Abstract
Intramedullary spinal cord abscess (ISCA) without meningitis is an extremely rare entity in the central nervous system, and it is often difficult to diagnose immediately, and no definitive imaging findings have been established. We experienced the case of a 61-year-old male who presented with a sudden onset back pain without fever following rapidly worsening paraparesis for 3 days, who subsequently become unable to walk. According to the initial MRI and 3D-CTA, the presumptive diagnosis was spinal infarction due to spinal artery embolism. However, his symptoms did not improve, despite the gradual changes in MRI following antiplatelet therapy. He underwent a biopsy in an attempt to prevent the lesion from progressing toward the upper spinal cord. The pathological examination revealed an intramedullary abscess, so we performed a midline myelotomy and drained the pus from the abscess. After surgery, MRI showed improvement, but the patient’s paraplegia persisted. To the best of our knowledge, this is the first case report of spinal cord abscess with the confirmation of spinal artery occlusion on angiography, which could have been caused by a bacterial embolism. We herein discuss its possible etiology and also review recent reports on ISCA.
Keywords: Intramedullary spinal cord abscess, Spinal infarction, Bacterial embolism, MRI findings
Introduction
An intramedullary spinal cord abscess (ISCA) is an uncommon entity described initially by Hart [28] in 1830. Since then, more than 120 cases have been reported by several reviews. The mortality of this entity has improved by the use of antibiotic agents, adequate radiographical diagnosis and immediate surgery. The first review of the literature [3] published between 1830 and 1944 reported a 90% mortality rate, while a recent review by Kurita et al. [34] of the literature published between 1998 and 2007 reported only 4% mortality.
Our case presented with an acute onset of paraparesis with a presumptive diagnosis of spinal infarction developing chronologically on magnetic resonance imaging (MRI), and the definitive diagnosis of an intramedullary abscess was finally made based on the findings of a biopsy. Following our case report, we present a review of the recent world literature and an analysis of the 54 cases reported between 1977 and 2009, including their diagnosis, pathogenesis and treatment approaches and outcomes.
Case report
A 61-year-old male with drug-controlled diabetes mellitus was admitted to another hospital with sudden onset lumbago, where he was diagnosed with lumbar spondylosis and prescribed analgesics. However, progressive paraparesis and urinary retention developed 3 days later, and he was transferred to our hospital. On admission, he could not walk due to severe paraparesis, of grade 1/5 power in both lower limbs. He had hypesthesia below the T10 (thoracic10th) level, and was afebrile. Nuchal rigidity was not obviously present. There was no history of infection, trauma, fever, or surgery of the thoraco-lumbar spine. The results of his laboratory studies were as follows: white blood cell (WBC) count 7,500 mm−3 with a normal differential, C-reactive protein (CRP) 0.67 mg/l, and hemoglobin A1c 6.9. MRI revealed intramedullary high intensity on the ventral aspect of the spinal cord at the T10-11 level on sagittal T2WI, and axial T2WI demonstrated high intensity chiefly on the bilateral anterior two-thirds of the gray matter (Fig. 1a, b). A diffusion-weighted image had bi-modal distribution of high intensity with peaks at the same level and conus, although there was difficulty with regard to its visual accuracy for a definitive diagnosis (Fig. 1c). Three-dimensional computed tomography angiography (3D-CTA) revealed the interruption of the spinal artery, which branched off from the intercostal artery at the level of the T10-11 on the right side, and there was no obvious Adamkiewicz artery or aortic dissection (Fig. 2). Therefore, we initially diagnosed spinal infarction caused by atherosclerotic or embolic occlusion of the spinal artery on the background of diabetes mellitus.
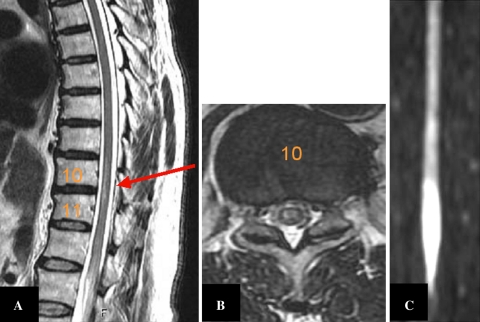
Fig. 1.
MR T2-weighted sagittal (a), axial (b: T10) and diffusion-weighted (c) images showing an anterior intramedullary high signal at the lower thoracic and conus level
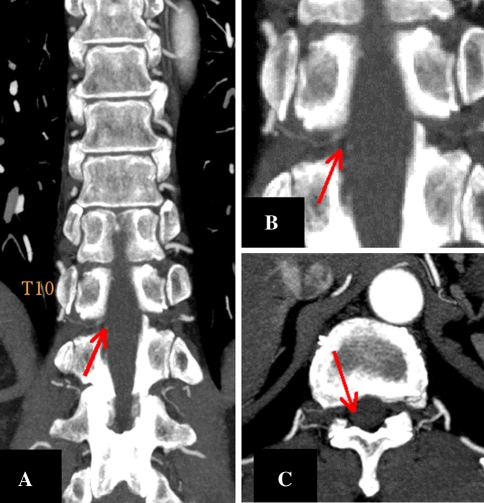
Fig. 2.
