Abstract
Rationale
Attempts to simultaneously control food intake and smoking may lead to smoking cessation failure. We sought to model this relationship using a human laboratory paradigm of smoking lapse behavior.
Objectives
We examined the combined effect of food and nicotine deprivation, compared to nicotine deprivation alone, on the ability to resist smoking and on subsequent ad libitum smoking.
Methods
In a between-subjects design, daily smokers (N = 30) were all deprived of nicotine for 18 hours and were either food deprived (12 hrs) or not during a laboratory session. Following exposure to individualized food cues, participants had the option of initiating tobacco self-administration or delaying up to 50 minutes in exchange for monetary reinforcement. Subsequently, the tobacco self-administration period consisted of one-hour in which participants could choose to smoke or receive monetary reinforcement for cigarettes not smoked.
Results
Smokers who had been deprived of food and nicotine smoked their first cigarette sooner and were more likely to smoke at some point during the laboratory session, compared to those who were only nicotine deprived. Those who were food and nicotine deprived smoked slightly more cigarettes than those who were nicotine deprived only, although this difference was not statistically significant. There were no sex differences in outcomes. Hunger and food craving ratings while trying to resist smoking were greater in the food + nicotine deprived group. Tobacco craving was predictive of outcome in both conditions.
Conclusions
These findings support the hypothesis that food deprivation can undermine a smoker’s ability to resist smoking.
Keywords: Tobacco, nicotine, smoking, lapse behavior, human laboratory, food, deprivation, cigarette, cue, craving
Introduction
Although the benefits of smoking cessation are well documented, many smokers are discouraged from quitting by the prospect of gaining weight. Concerns about weight gain are related to a lower likelihood of making a quit attempt (Klesges et al. 1988b; Pomerleau et al 2001; Weekley et al. 1992), attrition in cessation studies (Leeman et al. 2006) and relapse in some (Jeffery et al. 2000 [only in bivariate analyses in females]; Klesges et al., 1988a; Meyers et al. 1997), but not all studies (Borrelli and Mermelstein, 1998; French et al. 1992; Glasgow et al. 1999; Jeffery et al. 1997).
There is evidence that simultaneous food and smoking restriction is detrimental to smoking cessation. Perkins et al. (2001) randomized female, weight-concerned smokers to standard care, cognitive behavioral therapy (CBT) to reduce weight concerns or behavioral treatment to prevent weight gain (i.e., weight control). Compared to standard care, the CBT condition had higher rates of smoking cessation, whereas the weight control condition did not. These findings suggest that weight control did not benefit smoking cessation in this study.
Several hypotheses have been proposed to explain why weight concerned individuals are at risk of relapse and why interventions that propose dietary restraint may be counterproductive. One hypothesis is that “the combined psychological cost of weight control and smoking abstinence efforts outweighs the benefits of abstinence” (Hall et al. 1986, p. 346). There is a growing literature suggesting that self-control is a limited resource, thus efforts to control one behavior reduce the ability to inhibit a second behavior (Muraven and Baumeister 2000).
There has been a great deal of research concerning the mechanisms underlying post-cessation weight gain (Grunberg et al. 1992; Hall et al. 1986; Perkins et al. 1990; 1995), but relatively little laboratory research on the converse question of how food restriction affects smoking. Two laboratory studies with small sample sizes failed to find an effect of three days of restricted feeding on ad libitum smoking behavior (Lawson et al. 1997; Zacny and de Wit 1992). A larger study testing four types of six-day diets found—compared with a normal diet—slightly higher ad libitum smoking and expired breath carbon monoxide levels (CO) with a low-calorie/ balanced diet and slightly higher CO with a low carbohydrate, low calorie diet (Cheskin et al. 2005). Another small study (Zacny and de Wit 1990) and a larger study of females (Kendzor et al. 2008) found that CO levels, but not ad libitum smoking, were higher in a 24-hour food deprived condition, compared to normal food intake.
By manipulating food and nicotine deprivation in the same experiment, researchers can model cessation attempts when smokers simultaneously restrict their eating. Only two such studies were found (Alsene et al. 2003; Epstein et al. 1991). In Epstein et al., 8 female smokers were evaluated over four separate days in which they were either nicotine deprived (16 hours), food deprived (7 hours), both food and nicotine deprived, or not deprived. During sessions, smokers could work for either food or puffs of cigarettes on a progressive ratio schedule. Participants were more likely to work for food when food deprived and for cigarettes when smoking deprived. In the joint deprivation condition, participants initially worked for food, then shifted to smoking. These findings suggest that the reinforcing value of both food and cigarettes to smokers increases under deprivation.
