Abstract
Objectives
The objective of this study is to develop a simple risk score to predict 30-day mortality of aortic valve replacement (AVR).
Methods
In a development set of 673 consecutive patients who underwent AVR between 1990 and 1993, four independent predictors for 30-day mortality were identified: body mass index (BMI) ≥30, BMI <20, previous coronary artery bypass grafting (CABG) and recent myocardial infarction. Based on these predictors, a 30-day mortality risk score—the AVR score—was developed. The AVR score was validated on a validation set of 673 consecutive patients who underwent AVR almost two decennia later in the same hospital.
Results
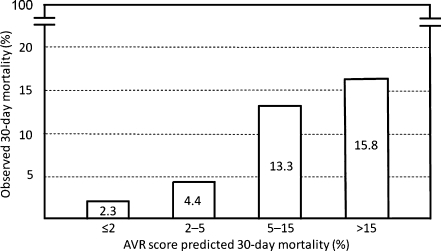
Thirty-day mortality in the development set was ≤2% in the absence of any predictor (class I, low risk), 2–5% in the solitary presence of BMI ≥30 (class II, mild risk), 5–15% in the solitary presence of previous CABG or recent myocardial infarction (class III, moderate risk), and >15% in the solitary presence of BMI <20, or any combination of BMI ≥30, previous CABG or recent myocardial infarction (class IV, high risk). The AVR score correctly predicted 30-day mortality in the validation set: observed 30-day mortality in the validation set was 2.3% in 487 class I patients, 4.4% in 137 class II patients, 13.3% in 30 class III patients and 15.8% in 19 class IV patients.
Conclusions
The AVR score is a simple risk score validated to predict 30-day mortality of AVR.
Keywords: Aortic valve stenosis, Aortic valve replacement, EuroSCORE, STS score
Introduction
The European System for Cardiac Operative Risk Evaluation (EuroSCORE) and its American counterpart, the Society of Thoracic Surgeons (STS) score are currently the most used risk scores to predict operative mortality of cardiac surgery [1, 2]. Besides being complex, these risk scores have not been especially developed for patients undergoing aortic valve replacement (AVR). Therefore, we aimed to develop a risk score that was both simple and specifically applicable to patients undergoing AVR. In addition, similarly to most cardiac surgery centres reporting 30-day mortality, we designed the AVR score to predict 30-day mortality. This is in contrast to the EuroSCORE and STS score, which predict mortality within 30 days or later if the patients are still hospitalised [3, 4].
Methods
Study population
The study population consisted of a development and validation set.
Development set
The development set comprised 673 consecutive adult patients who underwent their first AVR, either isolated or combined with concomitant coronary artery bypass grafting (CABG), in our hospital between 1 January 1990 and 31 December 1993.
Validation set
The validation set comprised 673 consecutive adult patients who underwent their first AVR, either isolated or combined with CABG, in the same hospital between 1 January 2007 and 16 February 2009.
Operative technique
In the development set, all aortic valve replacements were performed by a midsternal approach. In the validation set, however, 85 patients (12.6%) underwent minimal access AVR instead of standard median sternotomy, and in 188 patients (27.9%), minimal extracorporeal circulation was utilised instead of conventional cardiopulmonary bypass. In 82 patients from the validation set (12.2%), minimal access AVR was combined with minimal extracorporeal circulation, as described by Yilmaz et al. [5].
Statistical analysis
Multiple regression analysis (forward stepwise technique) was performed on the development set to identify independent predictors for 30-day mortality. A 30-day mortality risk score—the AVR score—was developed by calculating 30-day mortality for all possible combinations of independent predictors. Calculation of 30-day mortality was done according to the logistic regression equation with the following formula: 30-day mortality risk 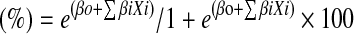 , where ‘e’ is the natural logarithm (2.718281828…), βo is the constant of the regression equation and βi is the set of β coefficients of the independent predictors Xi in the logistic regression equation. The AVR score was validated in the validation set. Discrimination of the AVR score was assessed in both the development and validation sets by the area under the receiver operating characteristic curve (c-statistic). Calibration of the AVR score was assessed by the Hosmer–Lemeshow goodness-of-fit test in the development set and by plotting observed versus predicted 30-day mortality graphically in the validation set. Binary (yes/no) variables were labelled as yes versus no or missing. Missing data on categorical variables were automatically categorised into the lowest risk category. Continuous outcomes and dichotomous variables were analysed using t tests and χ2 tests, respectively. Two-sided tests of significance are reported, and p values <0.05 were considered statistically significant. Data were analysed by SPSS version 17.0 for Windows (SPSS, Inc., Chicago, Illinois).
