Introduction
Recent meta-analyses have shown good long-term survival of patients with hypertrophic obstructive cardiomyopathy (HOCM) after alcohol septal ablation (ASA) comparable with surgical myectomy. However, gradient reduction after ASA was slightly less favourable [1, 2] and in a single-centre study a higher rate of aborted sudden cardiac death was found [3]. The following case report illustrates the importance of coronary anatomy and infarction location in determining the outcome after ASA.
Case report
A 53-year-old male patient was referred to undergo alcohol septal ablation (ASA) for symptoms due to hypertrophic obstructive cardiomyopathy despite medical therapy. His echocardiogram revealed a septal wall thickness of 18 mm, severe systolic anterior motion of the mitral valve and a severe posterolaterally directed mitral valve regurgitation. The gradient in the left ventricular outflow tract was 54 mmHg at rest and 100 mmHg after valsalva manoeuvres.
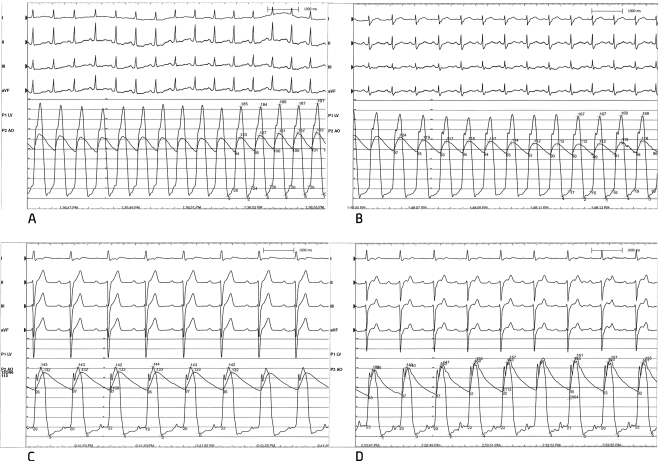
Coronary angiography revealed two septal branches of which the first was very small and could not be cannulated. Subsequently, the second branch (S2) was cannulated (Fig. 1) and myocardial contrast echocardiography (MCE) was used to delineate the perfused area across the septal contact area of the mitral valve. However, during probatory inflation of the balloon the invasively measured gradient was only reduced from 60 mmHg to 50 mmHg (Fig. 2a and 2b). Also after injection of 2 ml of alcohol, the gradient remained elevated at 50 mmHg. Therefore, after perseverance, the smaller and more proximal septal branch was successfully cannulated. After probatory inflation of the balloon, the gradient resolved completely and this septal branch was ablated as well (Fig. 2c). The second ablation resulted in complete resolution of the outflow tract gradient (Fig. 2d), but was offset by the necessity for pacemaker implantation due to total AV block. An MRI was performed three days after the procedure and revealed an infarction of the entire basal part of the septum (Fig. 3). At three months follow-up the echocardiogram revealed a gradient in the outflow tract of only 10 mmHg and the patient no longer experienced shortness of breath.
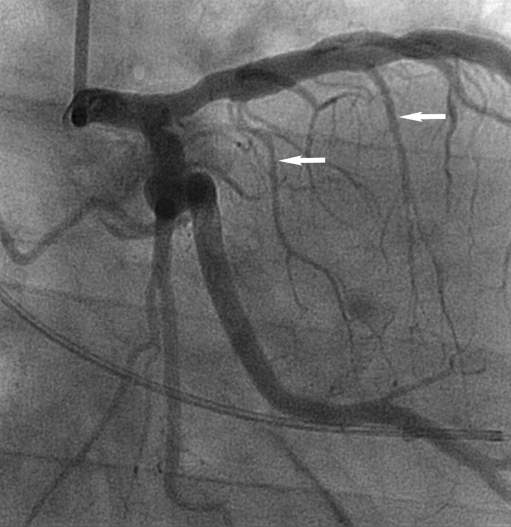
Fig. 1.
Coronary angiogram before ASA. Arrows indicate a proximal and more distal septal branch (S1 and S2)
Fig. 2.
Simultaneous pressure recordings of the pressures in the aorta and left ventricle. The difference between these two reflects the left ventricular outflow tract gradient. a = measurement at baseline. b = measurement during balloon inflation of S2, just before the first ablation. c = measurement during balloon inflation of S1, just before the second ablation. d = measurement after the ablation of both septal branches
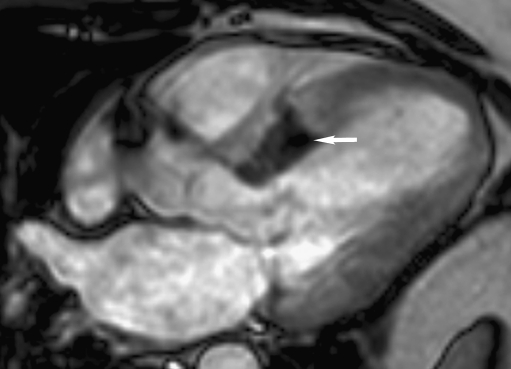
Fig. 3.