Alsene et al. (2003) tested the hypothesis that combined food and nicotine deprivation would increase smoking urges in 15 smokers (9 males) who participated in four laboratory sessions in which they were either food deprived, nicotine deprived, food and nicotine deprived, or non-deprived for 18 hours prior to the session. During the sessions, participants viewed slides of food cues, smoking cues, or neutral cues and rated their food and smoking urges. Food deprivation increased food craving in response to food cues and nicotine deprivation increased smoking craving in response to smoking cues. The highest smoking craving levels were in the combined food and nicotine deprived group. These data support the notion that restraining eating while abstaining from nicotine may increase relapse risk.
None of these studies, however, directly examined the influence of food deprivation on the ability to resist smoking, or early lapse behavior. The “first lapse” or “slip” is one of the best predictors of relapse (Brandon et al. 1990; Garvey et al. 1992; Kenford et al. 1994). Given the importance of early smoking lapse, our group developed a human laboratory model of smoking lapse behavior (McKee 2009; McKee et al. 2006). In this model, participants have the option of initiating a tobacco self-administration session or delaying initiation for up to 50 minutes in exchange for monetary reinforcement. The subsequent tobacco self-administration session is a one-hour period in which subjects could choose to smoke their preferred brand of cigarettes ad libitum or receive monetary reinforcement for cigarettes not smoked. This paradigm has been used to demonstrate that alcohol consumption, stress, and nicotine deprivation decrease ability to resist the first cigarette and increase the number of cigarettes smoked, thus modeling in the laboratory what has been demonstrated clinically (McKee, 2009; McKee et al. 2006).
The goal of the present study was to test the effect of a 12-hour period of food deprivation, in combination with 18 hours of nicotine deprivation, on ability to resist smoking and on number of cigarettes smoked in the laboratory. The opportunity to smoke came after a task designed to elicit food craving. The inclusion of this task ensured that all participants thought about food consumption for an extended period of time at least once during the session. In addition to testing the effect of food deprivation on smoking behavior, we examined the following potential mechanisms underlying a link between food and nicotine deprivation and smoking: tobacco and food craving, nicotine withdrawal and hunger. Our hypotheses were that food and nicotine deprived participants would smoke their first cigarette sooner and smoke more cigarettes in the laboratory than participants deprived of only nicotine.
Methods
Participants
Participants were recruited through advertisements in local newspapers, postings on websites (e.g., Craigslist), flyers and word of mouth. Smokers were eligible to enroll if they were 18 to 55 years old; not seeking smoking cessation treatment; smoked 10–30 cigarettes per day and presented with a CO reading ≥ 10ppm. Individuals were excluded if they reported a current medical condition that would contraindicate smoking or food deprivation (e.g., cardiac abnormalities or diabetes); presented with a body mass index < 18.5 or > 35; currently met criteria for substance abuse or dependence except for nicotine dependence and alcohol abuse; met lifetime criteria for an eating disorder; presented as suicidal, homicidal or with current severe mental illness; provided a urine test result indicative of illegal drug use; reported regular use of psychoactive drugs including anxiolytics and antidepressants or receipt of a prescription for any psychotropic drug in the prior 30 days; were pregnant or nursing; recently donated blood or participated in a study with additive blood sampling.
Thirty participants (16 male) completed the laboratory session that is the subject of the present report, as well as a subsequent smoking laboratory session involving nicotine deprivation and food deprivation/non-deprivation, the results of which are not presented here. Participants had an average age of 32.07 (SD = 11.33) and were mostly Caucasian (66.7%, 26.6% African-American, 6.7% “other”), with at least some college (58.6%, 41.4% high school or GED) and currently unmarried (86.7%). Mean household income was 4.93 (SD = 2.45) on a 9-point scale. A response of “5” equated to a range of $20,000–$39,000 per year. At initial screening, on average, they reported 17.55 cigarettes per day (SD = 4.26); gave expired carbon monoxide (CO) readings of 27.14 ppm (SD = 15.36) and Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991) scores of 4.87 (SD = 2.05).