3D-CTA showing the interruption of the spinal artery branching off from the intercostal artery in the spinal canal at the thoracic 10-11 level. No obvious Adamkiewicz artery or aortic dissection was recognized. Coronal image (a), magnification of a (b), and an axial image (c)
Two days later, the high intracordal T2WI signal had spread over the initial lesion (Fig. 3a), and therefore we administered more antiplatelet agents to prevent the development of spinal ischemia. However, the patient’s symptoms in his lower extremities had worsened to paraplegia at that point, although he was afebrile and had no signs of inflammation. A subsequent Gd-DTPA image 10 days after onset showed distinct enhancement in the initial lesion, which could not be observed at first (Fig. 3b). Finally, MRI 1 month after onset showed local cord expansion in a ‘hump’-like configuration at the T10/11 level (Fig. 3c), and some infectious disease, intramedullary tumor or demyelinating disease was suspected. Subsequent cerebro-spinal fluid (CSF) and blood cultures were sterile, the CSF cytodiagnosis was also negative, and a cardiovascular physician did not find any evidence of infectious endocarditis on the cardiac echo, and therefore a biopsy was performed in order to make a definitive diagnosis and to select the optimal treatment to prevent such inflammation from spreading to the cervical region.
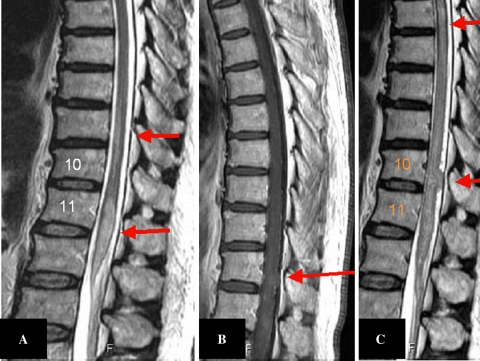
Fig. 3.
T2-weighted image on day 2 (a), the Gd-DTPA image on day 10 (b), and a T2-weighted image on day 30 (c) showing the chronological development of the lesion, which finally transformed to the dorsal mass-like `hump`
The patient underwent a laminectomy at T11-L1 and intradural exploration. The spinal cord was expanded. A yellowish soft mass exophyting through the dorsal aspect of the cord rostral adjacent conus medullaris coated with thin pia (Fig. 4a) was taken piece by piece, and intraoperative histopathological examination revealed an abscess. Purulent fluid was encountered by midline myelotomy from T11 to the conus. The cavity was irrigated with saline, and the wound was closed in layers. No drains were placed in the abscess cavity.
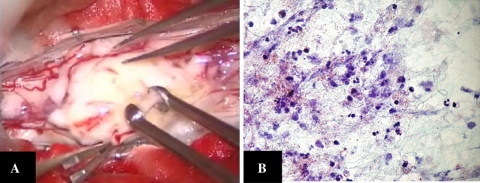
Fig. 4.
a Intraoperative view of the lesion showing the exophyting abscess coated by only the thin pia. b A histopathological examination showing the degenerative fibrinous matrix, macrophages, granulocytes, and no tumor cells (Papanicolaou stain ×80)
Pathological evaluation of the surgical specimen showed degenerative fibrinous matrix, macrophages, granulocytes, and no tumorous cells, thus indicating a definitive diagnosis of spinal abscess (Fig. 4b). The intraoperative pus culture was also sterile.
A temporal high fever developed on one postoperative day, but it subsided once antibiotics were instituted intrathecally, and the patient was given a 5-week postoperative regimen of antibiotics intravenously assuming gram-positive rods. MRI T2WI findings on postoperative day 35 showed a decreasing high signal in the spinal cord (Fig. 5), but the patient’s paraplegia persisted. He was thereafter transferred to another hospital to undergo rehabilitation.
Fig. 5.
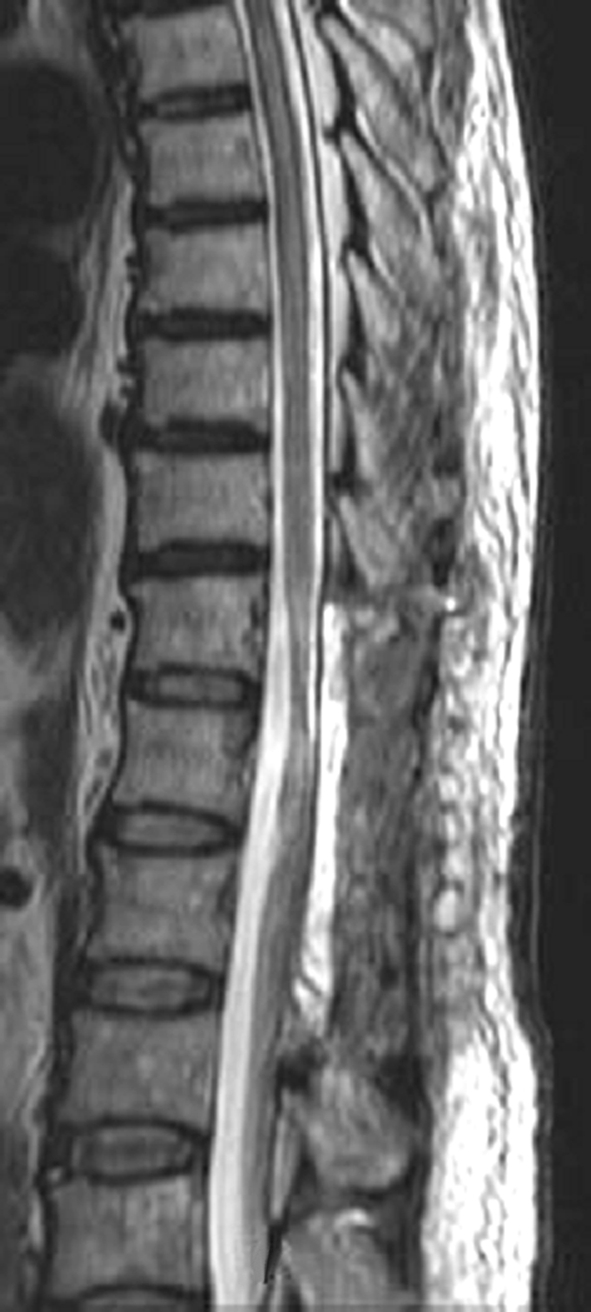
A T2-weighted image after treatment showing the decrease in the intracordal high signal
Review
A MEDLINE search of the literature was conducted using the keywords “intramedullary”, “spinal cord”, and “abscess”. Fifty-four cases of ISCA were identified between the years of 1977 and 2009 (Table 1) [1, 2, 4–10, 12–24, 26, 27, 29–43, 45–56].
