Abstract
The authors present the case of an inflammatory myofibroblastic tumor that involves the cervical spinal cord meninges, presenting in a manner mimicking en plaque meningioma, which has never been previously reported. During the first surgical procedure, which did not involve exploration of the intradural space, inflammatory epidural tissue was found. We performed a second operation to remove the tumor that was finally intradural, dural-based and very tough. Imaging studies, surgical findings, and histopathological examinations were used to support the diagnosis. Intradural extramedullary inflammatory myofibroblastic tumor is a rare entity that has only been described nine times in the literature. Surgery remains the treatment of choice. Although histologically benign, spinal inflammatory myofibroblastic tumor can be aggressive and requires a large resection and long-term follow-up of the entire central nervous system with magnetic resonance imaging.
Keywords: Inflammatory myofibroblastic tumor, Cervical spine, Intradural mass, Inflammatory pseudotumor, Plasma cell granuloma
Introduction
Inflammatory myofibroblastic tumor (IMT) is a histologically benign ubiquitous lesion usually described in the lung [1] and the orbit. Although it has been described using various terms, such as “inflammatory pseudotumor” or “plasma cell granuloma”, IMT is the more appropriate term as recognized in the 2002 World Health Organization Classification [2]. Spinal meningeal involvement is rare. To the best of our knowledge, only nine cases of intradural extramedullary spinal lesions [3–11] have been reported in the literature. We report the case of an intradural extramedullary IMT of the cervical spine in a 43-year-old male. The aims of this report are to describe a new case of this rarely reported location of IMT and to place emphasis on clinical, radiological, and surgical findings through a literature review.
Case report
A 43-year-old male had a 4-month history of nocturnal posterior cervical pain. His past medical history was uneventful. He reported a progressive radicular pain in his left upper limb at C6 and C7. He had developed paresthesia in the first three left fingers. His neurological examination showed weakness in pronation and the interosseous and lumbrical muscles of the left hand. Tricipital and ulnar pronator reflexes were bilaterally absent. There was no sign of spinal cord compression.
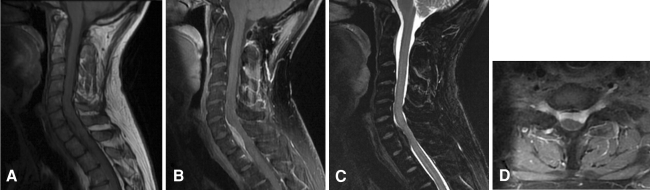
X-rays of the cervical and thoracic spine revealed a loss of cervical lordosis. The Computed Tomography (CT) scan of the cervical and thoracic spine was normal. The results of laboratory tests including blood cell count, C-reactive protein and erythrocyte sedimentation rate were normal. Magnetic Resonance Imaging (MRI) showed a mass at C6–T1 (Fig. 1), anterior to the spinal cord, infiltrating bilaterally C7 and T1 neural foramina. The mass was isointense to the spinal cord on T1-weighted images with homogeneous contrast enhancement, and hypointense on T2-weighted images.
Fig. 1.
Preoperative magnetic resonance imaging (MRI). a Sagittal T1-weighted image showing a tumor isointense to the spinal cord, at C6 to T1. b Homogeneous enhancement of the tumor after injection of gadolinium. c Sagittal T2-weighted image: the tumor appears as very low signal intensity. d Axial post-contrast T1-weighted image: bilateral foraminal contrast enhancement interpreted as extension of the tumor to the C7 neural foramina
The tumor was initially considered epidural and the patient was operated via a posterior approach with conservation of the posterior joints. During surgery, the anterior epidural tissues were inflammatory with thickening of the anterior dura mater. Histopathological examination of the epidural specimen showed non-specific diffuse chronic inflammation of a fibrous tissue.