Procedures
This study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Screening session
Written informed consent was obtained from all participants. The Structured Clinical Interview for DSM-IV (SCID; First et al. 1995) was used to exclude individuals who met diagnostic criteria for alcohol dependence or other Axis I disorders. The FTND was included in a self-report battery. The Timeline Followback interview (TLFB; Sobell and Sobell 2003) was used to assess past 30-day smoking. CO readings (Micro CO Meter, MicroDirect, Lewiston, ME, USA), breath alcohol (Alco-Sensor III, Intoximeter, St. Louis, MI, USA), urine toxicology and urine pregnancy tests for women were conducted. Participants were paid $20 for attendance if breath alcohol was 0.00% and urine toxicology was negative.
Laboratory session
Each participant completed a 12-h, 45-min laboratory session (i.e., food and nicotine deprivation or nicotine deprivation only), which took place in a patient room at the Hospital Research Unit of Yale-New Haven Hospital. Prior to the session, participants were asked to abstain from food and cigarettes beginning at bedtime the night before, but no later than 1:00am. Participants were paid $10 for on-time arrival to the research unit and $125 for completion of the laboratory session.
Table 1 shows key laboratory procedures and time points when self-reports were administered. Overnight abstinence from cigarettes was confirmed biochemically with CO readings (i.e., no more than 50% of initial screening level) at the outset of the laboratory session and by serum nicotine at 6:30pm (Table 1). Serum nicotine was measured by reversed-phase HPLC with UV detection, modified from the literature (Hariharan et al. 1988) to include a micro acid back extraction cleanup step which allows for cleaner chromatograms. The lower limit of quantitation for nicotine was set to 4 ng/ml. Serum nicotine levels for all participants were below this level. Two participants reported overnight abstinence that could not be confirmed via CO readings, however they were retained in the study because their serum nicotine levels were less than 4 ng/ml prior to initiation of the smoking period, consistent with extended abstinence. Participants were paid $20 for biochemically-verified overnight abstinence in the earlier portion of the study (n = 16), which was increased to $50 in the latter portion (n = 14) to provide additional incentive for overnight abstinence. There were no differences in any of the main outcome analyses based on participation before or after the compensation change.
Table 1.
Outline of key laboratory session procedures and administration of self-reports
| Time | Procedure |
|---|---|
| 8:30am | Participants arrive. Cigarettes, lighters and matches confiscated. Baseline assessments of breath CO, breath alcohol, urine toxicology, urine pregnancy screen, height and weight obtained. |
| 9:00am | Participants in both study conditions provided a standard breakfast |
| 12:00pm | Lunch given to participants in nicotine deprived alone condition |
| 6:00pm | Dinner given to participants in nicotine deprived alone condition |
| 6:30pm | Blood draw to assay serum nicotine |
| 6:45pm | Pre-food craving priming task self-report packet, then food craving priming task (assessment of food craving during task) |
| 6:55pm | Post-food craving priming task/pre-delay period self-report packet (withdrawal measure omitted) |
| 7:05pm | Delay period begins, participants can smoke at any time they choose |
| 7:55pm/end of delay | End of delay period/+0 self-report packet, then smoking self-administration period begins |
| 30-min later | +30 self-report packet (withdrawal measure omitted) |
| 60-min later | + 60 self-report packet |
| 9:15pm | Dismissal |
Baseline assessment period
Baseline breath alcohol, pregnancy, and toxicology taken at the outset were all required to be negative. A small breakfast including one cup of caffeinated coffee was provided to participants at 9:00am regardless of study condition to standardize time and amount of last food consumption. From 12:30pm–6:45pm, vital signs were collected and self-reports were administered at regular intervals (every 1–2 hours). During the 10-h period from 8:30am–6:30pm, participants were not able to smoke but were able to watch TV, listen to the radio, read and do puzzles or written work.
Study conditions
Participants randomized to nicotine deprivation only could choose from the same meal menu as patients at the hospital and could select any food other than caffeinated beverages. They also had open access to snacks (e.g., yogurt, graham crackers) and drinks (e.g., milk, juice, decaffeinated coffee and caffeine free soda) provided by the hospital. No outside food or drink was allowed during the laboratory session, regardless of condition. For participants randomized to the food deprivation condition, no food of any kind was provided after breakfast. Participants had open access only to water and black decaffeinated coffee.