, where ‘e’ is the natural logarithm (2.718281828…), βo is the constant of the regression equation and βi is the set of β coefficients of the independent predictors Xi in the logistic regression equation. The AVR score was validated in the validation set. Discrimination of the AVR score was assessed in both the development and validation sets by the area under the receiver operating characteristic curve (c-statistic). Calibration of the AVR score was assessed by the Hosmer–Lemeshow goodness-of-fit test in the development set and by plotting observed versus predicted 30-day mortality graphically in the validation set. Binary (yes/no) variables were labelled as yes versus no or missing. Missing data on categorical variables were automatically categorised into the lowest risk category. Continuous outcomes and dichotomous variables were analysed using t tests and χ2 tests, respectively. Two-sided tests of significance are reported, and p values <0.05 were considered statistically significant. Data were analysed by SPSS version 17.0 for Windows (SPSS, Inc., Chicago, Illinois).
Candidate independent predictors for 30-day mortality
The candidate independent predictors for 30-day mortality in the development set were based on previous research, including EuroSCORE and STS score [1, 2, 6–8], and defined as: age, categorised as <70 years (reference), 70–80 years, and ≥80 years; gender; previous CABG; previous percutaneous coronary intervention; previous balloon aortic valvuloplasty; previous valve surgery (surgical or percutaneous cardiac valve replacement or repair, excluding previous AVR); previous cardiac surgery (requiring opening of the pericardium); previous ischaemic stroke; recent myocardial infarction (≤6 weeks before operation); old myocardial infarction (>6 weeks before operation); hypertension (blood pressure >140/90 mmHg or use of antihypertensive medication); chronic lung disease (chronic obstructive pulmonary disease, asthma, or pulmonary fibrosis); carotid disease (transient ischaemic attack, carotid endarterectomy, carotid occlusion, or >50% stenosis); peripheral arterial disease (claudication, surgical or percutaneous intervention to the extremities, excluding carotid disease); diabetes (insulin-dependent or non-insulin-dependent); atrial fibrillation (paroxysmal or persistent/permanent); serum creatinine ≥177 μmol/l (2.0 mg/dl); serum creatinine >200 μmol/l (2.3 mg/dl); dialysis; immunosuppressive therapy (excluding topical applications, one time systemic therapy, or preoperative protocol); body mass index (BMI), categorised as BMI 20–30 (reference), BMI <20, and BMI ≥30; active endocarditis (still on antibiotic treatment at time of surgery); unstable angina; left main disease; New York Heart Association class; predominant aortic stenosis; predominant aortic regurgitation; bicuspid aortic valve; moderate left ventricular function (ejection fraction 30–50%); poor left ventricular function (ejection fraction <30%); severe pulmonary hypertension (mean pulmonary artery pressure >40 mmHg); urgent operation (within days); emergency operation (before next working day) and concomitant CABG.
Results
Preoperative and operative characteristics of the 673 patients in the development set and 673 patients in the validation set are depicted in Table 1. The patients in the validation set were older and had more adverse risk factors, resulting in a higher EuroSCORE and STS score. Four patients (0.6%) in the development set and two (0.3%) in the validation set died during the operation. Three patients (0.4%) in the development set and one (0.1%) in the validation set survived the operation, but died within 72 h from operation. Twenty-six patients (3.9%) in the development set and 31 (4.6%) in the validation set died while they were still admitted (in-hospital mortality). Thirty-day mortality in the development set was 2.8% (19 deaths) and 3.6% (24 deaths) in the validation set. Mortality within 30 days from AVR or later if the patients are still hospitalised (operative mortality as defined by the EuroSCORE and STS score) was 3.9% (26 deaths) in the development set and 4.6% (31 deaths) in the validation set.
