higher hepatic content of free cholesterol.
Table 1. Blood and hepatic parameters.
| CT | HF | HF-AX | |
| Serum | |||
| - fasting glycemia (mM) | 7.49±0.28 | 9.58±0.41* | 8.44±0.41 |
| - fasting insulinemia (mM) | 83.9±12.6 | 226.3±32.2* | 156.1±16.6* |
| - insulin resistance index | 30030±963 | 113500±19440* | 71990±7423*§ |
| - adiponectin (pg/ml) | 9.36±0.40 | 9.38±0.46 | 7.76±0.55 *§ |
| - triglycerides (mM) | 1.47±0.11 | 1.71±0.13 | 1.54±0.11 |
| - non esterified fatty acids (mM) | 0.27±0.03 | 0.31±0.03 | 0.29±0.04 |
| - total cholesterol (mM) | 1.75±0.06 | 3.00±0.03 * | 2.41±0.06 *§ |
| - LDL-cholesterol (mM) | 0.31±0.05 | 1.22±0.08 * | 0.90±0.06 *§ |
| - HDL-cholesterol (mM) | 0.78±0.02 | 1.00±0.03 * | 0.82±0.02 § |
| Liver lipid content, nmol/mg protein | |||
| - triglycerides | 60.9±3.0 | 77.8±5.3 * | 66.1±8.0 |
| - total cholesterol | 41.1±4.1 | 74.2±11.0 * | 73.2±10.5 * |
| - free cholesterol | 27.0±2.2 | 40.3±4.6 * | 27.8±1.3 § |
| - esterified cholesterol | 14.1±3.2 | 33.9±6.6 | 45.5±9.3 * |
Mice were fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks * p<0.05 versus CT and §p<0.05 versus HF (ANOVA). LDL, low density liprotein; HDL, high density lipoprotein.
AX did not change significantly fasting glycemia or insulinemia. Interestingly, AX feeding improved the insulin resistance index as compared to the HF group. However, we observed a lower concentration of adiponectin after AX supplementation (Table 1). AX supplementation furthermore decreased hypercholesterolemia (both LDL- and HDL-cholesterol) and also led to a shift from hepatic free cholesterol towards esterified cholesterol, without changing total cholesterol or triglyceride level in the tissue (Table 1).
Supplementation with arabinoxylan increased the proportion of rumenic acid in the subcutaneous adipose tissue reflecting changes in gut bacterial metabolism
The fatty acid profile in adipose tissue is influenced by that of the dietary lipids [32], [33]. In addition, specific fatty acids, such as vaccenic acid (C18:1 t11) and conjugated linoleic acids, the major being the rumenic acid (C18:2 c9,t11), may be formed upon bacterial metabolism (biohydrogenation) of linoleic acid in the intestine [34]. Therefore, we analyzed the fatty acid patterns in subcutaneous adipose tissue of mice (Table 2). HF feeding induced drastic changes in the fatty acid composition of the adipose tissue with a higher proportion of monounsaturated fatty acids (MUFA), at the expence of both polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA). Interestingly, HF feeding induced a significant increase in both vaccenic acid and rumenic acid. Supplementation with AX significantly decreased the proportion of palmitic acid (C16:0) whereas it further increased the rumenic acid content as compared to the HF treatment.
Table 2. Fatty acid pattern of the subcutaneous adipose tissue from mice fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks.
| CT | HF | HF-AX | |||||||
| SFA | 28.197 | ± | 0.614 | 25.998 | ± | 0.180* | 25.010 | ± | 0.322*§ |
| C6:0 | 0.017 | ± | 0.002 | 0.007 | ± | 0.001* | 0.006 | ± | 0.001* |
| C8:0 | 0.009 | ± | 0.001 | 0.004 | ± | 0.001* | 0.004 | ± | 0.001* |
| C10:0 | 0.045 | ± | 0.003 | 0.039 | ± | 0.003 | 0.040 | ± | 0.002 |
| C12:0 | 0.119 | ± | 0.009 | 0.080 | ± | 0.006* | 0.082 | ± | 0.004* |
| C13:0 | 0.014 | ± | 0.001 | 0.010 | ± | 0.001* | 0.011 | ± | 0.001 |
| C14:0 | 1.563 | ± | 0.057 | 1.010 | ± | 0.017* | 0.997 | ± | 0.024* |
| C15:0 iso | 0.013 | ± | 0.001 | 0.003 | ± | 0.000* | 0.003 | ± | 0.001* |
| C15:0 anteiso | 0.013 | ± | 0.001 | 0.003 | ± | 0.001* | 0.004 | ± | 0.001* |
| C15:0 | 0.169 | ± | 0.005 | 0.106 | ± | 0.001* | 0.107 | ± | 0.003* |
| C16:0 iso | 0.133 | ± | 0.003 | 0.067 | ± | 0.003* | 0.069 | ± | 0.003* |