Food craving priming task
At the initial screening, participants rated their liking for 18 snacks (e.g., pretzels, brownies) on a five-point scale from “not at all” to “extremely.” Five highly rated snacks were used as food cues in a craving priming task conducted shortly before participants had the opportunity to smoke. The snacks were placed in a basket in front of the participant. Participants held each snack one at a time for 30 seconds and were instructed to think about what it would be like to eat the snack, how it would taste and how much they would enjoy eating it. Similar instructions were used by Sobik et al. (2005). After 30 seconds, participants rated their craving for each snack on a 0–100 visual analog scale (VAS). This process continued with all five snacks. The snack basket remained in the room with the participant for the rest of the session. Regardless of condition, participants were not allowed to eat the snacks, although they were allowed to take them at the end of the session.
Delay period
At 7:05pm., participants were presented a tray containing eight cigarettes of their preferred brand and an ashtray. Participants were instructed that they could begin smoking at any point over the next 50 min. For each 5-min that they delayed or “resisted” smoking, they earned $1 for a maximum of $10 over the next 50 min. We recorded the time (in minutes) until participants announced to the experimenter that they wanted to smoke (range 0–50 min). For participants opting not to smoke for the entire delay period, the length of their delay was entered as 50 min. At the end of the delay period, participants completed a self-report packet, gave a breath CO reading and then were handed a lighter by the experimenter.
Smoking self-administration period
The smoking self-administration period was 60 min in length and started once participants decided to end the delay period (or delayed for the full 50-min). Participants were instructed to “smoke as little or as much as you wish.” All cigarettes were smoked using topography equipment. About halfway through data collection we learned that the equipment could not be calibrated properly and would not yield valid readings, however we continued to use the equipment so that the smoking experience would be consistent across all participants. They were further instructed that for each cigarette they lit, it would cost them $1 of their $8 “smoking tab.” Money earned for delaying smoking and any unused portion of the “smoking tab” was paid to the participants at the end of the laboratory session.
Measures administered during the laboratory session
Tobacco craving was assessed with the Questionnaire of Smoking Urges-Brief (QSU-B; Cox et al. 2001), made up of 10 items to evaluate urges to smoke in response to positive (Factor 1; α = .92) or negative (Factor 2; α = .90) reinforcement, rated using a VAS with a 0–100 range. DSM-IV nicotine withdrawal symptoms were assessed with six items from the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami 1986). Participants rated items on a 0 (none) to 4 (severe) scale as to their degree of current withdrawal. The items “craving for cigarettes” and “increased appetite” were omitted to keep the withdrawal construct separate from the tobacco craving and hunger constructs (α = .90). Withdrawal was not assessed at each time point (see Table 1). Hunger and food craving were assessed to provide manipulation checks for the food deprivation condition and for the food craving priming task. Hunger was rated using three items from the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) rated on a 0–100 VAS anchored with “strongly disagree” and “strongly agree” (“I want to nibble on snacks and sweets;” “I feel hungry;” “I have been thinking about food a lot;” α = .95). Participants reported their current level of craving for five preferred snacks using a 0–100 VAS anchored with “not at all” and “extremely” (α = .92). Scores for all measures were derived by taking a mean of the individual items, except for withdrawal, which was a sum score.
Analysis plan
Independent samples t-tests were used to assess differences in the primary outcome variables—length of the delay to the first cigarette and number of cigarettes smoked ad libitum in the self-administration period—by study condition. No significant differences by study condition were found on self-report measures administered at initial screening, thus none of these variables were held constant in any subsequent analyses.
Mixed model ANOVAs were used to test for main effects of time and study condition and for time × condition interactions on self-report measures administered during the laboratory session (i.e., secondary outcomes). No significant effects of sex were detected for any of these analyses, thus sex was omitted from the results reported below. Three separate sets of analyses were conducted to compare scores at the following time points: 1) Pre to post-food craving priming task (except for food craving, which was assessed during the task); 2) Pre to post-delay period. The post-food craving time point was used for the pre-delay time point; 3) The smoking self-administration period (+0, +30 minutes, +60 minutes). The post-delay period time point was used as the +0 time point.