Table 1.
Pre-operative and operative patient characteristics
| Variable | Development set (n = 673) | Validation set (n = 673) | P value |
|---|---|---|---|
| Age, years | 65.4 ± 10.7 | 70.6 ± 10.6 | <0.001 |
| Male | 385 (57.2) | 404 (60.0) | 0.319 |
| STS score | 1.9 ± 1.6 | 2.2 ± 1.7 | 0.004 |
| Logistic EuroSCORE | 5.0 ± 5.1 | 8.1 ± 7.3 | <0.001 |
| Additive EuroSCORE | 5.5 ± 2.4 | 6.5 ± 2.6 | <0.001 |
| NYHA class | 2.5 ± 0.9 | 2.7 ± 0.7 | 0.001 |
| Missing | 4 (0.6) | 547 (81.3) | |
| Predominant aortic stenosis | 562 (83.5) | 593 (88.1) | 0.019 |
| Predominant aortic regurgitation | 111 (16.5) | 80 (11.9) | 0.019 |
| Previous intervention | |||
| CABG | 26 (3.9) | 39 (5.8) | 0.126 |
| PCI | 16 (2.4) | 60 (8.9) | <0.001 |
| Balloon aortic valvuloplasty | 3 (0.4) | 2 (0.3) | 1.000 |
| Valve surgerya | 9 (1.3) | 7 (1.0) | 0.802 |
| Cardiac surgerya | 37 (5.5) | 47 (7.0) | 0.311 |
| Previous ischaemic stroke | 27 (4.0) | 28 (4.2) | 0.892 |
| Recent myocardial infarctiona | 12 (1.8) | 6 (0.9) | 0.235 |
| Old myocardial infarctiona | 83 (12.3) | 63 (9.4) | 0.096 |
| Hypertensiona | 333 (49.5) | 267 (39.7) | <0.001 |
| Missing | − | 402 (59.7) | |
| Chronic lung diseasea | 79 (11.7) | 97 (14.4) | 0.169 |
| Carotid diseasea | 63 (9.4) | 109 (16.2) | <0.001 |
| Peripheral arterial diseasea | 47 (7.0) | 66 (9.8) | 0.076 |
| Insulin-dependent diabetes | 18 (2.7) | 38 (5.6) | 0.009 |
| Non-insulin-dependent diabetes | 49 (7.3) | 94 (14.0) | <0.001 |
| Paroxysmal atrial fibrillation | 66 (9.8) | 57 (8.5) | 0.449 |
| Persistent/permanent atrial fibrillation | 38 (5.6) | 51 (7.6) | 0.188 |
| Serum creatinine (μmol/l) | 97.2 ± 44.2b | 88.4 ± 35.4c | <0.001 |
| Dialysis | 3 (0.4) | 7 (1.0) | 0.342 |
| Immunosuppressive therapya | 16 (2.4) | 23 (3.4) | 0.330 |
| Body mass index | 26 ± 3 | 27 ± 4 | <0.001 |
| <20 | 9 (1.3) | 6 (0.9) | 0.605 |
| 20–30 | 586 (87.1) | 520 (77.3) | <0.001 |
| ≥30 | 78 (11.6) | 147 (21.8) | <0.001 |
| Active endocarditis | 4 (0.6) | 12 (1.8) | 0.075 |
| Unstable angina | 36 (5.3) | 8 (1.2) | <0.001 |
| Left main disease | 13 (1.9) | 12 (1.8) | 1.000 |
| Aortic valve area (cm2)d | 0.73 ± 0.28 | 0.80 ± 0.22 | <0.001 |
| Missing | 327 (48.6) | 109 (16.2) | |
| Left ventricular ejection fractiond | |||
| ≥50% | 566 (84.1) | 519 (77.1) | 0.001 |
| 30–50% | 78 (11.6) | 109 (16.2) | 0.018 |
| <30% | 29 (4.3) | 45 (6.7) | 0.072 |
| Severe pulmonary hypertensiona | 24 (3.6) | 32 (4.8) | 0.339 |
| Missing | 102 (15.2) | − | |
| Urgent/emergency operationa | 28 (4.2) | 20 (3.0) | 0.244 |
| Concomitant CABG | 194 (28.8) | 300 (44.6) | <0.001 |
Values are presented as mean ± standard deviation or n (%)
CABG coronary artery bypass grafting, PCI percutaneous coronary intervention, NYHA New York Heart Association, STS Society of Thoracic Surgeons
aSee text for explanation
b1.1 ± 0.5 mg/dl
c1.0 ± 0.4 mg/dl
dOn echocardiography