Table 1.
Clinical data about intramedullary spinal cord abscesses in the published literature
| Case | Author | Age | Sex | Onset | T2WI high | Gd-DTPA | DWI | Op | Anti-biotics | Prognosis | Causes of abscess |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Our case | 61 | M | Acute | T10-conus | Homogenius | High | + | + | Poor | Embo. to A-K a. |
| 2 | DiTullio [19] | 0 | F | Acute | nm | nm | nm | + | + | Poor | Dermal sinus |
| 3 | Kurita [34] | 58 | M | Acute | C1-T1 | Ring | nm | + | + | Good | Cryptogenic |
| 4 | Menezes [39] | 48 | M | Subacute | nm | nm | nm | + | + | Good | Cryptogenic |
| 5 | Maheswaran [36] | 1 | M | Acute | nm | nm | nm | + | + | Good | Dermal sinus |
| 6 | Marwah [37] | 3 | F | Chronic | nm | nm | nm | + | + | Good | Cryptogenic |
| 7 | Fortuna [24] | 21 | M | Chronic | nm | nm | nm | + | + | Good | Trauma |
| 8 | Chu [13] | 69 | M | Subacute | C2-7 | Ring | nm | – | + | Poor | Cryptogenic |
| 9 | Novati [45] | 24 | M | Acute | C3 | Ring | nm | – | + | Good | Sepsis |
| 10 | Vajramani [53] | 40 | F | Chronic | T10-conus | nm | nm | + | + | Poor | Sepsis |
| 11 | Helvaci [29] | 15 | F | Acute | T11,12 | nm | nm | + | + | Good | Sepsis |
| 12 | Mukunda [43] | 52 | M | Subacute | L1 | nm | nm | + | + | Good | Cryptogenic |
| 13 | Applebee [3] | 47 | F | Acute | T8 | Ring | nm | + | + | Poor | Sepsis |
| 14 | Chidambaram [12] | 3 | M | Acute | T12-S1 | Ring | nm | + | – | Good | Cryptogenic |
| 15 | Crema [15] | 66 | M | nm | T3-6 | Ring | nm | – | + | Poor | Spondylodiskitis |
| 16 | Derkinderen [17] | 50 | M | Subacute | C1-6 | nm | nm | + | + | Poor | Surgery |
| 17 | Desai [18] | 22 | F | Acute | C1-conus | Ring | nm | nm | + | Poor | Cryptogenic |
| 18 | Durmaz [20] | 59 | M | Acute | C2-4 | Ring | nm | + | + | Dead | Sepsis |
| 19 | Elmaci [21] | 49 | F | Chronic | C2, 3 | Ring | nm | + | + | Poor | Sepsis |
| 20 | Guzel [26] | 1 | nm | Subacute | T3-11 | Ring | nm | + | + | Poor | Cryptogenic |
| 21 | Lascux [35] | 56 | M | Acute | nm | Ring | nm | – | + | Good | Sepsis |
| 22 | Morandi [40] | 4 | M | Acute | T-L | Ring | nm | + | + | Good | Dermal sinus |
| 23 | Morimoto [41] | 0 | F | nm | L2-conus | Ring | nm | + | + | Good | Dermal sinus |
| 24 | Simon [49] | 1 | M | Subacute | T12-conus | Ring | nm | + | + | Poor | Dermal sinus |
| 25 | Sverzut [50] | 42 | M | Subacute | C1-T3 | Ring | nm | + | + | Poor | Cryptogenic |
| 26 | Tsurubuchi [52] | 1 | F | Subacute | Medulla-conus | Ring | nm | + | + | Good | Dermal sinus |
| 27 | Weng [55] | 28 | M | Subacute | nm | Ring | nm | + | + | Good | Cryptogenic |
| 28 | Yuceer [56] | 4 | M | Subacute | nm | Homogenius | nm | + | + | Good | Sepsis |
| 29 | Vora [54] | 54 | M | Subacute | Medulla-C6 | Ring | nm | + | + | Dead | Sepsis |
| 30 | Bean [6] | 15 | M | Acute | nm | nm | nm | + | + | Good | Dermal sinus |
| 31 | Maurice-Williams [38] | 21 | M | Chronic | nm | nm | nm | + | + | Good | Dermal sinus |
| 32 | Maurice-Williams [38] | 20 | F | Subacute | nm | nm | nm | + | + | Good | Dermal sinus |
| 33 | Blacklock [8] | 71 | M | Acute | nm | nm | nm | + | + | Good | Cryptogenic |
| 34 | Carus [10] | 61 | M | Chronic | T7-10 | nm | nm | + | + | Poor | Cryptogenic |
| 35 | Koppel [32] | 50 | M | Acute | C4-7 | nm | nm | + | + | Poor | Cryptogenic |
| 36 | Benzil [7] | 2 | F | Acute | nm | Ring | nm | + | + | Good | Dermal sinus |
| 37 | Rogg [47] | 2 | F | Acute | nm | Ring | nm | + | + | Good | Dermal sinus |