| C16:0 | 22.422 | ± | 0.459 | 19.844 | ± | 0.138* | 18.631 | ± | 0.338*§ |
| C17:0 iso | 0.150 | ± | 0.003 | 0.143 | ± | 0.003 | 0.138 | ± | 0.003* |
| C17:0 anteiso | 0.683 | ± | 0.015 | 0.737 | ± | 0.014 | 0.798 | ± | 0.032* |
| C17:0 | 0.181 | ± | 0.005 | 0.250 | ± | 0.004* | 0.248 | ± | 0.004* |
| C18:0 | 2.525 | ± | 0.136 | 3.631 | ± | 0.088* | 3.791 | ± | 0.177* |
| C20:0 | 0.140 | ± | 0.005 | 0.065 | ± | 0.004* | 0.080 | ± | 0.007* |
| MUFA | 44.442 | ± | 0.260 | 48.981 | ± | 0.133* | 49.778 | ± | 0.438* |
| C14:1 C9 | 0.153 | ± | 0.008 | 0.062 | ± | 0.010* | 0.052 | ± | 0.003* |
| C16:1C9 | 8.631 | ± | 0.207 | 4.727 | ± | 0.160* | 4.407 | ± | 0.229* |
| C18:1T9 | 0.040 | ± | 0.003 | 0.224 | ± | 0.008* | 0.240 | ± | 0.007* |
| C18:1T11 | 0.055 | ± | 0.004 | 0.192 | ± | 0.007* | 0.194 | ± | 0.005* |
| C18:1C9 | 32.341 | ± | 0.214 | 41.253 | ± | 0.143* | 42.283 | ± | 0.593* |
| C18:1C11 | 3.222 | ± | 0.049 | 2.520 | ± | 0.019* | 2.602 | ± | 0.023*§ |
| PUFA | 27.361 | ± | 0.583 | 25.021 | ± | 0.164* | 25.211 | ± | 0.375* |
| C18:2C9C12 | 25.698 | ± | 0.544 | 23.115 | ± | 0.146* | 23.436 | ± | 0.294* |
| C18:3C9C12C15 | 0.859 | ± | 0.032 | 1.081 | ± | 0.026* | 0.991 | ± | 0.062 |
| C18:2C9T11 | 0.090 | ± | 0.005 | 0.171 | ± | 0.002* | 0.181 | ± | 0.003*§ |
| C20:3C11C14C17 | 0.013 | ± | 0.001 | 0.061 | ± | 0.001* | 0.060 | ± | 0.002* |
| C20:4C5C8C11C14 | 0.323 | ± | 0.011 | 0.362 | ± | 0.007 | 0.332 | ± | 0.017 |
| C20:5C5C8C11C14C17 | 0.041 | ± | 0.005 | 0.020 | ± | 0.001* | 0.013 | ± | 0.003* |
| C22:5C7C10C13C16C19 | 0.063 | ± | 0.002 | 0.065 | ± | 0.002 | 0.060 | ± | 0.003 |
| C22:6C4C7C10C13C16C19 | 0.264 | ± | 0.012 | 0.141 | ± | 0.003* | 0.132 | ± | 0.008* |
Values are means ± SEM (g/100 g of identified fatty acid methyl esters). SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
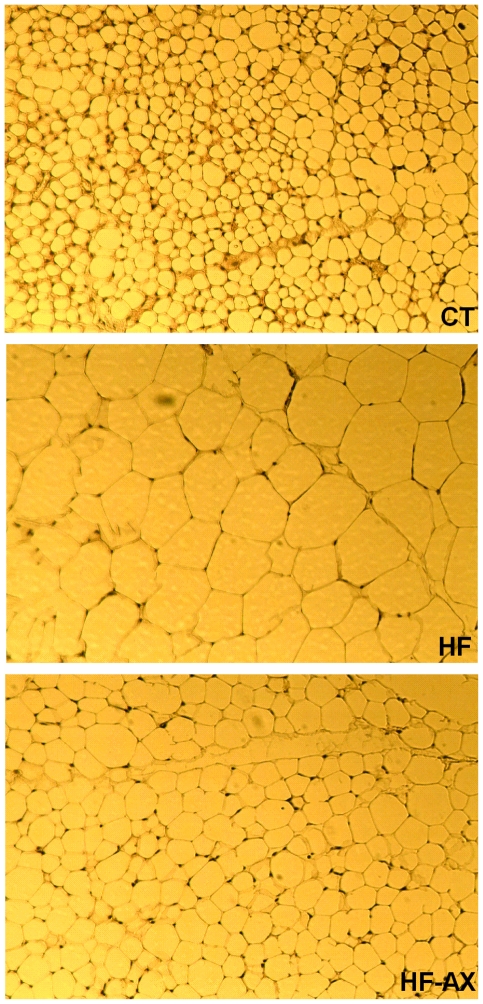
Supplementation with arabinoxylan reduced adipocyte size and decreased HF-induced gene expression in the subcutaneous adipose tissue
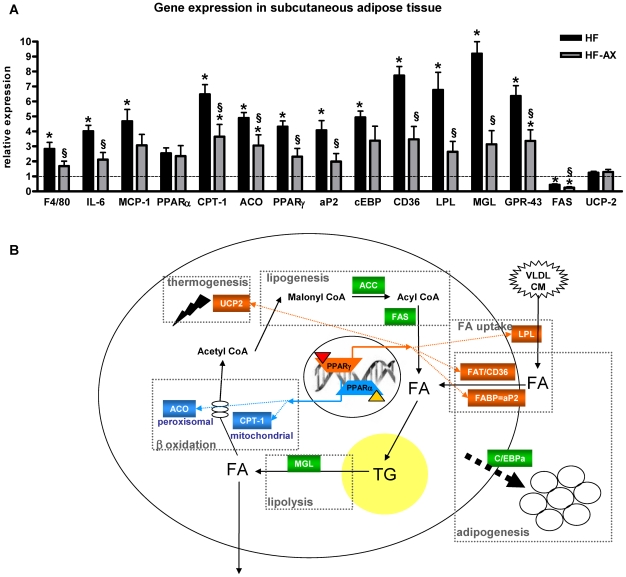
Histological analysis revealed that the adipocyte size in subcutaneous adipose tissues were increased in the HF-fed mice versus controls, whereas the AX treatment normalized this parameter (Figure 4; adipocyte number per field: 339±49, 71±13* and 177±21*§ for CT, HF and HF-AX, respectively; ANOVA, * p<0.05 versus CT and §p<0.05 versus HF). HF feeding increased the expression of genes controlling inflammation (F4/80, IL-6, MCP-1), PPAR-α dependent-fatty acid oxidation (CPT-1, ACO), PPARγ-dependent differentiation and/or fatty acid uptake (C/EBPα, FAT/CD36, aP2, LPL), and lipolysis (MGL) (Figure 5). Furthermore, the mRNA content of GPR43 -a receptor activated by short-chain fatty acids and implicated in the regulation of lipolysis and adipocyte differentiation- was significantly increased upon HF feeding. Interestingly, the AX treatment hugely decreased the expression of the most of these genes in the subcutaneous tissue. Of particular interest, AX treatment decreased the serum concentrations of 2 inflammatory markers that were downregulated in the adipose tissue through AX treatment, namely IL6 (53.9±11.5 pg/ml and 21.9±7.7 pg/ml for HF and HF-AX groups, respectively; t test p<0.05) and MCP-1 (32.1±5.3 pg/ml and 12.3±2.4 pg/ml for HF and HF-AX groups, respectively; t test p<0.05). In addition, AX supplementation inhibited the expression of fatty acid synthase (FAS), a lipogenic enzyme that was already downregulated by the HF diet. In accordance with its expression, we confirmed that FAS activity was downregulated through AX supplementation in adipose tissue since its activity was significantly lower as compared to HF group (32.5±7.1, 25.5±7.5 and 7.1±1.3*§ for CT, HF and HF-AX respectively; ANOVA: *p<0.05 versus CT and §p<0.05 versus HF). By contrast, it did not affect the expression of the uncoupling protein UCP-2.