Exploratory analyses were also conducted. Within each condition, Pearson correlation coefficients were used to assess associations between the outcome variables (i.e., length of delay, amount smoked) and self-report variables assessed at initial screening and during the laboratory session. We omitted correlations with self-reports collected during the self-administration period to avoid results being confounded by varying levels of smoking across participants.
Results
Primary outcome analyses
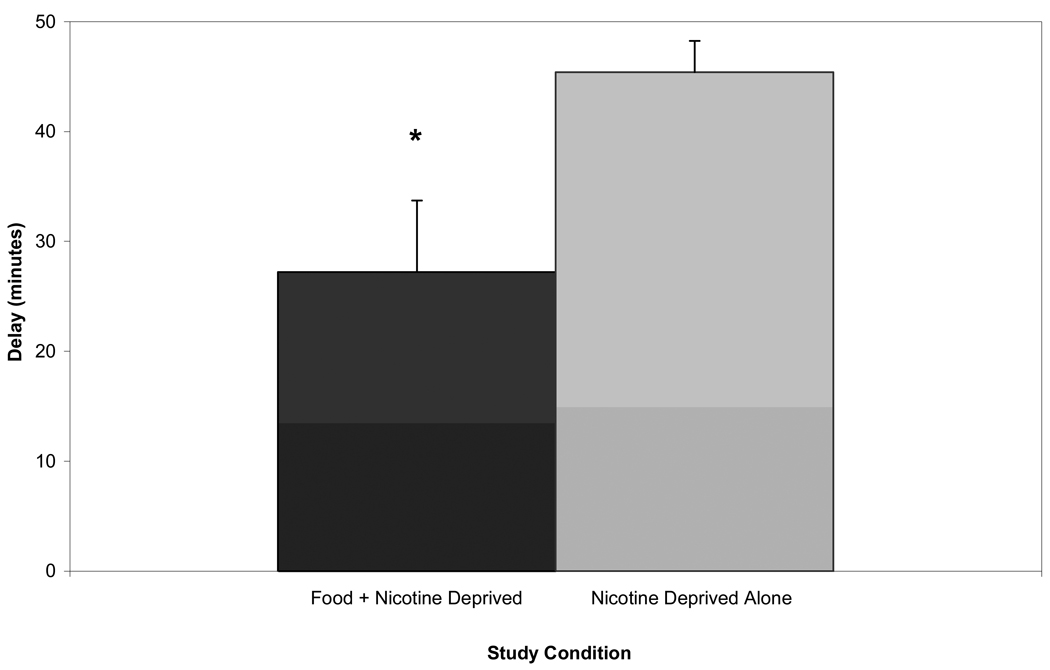
Participants in the food + nicotine deprivation condition smoked their first cigarette sooner than participants in the nicotine deprivation only condition (Figure 1), t(19.15) = 2.56, p = .019, d = 0.93. The food + nicotine deprived participants also smoked slightly, but not significantly, more than participants deprived of nicotine only (M = 1.80, SE = 0.24; M = 1.13, SE = 0.27, respectively), t(28) = 1.82, p = .079, d = 0.67. In the food + nicotine deprivation condition, 14/15 participants smoked at least one cigarette, compared to only 9/15 in the nicotine deprived only condition, z = 2.345, p < .005, d = 0.88.
Fig. 1.
Mean length of delay (minutes) to start smoking by study condition with standard errors. * indicates difference between conditions at p ≤ .05
Changes in craving, withdrawal and hunger by condition across the laboratory session
There were no significant differences between conditions for tobacco craving or withdrawal and no significant time × condition interactions for tobacco craving in the three sets of analyses conducted.