| 38 | Babu [4] | 72 | M | Acute | nm | Homogenius | nm | + | + | Poor | Cryptogenic |
| 39 | Amacher [1] | 0 | M | nm | nm | Ring | nm | + | nm | Poor | Dermal sinus |
| 40 | Tewari [51] | 11 | M | Chronic | nm | nm | nm | + | nm | Poor | Cryptogenic |
| 41 | Erlich [22] | 59 | M | Acute | C4-6 | nm | nm | + | + | Dead | Sepsis |
| 42 | King [31] | 50 | M | Subacute | Medulla-C6 | Ring | nm | + | nm | Dead | Sepsis |
| 43 | Hardwidge [27] | 26 | M | Subacute | nm | nm | nm | + | + | Good | Dermal sinus |
| 44 | Cokca [14] | 17 | M | Subacute | nm | nm | nm | + | + | Good | Dermal sinus |
| 45 | Byrne [9] | 55 | F | Chronic | Conus | Spotty | nm | + | + | Poor | Cryptogenic |
| 46 | Byrne [9] | 68 | M | Chronic | Conus | Spotty | nm | + | + | Poor | Cryptogenic |
| 47 | Bartels [5] | 5 | M | Acute | T8 | Ring | nm | + | + | Good | Cryptogenic |
| 48 | Pfadenhauer [46] | 73 | M | Acute | T10-11 | Spotty | nm | – | + | Poor | Sepsis |
| 49 | Morrison [42] | 32 | M | Acute | nm | nm | nm | + | + | Good | Sepsis |
| 50 | David [16] | 27 | M | Acute | nm | Ring | nm | + | + | nm | Cryptogenic |
| 51 | Kurisu [33] | 1 | M | Chronic | Conus | Ring | nm | + | + | Good | Dermal sinus |
| 52 | Seizeur [48] | 69 | nm | Chronic | T10 | Homogenius | nm | + | nm | Good | Cryptogenic |
| 53 | Hott [30] | nm | nm | Acute | C2-T5 | Ring | nm | + | + | Poor | Sepsis |
| 54 | Fernandez [23] | nm | nm | nm | nm | nm | nm | nm | nm | nm | IE |
C cervical, T thoracic, L lumbar, nm not mentioned
Of the 54 patients, 37 (68.5%) were males, 13 (24.1%) females, and 4 were not described in the literature. The age at presentation ranged from 0 to 73 years. The median age at presentation was 32 years and its distribution had three peaks: in infants, in the second decade, and in the fifth decade. Nine patients were under 2 years old, and all but one of these (15%) was associated with a congenital dermal sinus [1, 7, 19, 33, 36, 41, 47, 49, 52]. Symptoms at the time of presentation were back pain, neck stiffness, paralysis, sensory disturbance, and urinary dysfunction among others, which sometimes was caused by meningitis or encephalitis, but a fever at the initial presentation was only seen in 23 (43.4%) of the 54 cases. The duration from the onset of symptoms to admission was acute (less than 1 week) in 24 patients, subacute in 9, chronic (more than 6 weeks) in 17, and not mentioned in 4 cases (44.4, 16.7, 31.5, 7.4%, respectively). With regard to background medical conditions, 5 patients (including our case) had diabetes mellitus [8, 10, 13, 20], 1 had human immunodeficiency virus [35] and 2 were heroin addicts [32, 50] (11.1, 1.9, 3.8%, respectively).
With regard to the cause of infection, 21 (38.9%) patients had cryptogenic sources including our case, 15 (27.8%) were related to a dermal sinus infection (with a past history of dermal sinus surgery in 9 of these), 14 (25.9%) suffered from apparent sepsis due to listeria, nocardia, brucellosis, or oral flora, 3 (5.6%) were extended from contiguous lesions, such as diskitis, trauma, or from surgery, and only 1 (1.9%) case had infectious endocarditis [23].
Imaging studies, such as plain radiography, myelography, computed tomography scans and MRI were performed for all patients. MRI results with administration of contrast material were available for 33 cases, and the findings were ring-enhancing margins with a central low signal in 26, poorly or well-defined marginal enhancement in 4, or spotty enhancement in 3 cases.