Figure 4. Histological pictures of subcutaneous adipose tissue.
Mice were fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks.
Figure 5. mRNA levels of key factors and metabolic network in the subcutaneous adipose tissue.
Expression of genes involved in subcutaneous adipose tissue metabolism (A). Mice were fed a standard (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks. Values are expressed relative to CT group (set at 1). *p<0.05 versus CT and §p<0.05 versus HF (ANOVA). Genes that regulate metabolic processes in white adipose tissue (B); some of them are dependent on PPARα (blue) or PPARγ (orange) activation by an endogenous ligand. PPARγ, peroxisome proliferator-activated receptor γ; aP2, adipocyte fatty acid binding protein; C/EBPα, CCAAT enhancer binding protein α; GPR43, G protein-coupled receptor 43; LPL, lipoprotein lipase; CD-36, cluster of differenciation 36; FAS, Fatty acid synthase; ACC, AcylCoa carboxylase; PPARα, peroxisome proliferator-activated receptor-alpha ; CPT-1, carnitine palmitoyl transferase-1 ; ACO, AcylCoA oxydase; MGL, monoacylglycerol lipase; UCP-2, uncoupling protein-2; VLDL, very low density lipoprotein; CM, chylomicron; FA, fatty acids; TG, triglycerides.
Given that AX treatment decreased genes involved in fatty acid uptake, we decided to explore some markers of lipid metabolism in the liver and the muscle (Table S2). In the vastus lateralis muscle, there were no changes in mRNA expression of genes involved in fatty acid oxidation (ACO, PPARα, PPARδ, CPT1) between the groups. In the liver, HF feeding induced the expression of the genes encoding sterol regulatory element-binding protein-1c (SREBP-1c) and its downstream regulated protein FAS. AX supplementation did not modify significantly lipogenic gene expression but it lowered HF-induced PPARα expression with no consequences on genes involved in fatty acid oxidation (CPT1 or ACO). Of note, there was no change in the expression of genes involved in cholesterol metabolism, whatever the dietary treatments.
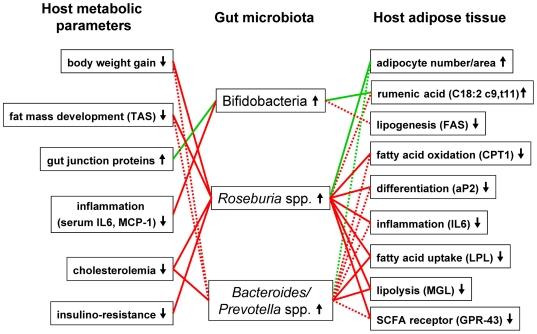
Improved metabolism following arabinoxylan supplementation was correlated with the increase in bifidobacteria or the restoration of Roseburia spp. and Bacteroides/Prevotella spp
To determine whether modifications of the gut microbiota in mice fed AX were associated with an improvement in obesity and lipid metabolism, a correlation analysis was performed between bacteria and host metabolic parameters that were significantly affected through AX supplementation (Figure 6 and Table S3). The analysis revealed negative correlations (p<0.001) between Roseburia spp, and fat mass development, body weight gain, cholesterolemia, insulinoresistance index, and expression of several genes that mediate differentiation and/or fatty acid uptake (PPARγ, aP2, FAT/CD36, LPL, FIAF), fatty acid oxidation (CPT-1, ACO), short-chain fatty acid response (GPR43), and inflammation (IL6, F4/80) in the subcutaneous adipose tissue. Some of these metabolic parameters were also inversely correlated with the number of Bacteroides/Prevotella spp. (p<0.01) whereas none of these markers was significantly correlated with the number of total bifidobacteria. However, bifidobacteria levels were positively correlated with rumenic acid in the adipose tissue and with the mRNA levels of the tight junction proteins (ZO-1 and occludine) in the gut. The analysis revealed other interesting negative correlation with bifidobacteria such as the FAS activity/expression in the adipose tissue and circulating inflammatory markers (IL-6 and MCP-1).