AVR score development
Multiple regression analysis performed on the development set demonstrated the following variables to be independent predictors for 30-day mortality (in descending order of significance): BMI <20, previous CABG, recent myocardial infarction, and BMI ≥30 (Table 2). Calculation of predicted 30-day mortality for all possible combinations of independent predictors resulted in the AVR score, as shown in Table 3. Thirty-day mortality was ≤2% in the absence of any of these predictors (class I, low risk), 2–5% in the solitary presence of BMI ≥30 (class II, mild risk), 5–15% in the solitary presence of previous CABG or recent myocardial infarction (class III, moderate risk), and >15% in the solitary presence of BMI <20, or any combination of BMI ≥30, previous CABG, or recent myocardial infarction (class IV, high risk). The c-index of the AVR score was 0.73, implying the AVR score to accurately distinguish between patients who died versus those who survived in the development set. The p value of the Hosmer–Lemeshow test of the AVR score was 0.67, implying the AVR score to accurately predict 30-day mortality in the development set.
Table 2.
Independent predictors for 30-day mortality of AVR in the development set (n = 673)
| Predictor | β coefficient | Odds ratio (95% CI) | P value |
|---|---|---|---|
| BMI <20 | 3.093 | 22.04 (3.95–123.01) | <0.001 |
| Previous CABG | 2.386 | 10.87 (3.20–36.90) | <0.001 |
| Recent myocardial infarction | 2.185 | 8.90 (1.76–45.05) | 0.008 |
| BMI ≥30 | 1.309 | 3.70 (1.21–11.30) | 0.021 |
| Constant | −4.346 |
AVR aortic valve replacement, CI confidence interval, CABG coronary artery bypass grafting, BMI body mass index
Table 3.
The AVR score
| Predictor (BMI ≥30, previous CABG, recent myocardial infarction, BMI <20) | AVR score predicted 30-day mortality (%) | AVR score risk class |
|---|---|---|
| None | ≤2 | I (low risk) |
| BMI ≥30 | 2–5 | II (mild risk) |
| Previous CABG | 5–15 | III (moderate risk) |
| Recent myocardial infarction | 5–15 | III (moderate risk) |
| BMI <20 | >15 | IV (high risk) |
| Any combination of BMI ≥30, previous CABG, or recent myocardial infarction | >15 | IV (high risk) |
AVR aortic valve replacement, BMI body mass index, CABG coronary artery bypass grafting
AVR score validation
Observed 30-day mortality in the validation set was 2.3% in 487 class I patients (11 out of 487), 4.4% in 137 class II patients (6 out of 137), 13.3% in 30 class III patients (4 out of 30) and 15.8% in 19 class IV patients (3 out of 19). The c-index of the AVR score in the validation set was 0.66, implying a moderate ability of the AVR score to distinguish between patients who died versus those who survived. The AVR score predicted 30-day mortality in the validation set correctly, as shown by the calibration plot of observed versus AVR score predicted 30-day mortality, depicted in Fig. 1.
Fig. 1.