Twenty-six (47.6%) patients were treated within a week from onset and 48 (88.8%) underwent surgery, including drainage in 42 (77.8%) and additional removal of dermoid or epidermoid tissue related to a dermal sinus in 6 (11.1%). The other five patients were treated with antibiotics alone [13, 15, 35, 45, 46]. The treatment method was not mentioned in one case [23]. In total, antibiotics were used in 47 patients, not used in 1 [12], and their use was not mentioned for six patients (87.0, 1.9, 11.1%, respectively), and the period of antibiotic administration ranged from 2 weeks to 2 months.
The clinical outcomes were recorded for all but two patients. Four (7.4%) patients died because of abscess rupture inducing meningitis and brain abscess [22], listeria meningoencephalitis [31], central nervous system nocardiosis involving the bilateral hemisphere, cerebellum, and upper cervical spinal cord due to diabetes mellitus (despite a two-stage operation) [20], or sepsis after irradiation for a misdiagnosed metastasis of post-gastroesophageal surgery [54]. We divided the other cases into two groups according to their prognosis, which was good in 26 (48.1%) patients and poor in 21 patients (38.9%). Of the 26 patients in the good prognosis group, 8 (14.8%) patients fully recovered and 18 (33.3%) were able to walk without a cane with minimal neurological sequelae. Of these, all but 1 (96.2%) had received antibiotics, 24 (92.3%) underwent surgery, and the duration from onset to first treatment (surgery or antibiotics) ranged from 0 to 60 (mean 15.2) days. Twenty-one patients in the bad prognosis group had severe residual or persistent neurological deficits, such as paraparesis and paraplegia. Of these, the treatment strategy was not described for three patients, 17 (81.0%) had received antibiotics, and 15 (71.4%) underwent surgery. The duration from onset to first treatment ranged from 2 to 120 (mean 25.3) days. There was no significant difference between the good and poor prognosis groups with regard to the time until treatment (P = 0.26).
Discussion
ISCA are rare, accounting for about 120 cases identified in the literature, including those mainly derived from the dermal sinus in pediatric cases, and adults with infectious diseases of other origins. We believe that these are different entities, because the pathology of spina bifida was more complex, with concomitant other lesions (such as lipomas), which potentially led to a poorer prognosis. One review reported that 68% of children who had ISCA when they were younger than 5 had a preexisting spinal cord defect, whereas only 15% of those presenting at age 5 years or older had a preexisting defect, which was significantly lower [26]. Our review also revealed that there was a peak of age distribution in infants, and all of them except one had a dermal sinus.
Murphy et al. [44] described that the chronological changes of MRI findings in the spinal cord reveal the same progression that has been documented in the brain. In the early stage of infectious myelitis, MRI shows high signals in T2WI with poorly defined enhancement on postcontrast T1WI. Approximately 1 week after the initiation of treatment, the lesion became less diffusely hyperintense on T2WI, with more clearly defined marginal enhancement on postcontrast T1WI. Our case showed no enhancement at first, a slightly homogeneous enhancement at 10 days after admission, and finally an obvious enhancement of the lesion at 1 month after admission without the infusion of any antibiotics. The radiological differential diagnosis in the early stage includes spinal infarction, intramedullary abscess, low grade astrocytoma, and demyelinating diseases, such as neuromyelitis optica, and multiple sclerosis.
Spinal cord ischemia may occur in association with arterial spasms in the pia or occlusion of other spinal arteries [25]. Mori et al. [57] noted the case of an elderly female with sudden onset of hemiparesis whose MRI showed acute infarction (DWI/high, T2WI/isointensity) who then developed multiple brain abscesses (Gd-DTPA/ring enhancement) at 1 month after admission. There was no evidence of infectious endocarditis (IE) on cardiac echo, however, since she demonstrated both fever and Osler’s node, they concluded that she had a bacterial embolism based on IE. Thus, cases of ‘stroke-like’ sudden onset brain abscess have been reported by some authors. On the other hand, an acute onset ISCA with apparent IE has so far only been reported by one author [23]. However, there has been no past description showing radiographical confirmation of an embolism of the spinal artery that resulted in an intramedullary abscess that was confirmed by MRI and then was treated via surgery. Our case had neither obvious findings of IE on cardiac echo nor systemic findings on close inspection. Therefore, it is difficult to disclose the embolic source. However, since his combined findings of MRI and 3D-CTA showed typical spinal infarction at first presentation and his subsequent histopathological findings revealed an abscess in the same lesion, a bacterial embolic cluster on the background of diabetes mellitus might have occluded the branch of the artery. Diffusion-weighted imaging and 3D-CTA or conventional angiography was not available in any of the other reports. The source and biogenesis of the infection were still unknown; however, this sudden onset clinical course and radiographical findings could indicate that the bacterial embolism predisposed the patient to ISCA in the presence of diabetes mellitus without IE.
We delayed the accurate treatment of ISCA because of the initial misdiagnosis and the patient’s severe paraplegia. It might have been helpful to perform a PCR analysis of his cerebrospinal fluid to detect lower levels of bacteria or bacteria that are difficult to culture. Generally, early drainage and the infusion of antibiotics result in a good prognosis [11]. Simon et al. [49] reported that the good prognosis group in children without neurological sequelae had significantly more surgical drainage procedures performed within 5 days after onset of symptoms as compared with the children with a poor prognosis who developed neurological sequelae. Our review also revealed that the good clinical course group had earlier treatment than the poor group, but the difference was not significant. Recently, Kurita et al. [34] noted that there was no significant difference in the frequency of neurological sequelae between surgically and non-surgically treated patients, although the number of cases in their study was small, and the abscesses were significantly more extensive in the surgically treated group. It is therefore recommended that at present, patients should be treated with an appropriate treatment strategy according to the patient’s clinical state.