Figure 6. Interrelationship between gut microbiota composition and host metabolic parameters significantly modified by arabinoxylan supplementation.
Green connections indicate a positive correlation (Pearson r>0.5), while red connections show correlations that are inverse (Pearson r<0.5). Solid lines represent significance with p<0.001 and shared lines represent significance with p<0.01. aP2, adipocyte fatty acid binding protein; GPR43, G protein-coupled receptor 43; IL6, interleukin 6; LPL, lipoprotein lipase; FAS, Fatty acid synthase; CPT-1, carnitine palmitoyl transferase-1 ; MCP-1, monocyte chemoattractant protein-1; MGL, monoacylglycerol lipase; SCFA, short chain fatty acid.
Discussion
Over the past 5 years, animal and human studies have revealed a remarkable microbial influence on host metabolism, energy utilization and storage, and metabolic diseases [1], [35]–[37]. A shift in the composition the murine gut microbiota, such as a decrease in Bacteroidetes or an increase in Firmicutes, was reported to be induced by dietary interventions in favour of a HF diet [3], [28], [38]. The microbial-related response of mice towards a HF diet, in terms of fat mass development and metabolic diseases, is dependent on the diet composition [39]. Here, we show that feeding mice with a HF diet decreases the dominant members of the mouse intestinal microbiota - Bacteroides-Prevotella ssp. from the Bacteroidetes phylum together with the specific populations of clostridial cluster XIVa, i.e. Roseburia spp from the Firmicutes phylum. However, it slightly increased the number of total bifidobacteria. The composition of the HF diet as compared to the standard diet -in term of dietary fiber (concentration and nature)- is probably responsible for the important changes in the parameters related to the gut fermentation (caecal content weight and microbial composition). Indeed, according to the manufacturer, the control diet contains 84% of cereal products that brings 4% of cellulose as dietary fibre without taking into account other non digestible/fermentable carbohydrates such as arabinoxylan, resistant starch, fructooligosaccharides present in cereal in particular in bran fractions [8]. In contrast, the dietary fibre of the HF diet is originating from cellulose BW200 (6.5%) which is poorly fermentable. Moreover, the HF diet has only maltodextrin and sucrose as digestible carbohydrate sources whereas the control diet has native starch coming from cereals, bran and remilling; a part of this starch being resistant to digestion and consequently entering in the caeco-colon as fermentable fraction (resistant starch). Interestingly, AX added to the HF diet led to an increase of bifidobacteria and in particular of Bifidobacterium animalis subsp. Lactis. AX also restored to control level, the bacterial populations in the caecal content which were decreased upon HF feeding, namely the gram negative Bacteroides-Prevotella spp. and the gram positive bacteria Roseburia spp.
In previous studies, we demonstrated that feeding rats with the inulin-type prebiotic (oligofructose) protected against liver triglyceride accumulation induced by fructose and that the lower lipogenic capacity of the liver could be the key event in this protection; indeed FAS activity remained significantly lower in oligofructose-fed rats [40]–[42]. More recently, we found that a selective change in gut microbiota composition through inulin-type prebiotic treatment improves gut barrier functions through a GLP-2-dependent mechanism during obesity and diabetes [4]. As shown for oligofructose, the bifidogenic effect of AX demonstrated here, were accompanied by a decrease in FAS activity in the adipose tissue. Moreover, we observed an increase in both tight junction proteins and proglucagon expression after AX supplementation, suggesting an increase in gut barrier-related functions as a physiological consequence of the changes in the composition of gut microbiota. Of particular interest, we observed lower levels of inflammatory markers in the serum due to AX supplementation that could be the result of improvement of gut barrier functions as suggested by Cani et al. [3], [5]. Interestingly, those metabolic changes were correlated with the number of bifidobacteria in the caecal content confirming prebiotic properties of AX.
Here, we demonstrate for the first time that a prebiotic approach is also able to change the occurrence of PUFA metabolites in the white adipose tissue. Such an effect was previously obtained with a probiotic strategy, leading to modifications of fatty acid pattern in the adipose tissue (including higher concentrations of the n−3 fatty acids eicosapentaenoic acid and docosahexaenoic acid) [43]. AX increases the amount of a linoleic acid metabolite, namely rumenic acid which belong to the conjugated linoleic acid (CLA) family. CLAs comprise a set of positional (eg, 9,11; 10,12; 11,13) and geometric (cis or trans) isomers of linoleic acid with conjugated double bonds. CLA isomers have been shown to exert a variety of biological activities and some of them have been shown to exert anti-obesity effects [44]. Commensal bifidobacteria from the mammalian gut have been shown to generate CLA, predominantly rumenic acid -the c9,t11 isomer from free linoleic acid- whereas Roseburia spp. formed either vaccenic acid (18:1 t11) or a 10-hydroxy-18:1, two precursors of rumenic acid [34], [45], [46]. We observed a higher proportion of rumenic acid upon HF feeding alone, probably due to a higher concentration of its substrate (linoleic acid) in the HF diet versus control diet. AX supplementation further increased the rumenic acid proportion in host adipose tissue, suggesting a specific linoleic acid metabolism by CLA-producing bacteria such as bifidobacteria and indirectly Roseburia spp, which are both promoted upon AX feeding.