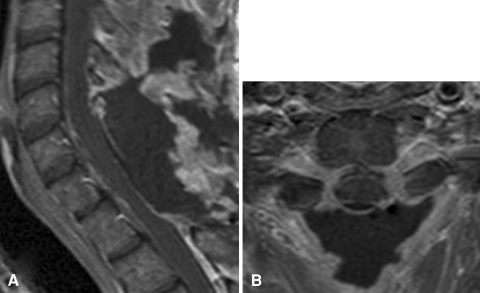
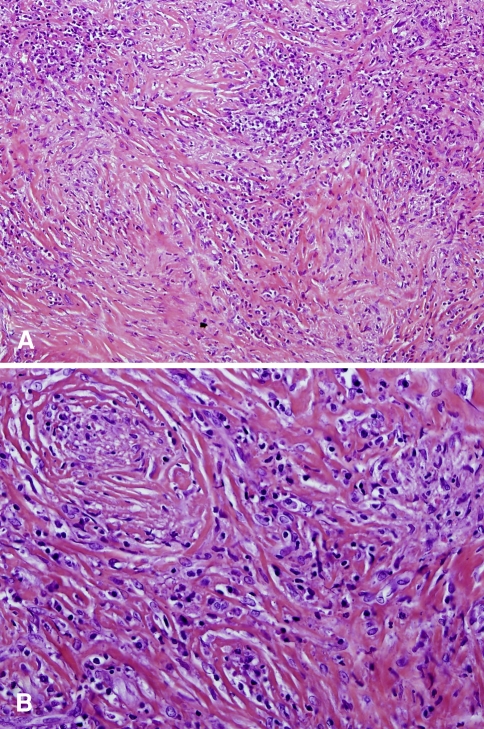
Postoperative MRI confirmed that the tumor had been missed. The neurological examination was unchanged. Therefore, the patient underwent a second surgical procedure 1 month later. Posterior intradural exploration revealed a tough, white, fibrous intradural extramedullary tumor (Fig. 2), anterior to the dentate ligament. A piecemeal resection was performed with optical magnification. The tumor was easily dissected from the spinal cord and nerve roots, but was firmly attached to the dura mater which was, however, finally spared. We could see the lateral tumor borders, anteriorly surrounding the C7 and T1 nerve roots bilaterally, with no extension to the foramina. Complete removal was confirmed by immediate postoperative MRI (Fig. 3). Histopathological examination showed a fibrous tissue mass infiltrated with a mixed population of lymphocytes, plasma cells and macrophages, thereby confirming the diagnosis of IMT (Fig. 4).
Fig. 2.
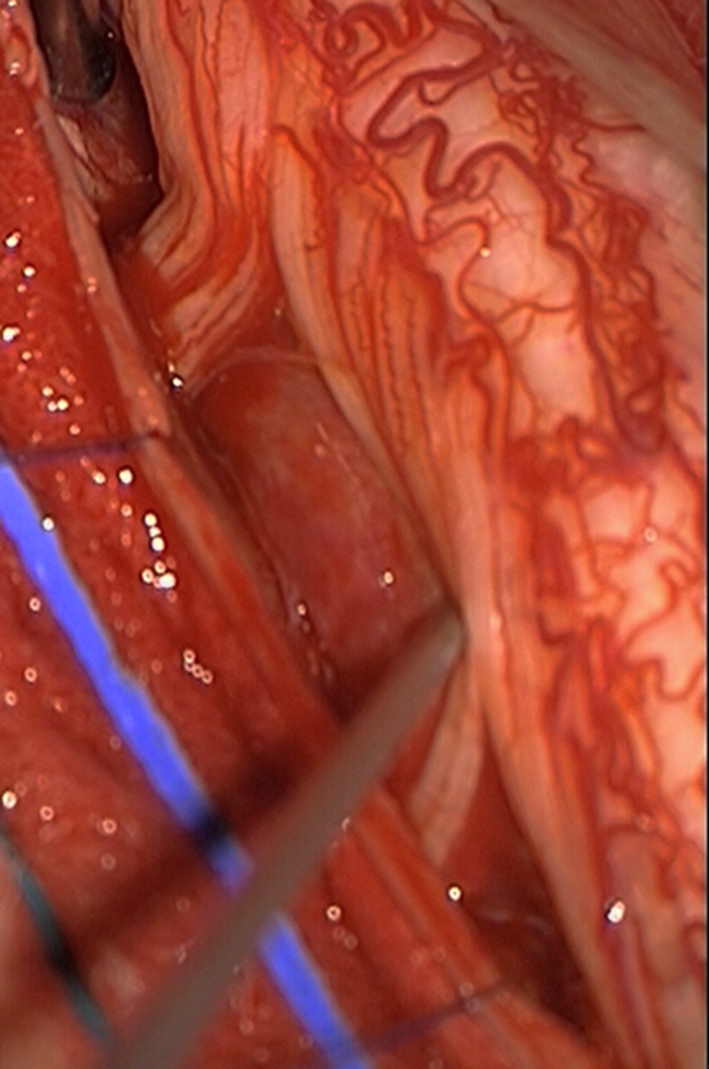
Intraoperative photograph. The tumor is located anterior to the spinal cord and nerve roots
Fig. 3.
Postoperative MRI on post-contrast T1-weighted sequences showing complete removal of the tumor. a sagittal image. b axial image
Fig. 4.