Acknowledgments
Conflict of interest None of the authors has any potential conflict of interest.
References
- 1.Amacher AL. Intramedullary epidermoid associated with an intramedullary abscess secondary to a dermal sinus [letter] Neurosurgery. 1992;31:979. [PubMed] [Google Scholar]
- 2.Applebee A, Ramundo M, Kirkpatrick BD, Fries TJ, Panitch H, et al. Intramedullary spinal cord abscess in a healthy woman. AAN. 2007;68:1230. doi: 10.1212/01.wnl.0000250231.86932.04. [DOI] [PubMed] [Google Scholar]
- 3.Arzt PK. Abscess within the spinal cord: review of the literature and report of three cases. Arch Neurol Psychiatry. 1944;51:533–543. [Google Scholar]
- 4.Babu R, Jafa JJ, Huang PP, Budzilovich GN, Ransohoff J, et al. Intramedullary abscess associated with a spinal cord ependymoma: case report. Neurosurgery. 1992;30:121–124. doi: 10.1227/00006123-199201000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Bartels RHMA, Gonera EG, Van der Sperk JAN, Thijssen HOM, Mullaart RA, Gabreels FJM, et al. Intramedullary spinal cord abscess: a case report. Spine. 1995;20:1199–1204. doi: 10.1097/00007632-199505150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Bean JR, Walsh JW, Blacker HM, et al. Cervical dermal sinus and intramedullary spinal cord abscess: case report. Neurosurgery. 1979;5:60–62. doi: 10.1227/00006123-197907010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Benzil DL, Epstein MH, Knuckey NW, et al. Intramedullary epidermoid associated with an intramedullary spinal abscess secondary to a dermal sinus. Neurosurgery. 1992;30:118–120. doi: 10.1227/00006123-199201000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Blacklock JB, Hood TW, Maxwell RE, et al. Intramedullary cervical spinal cord abscess: case report. J Neurosurg. 1982;57:270–273. doi: 10.3171/jns.1982.57.2.0270. [DOI] [PubMed] [Google Scholar]
- 9.Byrne RW, Von Roenn KA, Whisler WW, et al. Intramedullary abscess: a report of two cases and a review of the literature. Neurosurg. 1994;35:321–326. doi: 10.1227/00006123-199408000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Carus MEM, Anciones B, Castro A, Lara M, Isla A, et al. Intramedullary spinal cord abscess. J Neurol Neurosurg Psychiatry. 1992;55:225–226. doi: 10.1136/jnnp.55.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CT, Gold WL, et al. Intramedullary abscess of the spinal cord in the antibiotic era: clinical features, microbial etiologies, trends in pathogenesis, and outcomes. Clin Infect Dis. 1998;27:619–626. doi: 10.1086/514699. [DOI] [PubMed] [Google Scholar]
- 12.Chidambaram B, Balasubramaniam V, et al. Intramedullary abscess of the spinal cord. Pediatr Neurosurg. 2001;34:43–44. doi: 10.1159/000055991. [DOI] [PubMed] [Google Scholar]
- 13.Chu JY, Montanera W, Willinsky RA. Listeria spinal cord abscess-clinical and MRI findings. Can J Neurol Sci. 1996;23:220–223. doi: 10.1017/s0317167100038555. [DOI] [PubMed] [Google Scholar]
- 14.Cokca F, Meco O, Arasil E, Unlu A. An intramedullary dermoid cyst abscess due to Brucella abortus biotype 3 at T11–L2 spinal levels. Infection. 1994;22:359–360. doi: 10.1007/BF01715549. [DOI] [PubMed] [Google Scholar]
- 15.Crema MD, Pradel C, Marra MD, Arrive L, Tubiana JM, et al. Intramedullary spinal cord abscess complicating thoracic spondylodiscitis caused by Bacteroides fragilis. Skeletal Radiol. 2007;36:681–683. doi: 10.1007/s00256-006-0260-8. [DOI] [PubMed] [Google Scholar]
- 16.David C, Brasme L, Peruzzi P, Bertault R, Vinsonneau M, Ingrand D, et al. Intramedullary abscess of the spinal cord in a patient with a right-to-left shunt: case report. Clin Infect Dis. 1997;24:89–90. doi: 10.1093/clinids/24.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Derkinderen P, Duval X, Bruneel F, Laissy JP, Regnier B. Intramedullary spinal cord abscess associated with cervical spondylodiskitis and epidural abscess. Scand J Infect Dis. 1998;30:618–619. doi: 10.1080/00365549850161241. [DOI] [PubMed] [Google Scholar]
- 18.Desai KI, Muzumdar DP, Goel A, et al. Holocord intramedullary abscess: an unusual case with review of literature. Spinal Cord. 1999;37:866–870. doi: 10.1038/sj.sc.3100930. [DOI] [PubMed] [Google Scholar]