To date, only a few studies have investigated whether the consumption of wheat-derived AX could have potential beneficial effects for health. We show here that, in parallel to changes in gut bacterial population, the specific concentrate of water-extractable high molecular weight AX significantly decreased body weight gain and fat mass development in a model of HF diet-induced obesity. In addition, AX supplementation counteracted both the HF-induced hypercholesterolemia (total, LDL- and HDL-cholesterol) and the higher content of free cholesterol in the liver upon HF feeding. The hypocholesterolemic effect of AX has previously been observed in vivo in rats, an effect attributed to a decreased dietary cholesterol absorption and an increased fecal excretion of cholesterol and bile acids, leading -as a metabolic signature- to an increased expression of HMG-CoA reductase [13]. We may not exclude that the higher production of propionate upon AX fermentation could be implicated in the decrease in hepatic cholesterol synthesis and hypocholesterolemic effect [19]. However, the fact that we were unable to see any changes in hepatic HMG-CoA reductase expression is not in favor of this mechanism in our study.
The histological analysis of the subcutaneous adipose tissue showed that the adipocytes were larger upon the HF treatment and became smaller when the HF diet was combined with AX. PPARs are vital for adipogenesis (adipocyte differentiation), fatty acid uptake, lipogenesis and fatty acid oxidation (see fig. 5 and [47], [48]). PPARγ is abundantly expressed in adipose tissue, where it is a key regulator of adipocyte differentiation, whereas PPARα governs fatty acid oxidation [47]. Importantly, antagonism of PPARγ using a synthetic ligand suppresses the increased adiposity observed in HF induced obesity [47]. Recently, we have shown that inulin-type fructans with prebiotic properties counteract PPARγ-related adipogenesis in the white adipose tissue of HF fed mice [30]. Here, we demonstrate that the supplementation of the specific AX concentrate is also able to decrease the expression of most of the HF-induced metabolic genes in subcutaneous adipose tissue, in particular genes depending on PPARs-activation. Indeed, AX down-regulated genes involved in adipocyte differentiation, fatty acid uptake, fatty acid oxidation, lipolysis while it decreased fatty acid synthesis (expression and activity) that was already downregulated upon HF-feeding. Although we can attribute the antiobesity effect of AX (lower fat mass development) to a lower fatty acid synthesis (FAS expression and activity) or fatty acid uptake in white adipose tissue (downregulation of lipoprotein lipase, fatty acid translocase/CD36 and fatty acid binding protein (aP2)), we may not exclude an effect of AX on lipid absorption through either its intrinsic capacity of fat binding or modulation of pancreatic lipase activity [49]. Chitosan –coming from chitin- has the capacity to bind fatty acids and to increase the lipid content in the caecum [50], [51]. This mechanism seems also to be involved in the improvement of HF-induced metabolic alterations by AX. Indeed, the proportion of lipid accumulated in the caecum versus the lipid ingested during a 12 h-feeding period was increased due to AX or chitosan supplementation in the HF diet (figure S3A). Furthermore, in this experiment, AX or chitosan supplementation increased both the fatty acid content and the cholesterol content in the caecum of mice fed a HF diet (figure S3B and S3C). Although the host adiposity changes and hypocholesterolemic effect of AX could be the result of its direct fat binding capacity leading to fat leakage in faecal matter, we may not exclude other potential mechanisms. The study of VO2 and VCO2 by indirect calorimetry at rest and during physical activity could constitute one interesting perspective to investigate further. In fact, we postulate that the modulation of gut microbiota induced by AX supplementation observed in the present study, was involved in its anti-obesity action as well as in its cholesterol-lowering effects. This hypothesis is supported by the fact that the number of bacteria from the clostridial cluster XIVa, such as Roseburia spp. -and Bacteroides-Prevotella spp. to a lesser extent- showed inverse correlations with important markers of obesity and host lipid metabolism (fat mass development, body weight gain, cholesterolemia, and expression of several genes mediating differentiation and/or fatty acid uptake, fatty acid oxidation, short chain fatty acid response and inflammation in the subcutaneous adipose tissue). Recently, Martinez and coworkers [52] provided evidence that modulation of the gut microbiota-host metabolic interrelationship by dietary intervention has the potential to improve mammalian cholesterol homeostasis, which has relevance for cardiovascular health. Several mechanisms could explain how the gut microbiota may affect host lipid metabolism since gut bacteria are able 1) to regulate chylomicron formation and lipid uptake by affecting gut transit time and bile salt metabolism (modulation of the enterohepatic circulation of bile acids) [1], [53]; 2) to ferment complex polysaccharides into short chain fatty acids that may act either as lipogenic substrates in the liver or an inhibitor of cholesterologenesis and lipogenesis from acetate [54]; 3) to suppress the expression of fasting-induced adipose factor (FIAF) in the intestinal mucosa, a factor able to increase lipoprotein lipase (LPL) dependent triglyceride storage in adipose tissue and to reduce serum triglyceride level [1], [55], [56]. FIAF is indeed an important regulator of lipid metabolism and has been shown to increase total cholesterol and high-density lipoprotein (HDL) cholesterol levels when over-expressed in transgenic mice [57]. This last mechanism could be involved in the hypocholesterolemic effect of AX since FIAF expression in white adipose tissue was decreased after AX supplementation in the HF diet.