Pathology specimen, hematoxylin and eosin. a (original magnification ×100). There are compact fascicular and myxoid areas. b (original magnification ×200). Spindle cells are mixed with numerous lymphocytes and plasma cells
After surgery, there was a temporary worsening of weakness and paresthesia occurring in both upper extremities. The patient gradually improved. At the time of the most recent follow-up, 14 months after the last surgery, he still had paresthesia in the left index and interosseous and lumbrical muscle weakness in the left hand.
His brain MRI was normal. The thoracic and abdominal CT scan showed a 0.6 cm nodule in the upper lobe of the right lung. It was considered non-specific by pneumologists who suggested only a CT check-up at 6 months.
Discussion
Among the extrapulmonary locations [12, 13] of IMT, spinal involvement is very rare. To the best of our knowledge, since the first description by Eimoto et al. [5], only nine cases of intradural extramedullary [3–11] tumors have been reported in the literature (Table 1). A few other cases of epidural [14–17] or intramedullary [18–20] spinal IMT have been reported.
Table 1.
Cases of intradural extramedullary spinal inflammatory myofibroblastic tumor
| Age/sex | Location | Physical examination | Time to diagnosis (months) | MRI characteristics | Treatment | Postoperative neurological state | Recurrent (R)/multicentric (M) | Follow-up (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T1 Gado | T2 | |||||||||
| Eimoto et al. [5] | 37/M | C4–C5 | Spinal cord compression | 12 | NA | NA | NA | GTR + radiotherapy | No deficit | No | 9 |
| Mirra et al. [10] | 39/F | C7 | Spinal cord compression | NA | NA | NA | NA | GTR | Improvement until recurrence | R | 36 |
| Hsiang et al. [6] | 57/M | T3, T12–L3, falx cerebri | Radicular pain and spinal cord compression | 6 | NA | + | NA | GTR | No deficit | R and M | 84 (7 years) |
| Hsieh et al. [7] | 37/M | T5, T12–L1 | Back pain and spinal cord compression | 6 | Hypo | NA | NA | GTR | No deficit | M | 14 |
| Lacoste-Collin et al. [9] | 22/F | T9 then diffuse | Back pain and spinal cord compression | 3 | Iso | + | Iso-hypo | Sub-total resection | Slow recovery | R and M | 24 |
| Brandsma et al. [4] | 33/F | C6–C7, cranial nerves | Radicular pain and spinal cord compression | NA | NA | NA | NA | Surgery | No improvement | M | NA |
| Jeon et al. [8] | 60/F | Lumbar spine | Back pain | NA | NA | NA | NA | GTR | NA | No | NA |
| Boutarbouch et al. [3] | 30/F | C4–T2 | Spinal cord compression | 2 | Iso | NA | Hypo | GTR | Progressive recovery | No | 6 |
| Yoon et al. [11] | 56/F | L5 | Low back pain | 48 | Iso | + | Iso | GTR + interbody fusion | No pain, toe paresthesia | No | 24 |
| Present case | 43/M | C6–T1 | Radicular pain | 4 | Iso | + | Hypo | GTR | Incomplete recovery | No | 14 |
M male, F female, Gado gadolinium, NA not available, + enhancement after gadolinium infusion, hypo hypointense, iso isointense, GTR gross total resection
According to the previous cases reported, the time to diagnosis of intradural extramedullary spinal IMT ranges from 2 to 48 months. The present case was revealed by radicular pain, whereas the most common clinical features are spinal cord compression symptoms. The nocturnal character of the pain oriented us to an inflammatory lesion.
MRI features of intradural extramedullary IMT were poorly documented in four [3, 6, 9, 11] of the nine cases reported. In the present case, MRI showed a mass that was isointense on T1-weighted sequences and hypointense on T2-weighted sequences. Contrast enhancement was intense and homogeneous, similar to that of vascular or inflammatory lesions. The tumor was anterior to the spinal cord with scarcely demarcated lateral extension to the C7 and T1 neural foramina. The radiological features of the extramedullary tumor were discussed with neuroradiologists. The diagnosis suggested was either an epidural tumor such as a sarcoma, or an intradural tumor such as an en plaque meningioma.
Our first diagnosis was that of an epidural tumor based on MRI foraminal enhancement interpreted as lateral extension of the tumor, together with clinical features of an inflammatory tumor, and the fact that en plaque meningiomas of the spine are very rare [21–24], often occur in middle-aged female patients and are more likely to cause spinal cord compression than radicular symptoms [21]. The pitfall in our management was that as no tumor was found in the epidural space, intradural exploration should have followed. However, this was not the case as we believed the tumor to be an epidural sarcoma.