- 19.DiTullio MV. Intramedullary spinal abscess: a case report with a review of 53 previously described cases. Surg Neurol. 1977;7:351–354. [PubMed] [Google Scholar]
- 20.Durmaz R, Atasoys MA, Durmaz G, Adapinar B, Arslantas A, Aydinli A, Tel E, et al. Multiple nocardial abscess of cerebrum, cerebellum and spinal cord, causing quadriplegia. Clin Neurol Neurosurg. 2001;103:59–62. doi: 10.1016/S0303-8467(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 21.Elmaci I, Kurtkaya O, Peker S, Tuncer N, Adam B, Ozgen S, Ekinci G, Ture U, Pamir N, et al. Cervical spinal cord intramedullary abscess. Case report. J Neurosurg Sci. 2001;45:213–215. [PubMed] [Google Scholar]
- 22.Erlich JH, Rosenfeld JV, Fuller A, Brown GV, Wodak J, Tress BP, et al. Acute intramedullary spinal cord abscess: case report. Surg Neurol. 1992;38:287–290. doi: 10.1016/0090-3019(92)90043-M. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez RM, Lopez MF, Garcia MM, Hornedo MJ, Aguado JM, et al. Intramedullary cervical spinal cord abscess by viridans group Streptococcus secondary to infective endocarditis and facilitated by previous local radiotherapy. Intern Med. 2009;48(1):61–64. doi: 10.2169/internalmedicine.48.1548. [DOI] [PubMed] [Google Scholar]
- 24.Fortuna A, Contratti F, DiLorenzo N, et al. Cervical intramedullary abscess: extirpation by means of microsurgical techniques. J Neurosurg Sci. 1979;23:159–162. [PubMed] [Google Scholar]
- 25.Gero B, Sze G, Sharif H, et al. MR imaging of intradural inflammatory disease of the spine. AJNR. 1991;12:1009–1019. [PMC free article] [PubMed] [Google Scholar]
- 26.Guzel N, Eras M, Guzel DK, et al. A child with spinal intramedullary abscess. Childs Nerv Syst. 2003;19:773–776. doi: 10.1007/s00381-003-0802-5. [DOI] [PubMed] [Google Scholar]
- 27.Hardwidge C, Palsingh J, Williams B, et al. Pyomelia: an intramedullary spinal abscess complicating lumbar lipoma with spina bifida. Br J Neurosurg. 1993;7:419–422. doi: 10.3109/02688699309103498. [DOI] [PubMed] [Google Scholar]
- 28.Hart J. Case of encysted abscess in the center of the spinal cord. Dublin Hosp Rep. 1830;5:522–524. [Google Scholar]
- 29.Helvaci M, KasIrga E, Cetin N, Yaprak I, et al. Intramedullary spinal cord abscess suspected of Brucella infection. Pediatr Int. 2002;44:446–448. doi: 10.1046/j.1442-200X.2002.01569.x. [DOI] [PubMed] [Google Scholar]
- 30.Hott JS, Horn E, Sonntag VK, Coons SW, Shetter A, et al. Intramedullary histoplasmosis spinal cord abscess in a nonendemic region: case report and review of the literature. J Spinal Disord Tech. 2003;16(2):212–215. doi: 10.1097/00024720-200304000-00016. [DOI] [PubMed] [Google Scholar]
- 31.King SJ, Jeffree MA, et al. MRI of an abscess of the cervical spinal cord in a case of Listeria monocytogenes meningoencephalomelitis. Neuroradiology. 1993;35:495–496. doi: 10.1007/BF00588703. [DOI] [PubMed] [Google Scholar]
- 32.Koppel BS, Daras M, Duffy KR, et al. Intramedullary spinal cord abscess. Neurosurgery. 1990;26:145–146. doi: 10.1227/00006123-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Kurisu K, Hida K, Yano S, Yamaguchi S, Motegi Y, Kubota K, Iwasaki Y, et al. The case of a large intra and extra medullary abscess of the spinal cord due to dermal sinus. No Shinkei Geka (Jpn) 2008;36(12):1127–1132. [PubMed] [Google Scholar]
- 34.Kurita N, Sakurai Y, Taniguhi M, Terao T, Takahashi H, Mannen T. Intramedullary spinal cord abscess treated with antibiotic therapy: case report and review. Neurol Med Chir. 2009;49:262–268. doi: 10.2176/nmc.49.262. [DOI] [PubMed] [Google Scholar]
- 35.Lascux AS, Chevalier X, Brugieres P, Levy Y, et al. Painful neck stiffness secondary to an intramedullary abscess of the spinal cord in a HIV infected patient: a case report. J Neurol. 2002;249:229–230. doi: 10.1007/PL00007873. [DOI] [PubMed] [Google Scholar]
- 36.Maheswaran J, Rathinam PV, Lakshimi S, Inbasekaran V, et al. Intramedullary spinal cord abscess complicating a dermal sinus. J Indian Med Assoc. 1991;89:277. [PubMed] [Google Scholar]
- 37.Marwah RK, Khosla VK, Agarwal KC, Kataria S, et al. Intramedullary spinal cord abscess. Indian Pediatr. 1985;22:71–74. [PubMed] [Google Scholar]