Having a significant amount of excess body fat is a major problem that increases the risk of developing insulin resistance and type 2 diabetes. We have shown that the specific AX concentrate decreased HF-induced hyperglycemia and hyperinsulinemia, whereas its supplementation improved glucose tolerance after a glucose load (data not shown) but these effects were not significant. However, it is clear that the insulin resistance index was improved due to AX treatment as compared to the HF group, suggesting that the anti-obesity effect of AX was accompanied by a beneficial effect on insulin sensitivity. This result is in accordance with a clinical study demonstrating that consumption of the same AX concentrate for 6 weeks improved postprandial glycemia and insulinemia in overweight subjects with impaired glucose tolerance [16].
In conclusion, this study has shown in a 4-week study in mice, that supplementation of a concentrate of water-extractable high molecular weight AX in the diet counteracted HF-induced gut dysbiosis with a major effect on bacteria from clostridial cluster XIVa (restoration of Roseburia spp.), gram negative Bacteroides-Prevotella spp. and bifidobacteria (in particular, higher caecal content in Bifidobacterium animalis subsp. Lactis). This prebiotic effect was accompanied by a lower circulating inflammatory markers and by an increase in both tight junction markers and rumenic acid (18:2 c9,t11) in white adipose tissue. This last phenomenon reflects the influence of gut bacterial metabolism on host tissue. The gut microbiota changes due to the AX treatment were accompanied by an improvement of obesity and lipid-lowering effects together with an improvement of insulin resistance markers in HF diet-induced obesity. In addition, AX supplementation was able to decrease a number of PPARs- dependent genes within white adipose tissue, without inducing fat accumulation in the liver. Those metabolic effects may be related to the intrinsic fat binding capacity of AX. However, since it is known that gut bacteria could influence host lipid metabolism, we postulate that both hypocholesterolemic and anti-obesity effects conferred by AX are related, at least in part, to changes in gut microbiota.
Even if a direct extrapolation of the present study to human is still questionable due to differences in digestive tract structure and in gut microbiota, our results suggest that water extractable high molecular weight AX that is predominantly found in wheat endosperm can confer positive health impacts through gut microbiota modulation and may be a natural alternative in the prevention of obesity and cardiovascular diseases.
Supporting Information
Bacterial quantification in the caecum. Caecal bacterial content of total bacteria (A), Bifidobacterium spp. (B), Bacteroides-Prevotella spp. (C) and Roseburia spp. (D). Bacterial quantities are expressed as Log10 (bacterial cells/ total caecal content wet weight). Mice were fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks.*p<0.05 versus CT and §p<0.05 versus HF (ANOVA).
(TIF)
mRNA levels of key markers in jejunum related to gut barrier function. Mice were fed a standard (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks. Values are expressed relative to CT group (set at 1). *p<0.05 versus CT and §p<0.05 versus HF (ANOVA). ZO-1, zonula occludens-1.
(TIF)
Analysis of fat binding capacity of arabinoxylan (AX) in vivo. Eighteen male C57bl6/J mice (10 week old) were housed in groups of 3 per cage in a controlled environment with free access to HF diet. After 3 days for acclimatisation, the mice were divided into 3 groups (n = 6/group): a group fed with a HF diet, a group fed the same HF diet supplemented with 10% AX (HF-AX) and a group fed with the HF diet supplemented with 10% chitosan (KiOnutrime-Cs™ from KitoZyme sa, Belgium, HF-Cs). Food intake was recorded and mice were killed 12 h after access to the diets. Lipid content, fatty acids and cholesterol concentration in the caecal content were determined as previously described [50]. Proportion of caecal lipids versus ingested lipids (A), caecal pool of fatty acids (B) and caecal pool of cholesterol (C); *p<0.05 versus HF (ANOVA).
(TIFF)
The full composition of the control diet and the high fat diet.
(DOC)
Gene expression in the liver and in the muscle of mice fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks.
(DOC)
Analysis of correlation between the number of bacteria in the caecal content (expressed as Log10 (bacterial cells/ total caecal content wet weight) with metabolic parameters that were significantly affected by AX treatment.
(DOC)
Acknowledgments
We would like to thank Christine Turu and Eric Mignolet from the Institut des Sciences de la Vie (UCL) for their excellent technical assistance in the fatty acid profile analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work supported by a grant from the Walloon Region (General Directory of Agriculture, convention D31-1107, http://agriculture.wallonie.be). PDC is a Research Associate from the Fonds de la Recherche Scientifique (FRS-FNRS) Belgium (http://www.frs-fnrs.be/). SP and TVDW are Postdoctoral Researchers from the Research Foundation - Flanders (Fonds voor Wetenschappelijk Onderzoek [FWO] - Vlaanderen, http://www.fwo.be/). CD benefits from a Danone Institute grant (http://www.danoneinstitute.be/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Backhed F, Crawford PA. Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim Biophys Acta. 2010;1801:240–245. doi: 10.1016/j.bbalip.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 4.Cani PD, Possemiers S, Van de WT, Guiot Y, Everard A, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 6.Delzenne NM, Cani PD. Nutritional modulation of gut microbiota in the context of obesity and insulin resistance: Potential interest of prebiotics. International Dairy Journal. 2010;20:277–280. [Google Scholar]