Two different surgical approaches were discussed. An anterior approach would have allowed an en bloc resection to be performed, but the tumor was posterior to three vertebral bodies, imposing an extensive three-level corporectomy. We therefore chose the posterior approach with laminectomy and conservation of the posterior joints. A piecemeal resection was performed bilaterally to protect the spinal cord. The tumor was more fibrous and tougher than a meningioma, and resection was difficult.
Surgical excision is the best way to reach a precise diagnosis, as radiological findings cannot differentiate the tumor from a meningioma. Complete removal remains the recommended treatment, although other treatments such as steroid therapy [25], antibiotics, radiotherapy [26, 27], chemotherapy [5, 10, 13] or CO2 laser [28] have been tested on other locations of IMT with good results.
Of the nine cases of intradural extramedullary IMT reported in the literature, five were recurrent [3, 7–9] or multicentric [3, 8, 9, 12] in the central nervous system (CNS). We therefore believe it is important to perform an initial MRI check-up of the entire CNS and provide long-term follow-up. There is no apparent histological difference between recurrent and non-recurrent forms. Lacoste-Collin et al. [9] suggested studying the anaplastic lymphoma kinase (ALK) gene that may be correlated with a higher recurrence rate. The short follow-up of our case cannot exclude a recurrent form and we did not study the ALK gene.
IMT should be considered as a possible etiology of intradural extramedullary spinal mass. MRI features of intradural extramedullary spinal IMT, which have not been described previously, are not specific and surgery is the only way to reach a diagnosis. Surgeons should be aware of its possible tough consistency as it may influence the surgical approach. As recurrent or multicentric forms are common, long-term follow-up should be offered.
Conflict of interest
None.
References
- 1.Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973;31:191–208. doi: 10.1002/1097-0142(197301)31:1<191::AID-CNCR2820310127>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher C, Unni KK, Mertens F (2002) Pathology and genetics of tumors of soft tissue and bone. World Health Organization classification of tumors. IARC press, Lyon, France
- 3.Boutarbouch M, Arkha Y, Rifi L, Derraz S, El Ouahabi A, El Khamlichi A. Intradural cervical inflammatory pseudotumor mimicking epidural hematoma in a pregnant woman: case report and review of the literature. Surg Neurol. 2008;69:302–305. doi: 10.1016/j.surneu.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 4.Brandsma D, Jansen GH, Spliet W, Nielen K, Taphoorn MJ. The diagnostic difficulties of meningeal and intracerebral plasma cell granulomas–presentation of three cases. J Neurol. 2003;250:1302–1306. doi: 10.1007/s00415-003-0200-7. [DOI] [PubMed] [Google Scholar]
- 5.Eimoto T, Yanaka M, Kurosawa M, Ikeya F. Plasma cell granuloma (inflammatory pseudotumor) of the spinal cord meninges: report of a case. Cancer. 1978;41:1929–1936. doi: 10.1002/1097-0142(197805)41:5<1929::AID-CNCR2820410537>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Hsiang J, Moorhouse D, Barba D. Multiple plasma cell granulomas of the central nervous system: case report. Neurosurgery. 1994;35:744–747. doi: 10.1227/00006123-199410000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh PC, Lin CN (1995) Multicentric plasma cell granuloma of spinal cord meninges. Clin Orthop Relat Res (317):188–192 [PubMed]
- 8.Jeon YK, Chang KH, Suh YL, Jung HW, Park SH. Inflammatory myofibroblastic tumor of the central nervous system: clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol. 2005;64:254–259. doi: 10.1093/jnen/64.3.254. [DOI] [PubMed] [Google Scholar]
- 9.Lacoste-Collin L, Roux FE, Gomez-Brouchet A, Despeyroux ML, Uro-Coste E, Coindre JM, Delisle MB. Inflammatory myofibroblastic tumor: a spinal case with aggressive clinical course and ALK overexpression. Case report. J Neurosurg. 2003;98:218–221. doi: 10.3171/spi.2003.98.2.0218. [DOI] [PubMed] [Google Scholar]