- 38.Maurice-Williams RS, Pamphilon D, Coakham HB, et al. Intramedullary abscess—a rare complication of spinal dysraphism. J Neurol Neurosurg Psychiatry. 1980;37:242–244. doi: 10.1136/jnnp.43.11.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menezes AH, Graf CJ, Perret GE, et al. Spinal cord abscess: a review. Surg Neurol. 1977;8:461–467. [PubMed] [Google Scholar]
- 40.Morandi X, Mercier P, Fournier HD, Brassier G, et al. Dermal sinus and intramedullary spinal cord abscess. Report of two cases and review of the literature. Childs Nerv Syst. 1999;15:202–208. doi: 10.1007/s003810050370. [DOI] [PubMed] [Google Scholar]
- 41.Morimoto K, Takemoto O, Nakamura H, Takeuchi M, et al. Spinal dermal sinus associated with intramedullary abscess and dermoid. Pediatr Neurosurg. 2003;39:225–226. doi: 10.1159/000072478. [DOI] [PubMed] [Google Scholar]
- 42.Morrison CR, Brown J, Gooding RS, et al. Spinal cord abscess caused by Listeria monocytogenes. Arch Neurol. 1980;37:242–244. doi: 10.1001/archneur.1980.00500530081015. [DOI] [PubMed] [Google Scholar]
- 43.Mukunda BN, Shekar R, Bass S, et al. Solitary spinal intramedullary abscess caused by Nocardia asteroids. South Med J. 1999;92:1223–1224. doi: 10.1097/00007611-199912000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Murphy KJ, Brunberg JA, Quint DJ, Kazanjian PH, et al. Spinal cord infection: myelitis and abscess formation. Am J Neuroradiol. 1998;19:341–348. [PMC free article] [PubMed] [Google Scholar]
- 45.Novati R, Vigano MG, de Bona A, Nocita B, Finazzi R, Lazzarin A. Neurobrucellosis with spinal cord abscess of the dorsal tract: a case report. Int J Infect Dis. 2002;6:149–150. doi: 10.1016/S1201-9712(02)90079-2. [DOI] [PubMed] [Google Scholar]
- 46.Pfadenhauer K, Rossmanith T, et al. Spinal manifestation of neuroliseriosis. J Neurol. 1995;242:153–156. doi: 10.1007/BF00936888. [DOI] [PubMed] [Google Scholar]
- 47.Rogg JM, Benzil DL, Hass RL, Knuckey NW, et al. Intramedullary abscess: an unusual manifestation of a dermal sinus. Am J Neuroradiol. 1993;14:1393–1395. [PMC free article] [PubMed] [Google Scholar]
- 48.Seizeur R, Condette-Auliac S, Goutagny S, Pencalet P, Gaillard S. Chronic intramedullary abscess (Streptococcus sanguis). A case report and review of the literature. Neurochirurgie. 2006;52(6):542–546. doi: 10.1016/S0028-3770(06)71364-9. [DOI] [PubMed] [Google Scholar]
- 49.Simon JK, Lazareff JA, Diament MJ, Kennedy WA. Intramedullary abscess of the spinal cord in children: a case report and review of the literature. Pediatr Infect Dis J. 2003;22:186–192. doi: 10.1097/01.inf.0000048910.19136.49. [DOI] [PubMed] [Google Scholar]
- 50.Sverzut JM, Laval C, Smadja P, Gigaud M, Sevely A, Manelfe C, et al. Spinal cord abscess in a heroin addict case report. Neuroradiology. 1998;40:455–458. doi: 10.1007/s002340050623. [DOI] [PubMed] [Google Scholar]
- 51.Tewari MK, Devi BI, Thakur RC, Pathak A, Khandelwal N, Kak VK, et al. Intramedullary spinal cord abscess; a case report. Childs Nerv Syst. 1992;38:287–290. doi: 10.1007/BF00300801. [DOI] [PubMed] [Google Scholar]
- 52.Tsurubuchi T, Matsumura A, Nakai K, Fujita K, Enomoto T, Iwasaki N, Nose T, et al. Reversible holocord edema associated with intramedullary spinal abscess secondary to an infected dermoid cyst. Pediatr Neurosurg. 2002;37:282–286. doi: 10.1159/000066306. [DOI] [PubMed] [Google Scholar]
- 53.Vajramani GV, Ngmoti MB, Patil CS, et al. Neurobrucellosis presenting as an intra-medullary spinal cord abscess. Ann Clin Microbiol Antimicrob. 2005;4:14. doi: 10.1186/1476-0711-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vora YA, Raad II, McCutcheon IE (2004) Intramedullary abscess from group F Streptococcus. Surg Infect [Larchmt] 5:200–204 [DOI] [PubMed]
- 55.Weng TI, Shih FY, Chen WJ, Lin FY. Intramedullary abscess of the spinal cord. Am J Emerg Med. 2001;19:177–178. doi: 10.1053/ajem.2001.21343. [DOI] [PubMed] [Google Scholar]
- 56.Yuceer N, Senoglu M, Arda MN (2004) Intramedullary spinal cord abscess in a 4-year old child. Acta Neurochir [Wien] 146:1273–1274 [DOI] [PubMed]
- 57.Mori K, Miwa K, Hara S, Nakashima T, Ueda T, Yokoyama K, Sakai N. A case of a bacterial brain abscess presenting as symptoms of ‘sudden stroke-like’ onset. Noshinkei Geka. 2003;31(4):443–448. [PubMed] [Google Scholar]