- 7.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 8.Neyrinck AM, Delzenne NM. Potential interest of gut microbial changes induced by non-digestible carbohydrates of wheat in the management of obesity and related disorders. Curr Opin Clin Nutr Metab Care. 2010;13:722–728. doi: 10.1097/MCO.0b013e32833ec3fb. [DOI] [PubMed] [Google Scholar]
- 9.Hughes SA, Shewry PR, Li L, Gibson GR, Sanz ML, et al. In vitro fermentation by human fecal microflora of wheat arabinoxylans. J Agric Food Chem. 2007;55:4589–4595. doi: 10.1021/jf070293g. [DOI] [PubMed] [Google Scholar]
- 10.Vardakou M, Palop CN, Christakopoulos P, Faulds CB, Gasson MA, et al. Evaluation of the prebiotic properties of wheat arabinoxylan fractions and induction of hydrolase activity in gut microflora. Int J Food Microbiol. 2008;123:166–170. doi: 10.1016/j.ijfoodmicro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Grootaert C, Delcour JA, Courtin CM, Broekaert WF, Verstraete W, Van de Wiele T. Microbial metabolism and prebiotic potency of arabinoxylan oligosaccharides in the human intestine. Trends in Food Science & Technology. 2007;18:64–71. [Google Scholar]
- 12.Maes C, Delcour JA. Structural characterisation of water-extractable and water-unextractable arabinoxylans in wheat bran. Journal of Cereal Science. 2002;35:315–326. [Google Scholar]
- 13.Lopez HW, Levrat MA, Guy C, Messager A, Demigne C, Remesy C. Effects of soluble corn bran arabinoxylans on cecal digestion, lipid metabolism, and mineral balance (Ca, Mg) in rats. J Nutr Biochem. 1999;10:500–509. doi: 10.1016/s0955-2863(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 14.Lu ZX, Walker KZ, Muir JG, Mascara T, O'Dea K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am J Clin Nutr. 2000;71:1123–1128. doi: 10.1093/ajcn/71.5.1123. [DOI] [PubMed] [Google Scholar]
- 15.Lu ZX, Walker KZ, Muir JG, O'Dea K. Arabinoxylan fibre improves metabolic control in people with Type II diabetes. Eur J Clin Nutr. 2004;58:621–628. doi: 10.1038/sj.ejcn.1601857. [DOI] [PubMed] [Google Scholar]
- 16.Garcia AL, Otto B, Reich SC, Weickert MO, Steiniger J, et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur J Clin Nutr. 2007;61:334–341. doi: 10.1038/sj.ejcn.1602525. [DOI] [PubMed] [Google Scholar]
- 17.Monobe M, Maeda-Yamamoto M, Matsuoka Y, Kaneko A, Hiramoto S. Immunostimulating activity and molecular weight dependence of an arabinoxylan derived from wheat bran. Journal of the Japanese Society for Food Science and Technology-Nippon Shokuhin Kagaku Kogaku Kaishi. 2008;55:245–249. [Google Scholar]
- 18.Marzorati M, Possemiers S, Verstraete W. The use of the SHIME-related technology platform to assess the efficacy of pre- and probiotics. Agro Food Industry Hi-Tech. 2009;20:50–53. [Google Scholar]
- 19.Van den Abbeele P, Van de Wiele T, Grootaert C, Verstraete W, Gérard P, et al. Arabinoxylans and inulin modulate the luminal and mucosa-associated bacteria in vitro and in vivo. In: McCleary B, Jones JM, Topping D, van der Kamp JW, editors. Dietary Fibre - New frontiers for food and health. Wageningen Academic Publishers; 2009. pp. 233–249. [Google Scholar]
- 20.de Wiele TV, Boon N, Possemiers S, Jacobs H, Verstraete W. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. 2004;51:143–153. doi: 10.1016/j.femsec.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Possemiers S, Verthe K, Uyttendaele S, Verstraete W. PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. 2004;49:495–507. doi: 10.1016/j.femsec.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, et al. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine 1. J Nutr. 2006;136:1862–1867. doi: 10.1093/jn/136.7.1862. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 24.Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 25.Ventura M, Reniero R, Zink R. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Appl Environ Microbiol. 2001;67:2760–2765. doi: 10.1128/AEM.67.6.2760-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.bdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 28.Neyrinck AM, Possemiers S, Verstraete W, De BF, Cani PD, et al. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.10.008. In press. [DOI] [PubMed] [Google Scholar]
- 29.Linn TC. Purification and crystallization of rat liver fatty acid synthetase. Arch Biochem Biophys. 1981;209:613–619. doi: 10.1016/0003-9861(81)90320-9. [DOI] [PubMed] [Google Scholar]
- 30.Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Holle AV, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2010.05.009. In press. [DOI] [PubMed] [Google Scholar]
- 31.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki S, Ishikawa S, Arihara K, Itoh M. Molecular species-specific differences in composition of triacylglycerols of mouse adipose tissue and diet. Nutr Res. 2008;28:258–262. doi: 10.1016/j.nutres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Awad AB. Effect of dietary lipids on composition and glucose utilization by rat adipose tissue. J Nutr. 1981;111:34–39. doi: 10.1093/jn/111.1.34. [DOI] [PubMed] [Google Scholar]
- 34.Devillard E, McIntosh FM, Paillard D, Thomas NA, Shingfield KJ, et al. Differences between human subjects in the composition of the faecal bacterial community and faecal metabolism of linoleic acid. Microbiology. 2009;155:513–520. doi: 10.1099/mic.0.023416-0. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 36.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleissner CK, Huebel N, bd El-Bary MM, Loh G, Klaus S, et al. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr. 2010;104:919–929. doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- 40.Kok N, Roberfroid M, Delzenne N. Dietary oligofructose modifies the impact of fructose on hepatic triacylglycerol metabolism. Metabolism. 1996;45:1547–1550. doi: 10.1016/s0026-0495(96)90186-9. [DOI] [PubMed] [Google Scholar]