- 10.Mirra SS, Tindall SC, Check IJ, Brynes RK, Moore WW. Inflammatory meningeal masses of unexplained origin. An ultrastructural and immunological study. J Neuropathol Exp Neurol. 1983;42:453–468. doi: 10.1097/00005072-198307000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Yoon SH, Kim KJ, Chung SK, Kim HJ, Choe G, Chung SB, Jin YJ. Inflammatory myofibroblastic tumor in the intradural extramedullary space of the lumbar spine with spondylolisthesis: case report and review of the literature. Eur Spine J. 2009;19(Suppl 2):S153–S157. doi: 10.1007/s00586-009-1212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 13.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gilliard C, Coene B, Lahdou JB, Boutsen Y, Noel H, Godfraind C. Cervical epidural pseudotumor and multifocal fibrosclerosis. Case report and review of the literature. J Neurosurg. 2000;93:152–156. doi: 10.3171/spi.2000.93.1.0152. [DOI] [PubMed] [Google Scholar]
- 15.Roberts G, Farrell M, Allcutt D. Spinal inflammatory pseudotumours. Br J Neurosurg. 2001;15:197–198. doi: 10.1080/02688690120057691. [DOI] [PubMed] [Google Scholar]
- 16.Roberts GA, Eldridge PR, Mackenzie JM. Case report: inflammatory pseudotumour of the spine, with literature review. Br J Neurosurg. 1997;11:570–572. doi: 10.1080/02688699745754. [DOI] [PubMed] [Google Scholar]
- 17.Seol HJ, Kim SS, Kim JE, Lee SH, Won JY. Inflammatory pseudotumor in the epidural space of the thoracic spine: a case report and literature review of MR imaging findings. AJNR Am J Neuroradiol. 2005;26:2667–2670. [PMC free article] [PubMed] [Google Scholar]
- 18.Aizawa T, Sato T, Tanaka Y, Kishimoto K, Watanabe M, Kokubun S. Intramedullary plasma cell granuloma in the cervicothoracic spine. Case report. J Neurosurg. 2002;97:235–238. doi: 10.3171/spi.2002.97.2.0235. [DOI] [PubMed] [Google Scholar]
- 19.Kilinc M, Erturk IO, Uysal H, Birler K, Evrenkaya T, Akkalyoncu BB. Multiple plasma cell granuloma of the central nervous system: a unique case with brain and spinal cord involvement. Case report and review of literature. Spinal Cord. 2002;40:203–206. doi: 10.1038/sj.sc.3101271. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Epstein FJ, Rezai AR, Zagzag D. Nonneoplastic intramedullary spinal cord lesions mimicking tumors. Neurosurgery. 1998;43:788–794. doi: 10.1097/00006123-199810000-00034. [DOI] [PubMed] [Google Scholar]
- 21.Caroli E, Acqui M, Roperto R, Ferrante L, D’Andrea G. Spinal en plaque meningiomas: a contemporary experience. Neurosurgery. 2004;55:1275–1279. doi: 10.1227/01.NEU.0000143611.28034.B2. [DOI] [PubMed] [Google Scholar]
- 22.Frank BL, Harrop JS, Hanna A, Ratliff J. Cervical extradural meningioma: case report and literature review. J Spinal Cord Med. 2008;31:302–305. doi: 10.1080/10790268.2008.11760727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamache FW Jr, Wang JC, Deck M, Heise C (2001) Unusual appearance of an en plaque meningioma of the cervical spinal canal. A case report and literature review. Spine (Phila Pa 1976) 26:E87–E89 [DOI] [PubMed]
- 24.Messori A, Rychlicki F, Salvolini U. Spinal epidural en-plaque meningioma with an unusual pattern of calcification in a 14-year-old girl: case report and review of the literature. Neuroradiology. 2002;44:256–260. doi: 10.1007/s00234-001-0709-3. [DOI] [PubMed] [Google Scholar]
- 25.Albizzati C, Ramesar KC, Davis BC. Plasma cell granuloma of the larynx (case report and review of the literature) J Laryngol Otol. 1988;102:187–189. doi: 10.1017/s0022215100104487. [DOI] [PubMed] [Google Scholar]
- 26.Pettinato G, Manivel JC, Insabato L, Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol. 1988;90:627–632. doi: 10.1093/ajcp/90.5.627. [DOI] [PubMed] [Google Scholar]
- 27.Warter A, Satge D, Roeslin N. Angioinvasive plasma cell granulomas of the lung. Cancer. 1987;59:435–443. doi: 10.1002/1097-0142(19870201)59:3<435::AID-CNCR2820590315>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Satomi F, Mori H, Ogasawara H, Kumoi T, Uematsu K. Subglottic plasma cell granuloma: report of a case. Auris Nasus Larynx. 1991;18:391–399. doi: 10.1016/s0385-8146(12)80233-1. [DOI] [PubMed] [Google Scholar]