Calibration plot of observed versus AVR score predicted 30-day mortality of the 673 patients in the validation set who underwent AVR
EuroSCORE and STS score for the different subpopulations of the validation set
In the subpopulation of 487 class I patients with a predicted 30-day mortality of ≤2% and an observed 30-day mortality of 2.3%, the logistic EuroSCORE, additive EuroSCORE and STS score were 7.3 ± 6.5%, 6.3 ± 2.5% and 2.1 ± 1.6%, respectively. In the subpopulation of 137 class II patients with a predicted 30-day mortality of 2–5% and an observed 30-day mortality of 4.4%, the logistic EuroSCORE, additive EuroSCORE and STS score were 7.0 ± 5.6%, 6.3 ± 2.2% and 2.0 ± 1.5%, respectively. In the subpopulation of 30 class III patients with a predicted 30-day mortality of 5–15% and an observed 30-day mortality of 13.3%, the logistic EuroSCORE, additive EuroSCORE and STS score were 19.9 ± 8.7%, 10.4 ± 1.8% and 3.9 ± 2.3%, respectively. In the subpopulation of 19 class IV patients with a predicted 30-day mortality of >15% and an observed 30-day mortality of 15.8%, the logistic EuroSCORE, additive EuroSCORE and STS score were 17.9 ± 9.6%, 9.7 ± 2.4% and 3.3 ± 1.6%, respectively.
Discussion
In this study, the AVR score is presented, a simple risk score to predict 30-day mortality of AVR. This risk score can serve as an adjunct to the current but more complex risk scores, EuroSCORE and STS score, for providing a first, ‘quick-look’ impression of 30-day mortality of AVR.
The AVR score was developed in a development set and validated on a validation set of patients undergoing AVR. The development set, however, differed in several ways from the validation set. The patients in the validation set were operated upon almost two decennia later than the patients in the development set. Also, their risk profiles were higher, having a higher age and more adverse risk factors, resulting in a higher EuroSCORE and STS score. Despite these differences, the AVR score performed well. So, the predicting power of the independent AVR score predictors BMI ≥30, BMI <20, previous CABG, and recent myocardial infarction seemed to have remained constant over the last two decennia. Although the adverse effect of a low BMI on 30-day mortality of AVR was also recognised by Florath et al. [9], (extreme) obesity is more widely assumed to be an adverse risk factor [10, 11]. Several explanations for a low BMI being an adverse risk factor for early postoperative death have been proposed. Patients with a low percentage of body fat may have less nutritional reserve to cope with complications. Also, due to more haemodilution by a fixed bypass circuit during cardiopulmonary bypass, patients with a low BMI may experience greater postoperative weight gain and transfusion requirements [12]. A (very) high BMI may cause more postoperative death by complications such as deep sternal infection, renal failure, or respiratory failure [13]. Previous CABG is known to increase the risk of in-hospital mortality after redo cardiac surgery by intraoperative adverse events such as injury to bypass grafts, heart or great vessels [14]. Patients requiring AVR after previous CABG seem to be particularly at risk, possibly due to older age and the presence of left ventricular hypertrophy [15]. Recent myocardial infarction is acknowledged to be an adverse risk factor for early postoperative death after cardiac surgery, however only in patients with unstable angina undergoing CABG [16, 17]. To our knowledge, the presented study is the first to combine these separate risk factors into a simple risk score for patients undergoing AVR.
Limitations
The AVR score is not applicable to very high risk or very old patients because the number of these patients in the development set was relatively low: only four patients (0.6%) with STS score ≥10%, 11 (1.6%) with logistic EuroSCORE ≥20%, and 34 (5.1%) with age ≥80 years. Also, in contrast with the EuroSCORE and STS score, the AVR score is only applicable to patients undergoing AVR, with or without concomitant CABG. Both the EuroSCORE and STS score were based on thousands of patients referred by numerous centres. The AVR score was based on a relatively small number of patients from a single centre. As a logical consequence, the number of deaths was also small, hampering the power of this study.
Conclusion
The AVR score is a simple risk score to predict 30-day mortality of AVR.