MRI of the heart, three-chamber view. The white arrow indicates a basal infarction (dark, not perfused area)
Discussion
Patients with HOCM can exhibit severely reduced exercise tolerance. A prevalvular obstruction is caused by a thickened protruding septum and systolic anterior motion of the mitral valve (SAM). Gradient reduction can be achieved by surgical removal of part of the thickened septum or by ASA. With ASA, alcohol is selectively infused in an appropriate septal branch. This causes infarction and thinning of the septum. First probatory balloon inflation and later myocardial contrast echocardiography (MCE) were introduced for guidance and selection of the appropriate branch for ASA. Probatory balloon inflation in a septal branch leads to temporary ischaemia and reduction of the obstructive gradient but has a limited predictive value [4]. MCE delineates the septal perfusion area which should include the SAM induced mitral valve septal contact area. After the introduction of MCE, the outcome improved and complications were reduced [4, 5]. Despite these techniques, gradient reduction after ASA remains slightly less compared with surgical myectomy [1, 2]. This may be important since higher outflow gradients after ASA are associated with a less favourable long-term haemodynamic outcome [6]. Additionally, even though meta-analyses have shown comparable long-term outcome between ASA and myectomy, in a recently published single-centre study, a higher rate of aborted sudden cardiac death was found after ASA compared with myectomy and therefore myectomy was advised as the preferred treatment [3].
In this case report MCE revealed S2 (Fig. 1) as the appropriate target and this septal branch was therefore ablated. The sustained gradient after ablation of S2 was probably due to the fact that the basal part of the septum was still perfused by the more proximal branch S1. After ablation of S1 in addition to S2 an infarction was caused of the entire basal septum which can be seen on the MRI performed three days after the procedure (Fig. 3). For complete gradient resolution it was necessary to include the perfusion of the basal part of the septum that apparently still formed an obstruction and SAM in the outflow tract. At three-month follow-up, the patient was free from symptoms and the gradient in the outflow tract on echocardiogram was completely reduced to a provoked gradient of 10 mmHg. Complete gradient reduction was, however, offset by necessity for pacemaker implantation due to total AV block.
We conclude that coronary anatomy, infarct location, infarct size and technical difficulty for cannulation of the appropriate septal branch can be important determinants for a successful outcome after ASA. With the increasing rate of use of ASA compared with myectomy, future research is necessary to predict outcome after ASA in order to determine which patients are suitable for ASA.
Acknowledgments
Conflict of Interest All authors have no conflict of interest
Footnotes
Written informed consent was obtained.
Contributor Information
R. C. Steggerda, Phone: +31-065-5506972, Email: r.c.steggerda@mzh.nl
J. C. Balt, Phone: +30-609-9111, FAX: +31-306-034420
J. M. ten Berg, Phone: +30-609-9111, FAX: +31-306-034420
References
- 1.Mahboob A, Hisham D, Nasser ML. Hypertrophic obstructive cardiomyopathy-alcohol septal ablation vs. myectomy: a meta-analysis. Eur Heart J. 2009;30(9):1080–7. doi: 10.1093/eurheartj/ehp016. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, Tuczu EM, Desay MY, et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;55(8):823–34. doi: 10.1016/j.jacc.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Cate FJ, Soliman Ol, Michels M, et al. Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution. Circ Heart Fail. 2010;3(3):362–9. doi: 10.1161/CIRCHEARTFAILURE.109.862359. [DOI] [PubMed] [Google Scholar]
- 4.Faber L, Ziemssen P, Seggewiss H. Targeting percutaneous transluminal septal abalation for hypertrophic obstructive cardiomyopathy by intraprocedural echocardiographic monitoring. J Am Soc Echocardiogr. 2000;13:1074–9. doi: 10.1067/mje.2000.108250. [DOI] [PubMed] [Google Scholar]
- 5.Faber L, Seggewiss H, Gleichmann U. Percutaneous Transluminal Septal Myocardial Ablation in Hypertrophic Obstructive Cardiomyopathy: Results With Respect to Intraprocedural Myocardial Contrast Echocardiography. Circulation. 1998;98:2415–21. doi: 10.1161/01.cir.98.22.2415. [DOI] [PubMed] [Google Scholar]
- 6.Faber L, Welge D, Fassbender D, et al. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response. Clin Res Cardiol. 2007;96(12):851–5. doi: 10.1007/s00392-007-0578-9. [DOI] [PubMed] [Google Scholar]