- 41.Delzenne NM, Williams CM. Prebiotics and lipid metabolism. Curr Opin Lipidol. 2002;13:61–67. doi: 10.1097/00041433-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Delzenne NM, Kok N. Effects of fructans-type prebiotics on lipid metabolism. Am J Clin Nutr. 2001;73:456S–458S. doi: 10.1093/ajcn/73.2.456s. [DOI] [PubMed] [Google Scholar]
- 43.Wall R, Ross RP, Shanahan F, O'Mahony L, O'Mahony C, et al. Metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am J Clin Nutr. 2009;89:1393–1401. doi: 10.3945/ajcn.2008.27023. [DOI] [PubMed] [Google Scholar]
- 44.Benjamin S, Spener F. Conjugated linoleic acids as functional food: an insight into their health benefits1. Nutr Metab (Lond) 2009;6:36. doi: 10.1186/1743-7075-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devillard E, McIntosh FM, Duncan SH, Wallace RJ. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J Bacteriol. 2007;189:2566–2570. doi: 10.1128/JB.01359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntosh FM, Shingfield KJ, Devillard E, Russell WR, Wallace RJ. Mechanism of conjugated linoleic acid and vaccenic acid formation in human faecal suspensions and pure cultures of intestinal bacteria. Microbiology. 2009;155:285–294. doi: 10.1099/mic.0.022921-0. [DOI] [PubMed] [Google Scholar]
- 47.Nakano R, Kurosaki E, Yoshida S, Yokono M, Shimaya A, et al. Antagonism of peroxisome proliferator-activated receptor gamma prevents high-fat diet-induced obesity in vivo. Biochem Pharmacol. 2006;72:42–52. doi: 10.1016/j.bcp.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Stienstra R, Duval C, Ller M, Kersten S. PPARs, Obesity, and Inflammation. PPAR Res. 2006;2007:95974. doi: 10.1155/2007/95974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lairon D, Lafont H, Vigne JL, Nalbone G, Leonardi J, et al. Effects of dietary fibers and cholestyramine on the activity of pancreatic lipase in vitro. Am J Clin Nutr. 1985;42:629–638. doi: 10.1093/ajcn/42.4.629. [DOI] [PubMed] [Google Scholar]
- 50.Neyrinck AM, Bindels LB, De BF, Pachikian BD, Cani PD, et al. Dietary supplementation with chitosan derived from mushrooms changes adipocytokine profile in diet-induced obese mice, a phenomenon linked to its lipid-lowering action. Int Immunopharmacol. 2009;9:767–773. doi: 10.1016/j.intimp.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Wydro P, Krajewska B, Hac-Wydro K. Chitosan as a lipid binder: a langmuir monolayer study of chitosan-lipid interactions. Biomacromolecules. 2007;8:2611–2617. doi: 10.1021/bm700453x. [DOI] [PubMed] [Google Scholar]
- 52.Martinez I, Wallace G, Zhang C, Legge R, Benson AK, et al. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl Environ Microbiol. 2009;75:4175–4184. doi: 10.1128/AEM.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Delzenne NM, Daubioul C, Neyrinck A, Lasa M, Taper HS. Inulin and oligofructose modulate lipid metabolism in animals: review of biochemical events and future prospects. Br J Nutr. 2002;87(Suppl 2):S255–S259. doi: 10.1079/BJNBJN/2002545. [DOI] [PubMed] [Google Scholar]
- 55.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aronsson L, Huang Y, Parini P, Korach-Andre M, Hakansson J, et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS One. 2010;5. pii:e13087. doi: 10.1371/journal.pone.0013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mandard S, Zandbergen F, van SE, Wahli W, Kuipers F, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial quantification in the caecum. Caecal bacterial content of total bacteria (A), Bifidobacterium spp. (B), Bacteroides-Prevotella spp. (C) and Roseburia spp. (D). Bacterial quantities are expressed as Log10 (bacterial cells/ total caecal content wet weight). Mice were fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks.*p<0.05 versus CT and §p<0.05 versus HF (ANOVA).
(TIF)
mRNA levels of key markers in jejunum related to gut barrier function. Mice were fed a standard (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks. Values are expressed relative to CT group (set at 1). *p<0.05 versus CT and §p<0.05 versus HF (ANOVA). ZO-1, zonula occludens-1.
(TIF)
Analysis of fat binding capacity of arabinoxylan (AX) in vivo. Eighteen male C57bl6/J mice (10 week old) were housed in groups of 3 per cage in a controlled environment with free access to HF diet. After 3 days for acclimatisation, the mice were divided into 3 groups (n = 6/group): a group fed with a HF diet, a group fed the same HF diet supplemented with 10% AX (HF-AX) and a group fed with the HF diet supplemented with 10% chitosan (KiOnutrime-Cs™ from KitoZyme sa, Belgium, HF-Cs). Food intake was recorded and mice were killed 12 h after access to the diets. Lipid content, fatty acids and cholesterol concentration in the caecal content were determined as previously described [50]. Proportion of caecal lipids versus ingested lipids (A), caecal pool of fatty acids (B) and caecal pool of cholesterol (C); *p<0.05 versus HF (ANOVA).
(TIFF)
The full composition of the control diet and the high fat diet.
(DOC)
Gene expression in the liver and in the muscle of mice fed a standard diet (CT), a high fat diet (HF) or a high fat diet supplemented with 10% arabinoxylan (HF-AX) for 4 weeks.
(DOC)
Analysis of correlation between the number of bacteria in the caecal content (expressed as Log10 (bacterial cells/ total caecal content wet weight) with metabolic parameters that were significantly affected by AX treatment.
(DOC)