Acknowledgements
We would like to thank Yvonne van Hees, MSc for her efforts in developing the databases and entering data. Furthermore, we are grateful to Diane Vermeulen, MD, MSc for her efforts in entering data, and to Geert J.M.G. van der Heijden, MD, MSc and Henry A. van Swieten, MD, PhD for their efforts in developing the databases.
Disclosures This work was supported by Stichting Hartenzorg Sint Antonius, Nieuwegein; Stichting Nuts Ohra; and Jacques de Jong Stichting, all from the Netherlands.
Footnotes
Presented at the scientific spring meeting of the Dutch Society of Thoracic Surgery (NVT) on 21 May 2010 in Utrecht, the Netherlands.
References
- 1.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/S1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 2.Edwards FH, Clark RE, Schwartz M. Coronary artery bypass grafting: the Society of Thoracic Surgeons National Database experience. Ann Thorac Surg. 1994;57:12–19. doi: 10.1016/0003-4975(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 3.Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–823. doi: 10.1016/S1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 4.Shahian DM, O’Brien SM, Filardo G, Society of Thoracic Surgeons Quality Measurement Task Force et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1–coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz A, Rehman A, Sonker U, et al. Minimal access aortic valve replacement using a minimal extracorporeal circulatory system. Ann Thorac Surg. 2009;87:720–725. doi: 10.1016/j.athoracsur.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Christakis GT, Weisel RD, David TE, et al. Predictors of operative survival after valve replacement. Circulation. 1988;78(3 Pt 2):I25–I34. [PubMed] [Google Scholar]
- 7.Rankin JS, Hammill BG, Ferguson TB, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006;131:547–557. doi: 10.1016/j.jtcvs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 8.Tjang YS, Hees Y, Körfer R, et al. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg. 2007;32:469–474. doi: 10.1016/j.ejcts.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Florath I, Albert AA, Rosendahl UP, et al. Body mass index: a risk factor for 30-day or six-month mortality in patients undergoing aortic valve replacement? J Heart Valve Dis. 2006;15:336–344. [PubMed] [Google Scholar]
- 10.Villavicencio MA, Sundt TM, 3rd, Daly RC, et al. Cardiac surgery in patients with body mass index of 50 or greater. Ann Thorac Surg. 2007;83:1403–1411. doi: 10.1016/j.athoracsur.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 11.Florath I, Rosendahl UP, Mortasawi A, et al. Current determinants of operative mortality in 1400 patients requiring aortic valve replacement. Ann Thorac Surg. 2003;76:75–83. doi: 10.1016/S0003-4975(03)00341-2. [DOI] [PubMed] [Google Scholar]
- 12.Engelman DT, Adams DH, Byrne JG, et al. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999;118:866–873. doi: 10.1016/S0022-5223(99)70056-5. [DOI] [PubMed] [Google Scholar]
- 13.Tyson GH, 3rd, Rodriguez E, Elci OC, et al. Cardiac procedures in patients with a body mass index exceeding 45: outcomes and long-term results. Ann Thorac Surg. 2007;84:3–9. doi: 10.1016/j.athoracsur.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Roselli EE, Pettersson GB, Blackstone EH, et al. Adverse events during reoperative cardiac surgery: frequency, characterization, and rescue. J Thorac Cardiovasc Surg. 2008;135:316–323. doi: 10.1016/j.jtcvs.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Odell JA, Mullany CJ, Schaff HV, et al. Aortic valve replacement after previous coronary artery bypass grafting. Ann Thorac Surg. 1996;62:1424–1430. doi: 10.1016/0003-4975(96)00635-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Murrell HK, Strony J, et al. Risk analysis of coronary bypass surgery after acute myocardial infarction. Surgery. 1997;122:675–681. doi: 10.1016/S0039-6060(97)90073-8. [DOI] [PubMed] [Google Scholar]
- 17.Fremes SE, Goldman BS, Weisel RD, et al. Recent preoperative myocardial infarction increases the risk of surgery for unstable angina. J Card Surg. 1991;6:2–12. doi: 10.1111/j.1540-8191.1991.tb00557.x. [DOI] [PubMed] [Google Scholar]