Changes with food craving priming task
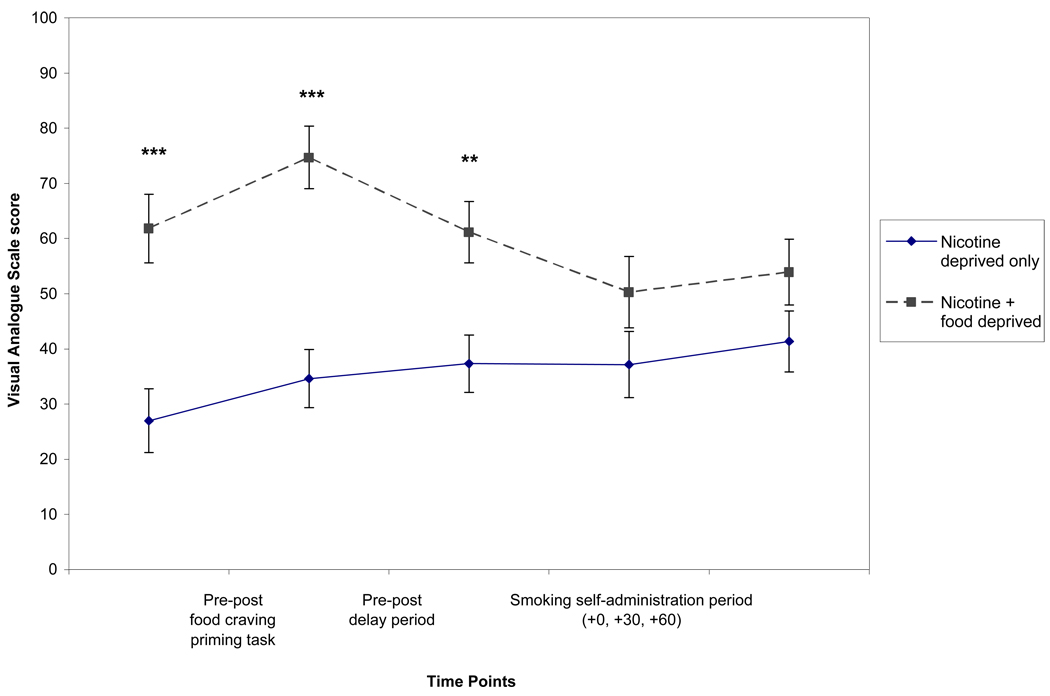
Hunger and food craving increased significantly with the food craving priming task and both were higher in the food + nicotine deprived condition than in nicotine deprivation only condition. Figure 2 shows only changes in hunger ratings by condition, however food craving ratings showed a similar pattern.
Fig. 2.
Hunger items from the Wisconsin Smoking Withdrawal Scale. There were significant main effects of condition pre-post food craving task (p ≤ .001), pre-post delay period (p ≤ .001) and across the smoking self-administration period (p ≤ .05). There was a significant main effect of time pre-post food craving priming task (p ≤ .01) and significant time × condition interactions pre-post delay period and across the smoking self-administration period (both p’s ≤ .05).
Changes from pre to post delay period
Hunger and food craving remained significantly higher in the food + nicotine deprived condition with mean levels similar to those observed with the food craving priming task. Figure 2 shows the change in hunger ratings by condition from pre to post delay period. Food craving ratings (not shown) displayed a similar pattern. There was a significant time × condition interaction for hunger, due to a decrease in ratings in the food + nicotine deprivation condition, but not in the nicotine deprivation only condition (Figure 2).
Changes across the self-administration period
There was a significant time × condition interaction for withdrawal with only participants in the food + nicotine deprivation condition showing a decrease from the start to the end of the 60-min period. Hunger ratings continued to be significantly higher in the food + nicotine deprivation condition than in the nicotine deprivation only condition. As shown in Figure 2, from 0 to 30 min into the self-administration period, there was a decline in hunger ratings in the food + nicotine deprivation condition, unlike in the nicotine deprivation only condition (i.e., a significant time × condition interaction).
Exploratory analyses
There was one significant correlation with initial screening assessments. FTND score was negatively correlated with length of delay to the first cigarette in the food + nicotine deprivation (r = −.68, p = .006) but not in the nicotine deprivation only condition (r = −.37, p = .169).
Regarding assessments collected during the laboratory session, in the nicotine deprived only condition, QSU-B Factor 1 scores had significant positive correlations with number of cigarettes smoked at all three time points considered (r’s between .52 and .62, p’s between 0.048 and 0.013). QSU-B Factor 2 scores correlated significantly to near significantly with length of delay in both conditions (r values between −.49 and −.74, p values between .061 and 0.002) . In the nicotine deprived only condition, food craving ratings taken before the food craving priming task were significantly and positively correlated with length of delay (r = .52, p = .05).
Discussion
As hypothesized, food deprivation, combined with nicotine deprivation, made resisting smoking more difficult than nicotine deprivation alone. Given that the ability to resist smoking in this laboratory model can be considered an indicator of self-control (McKee, 2009), this finding suggests that food deprivation, in addition to nicotine deprivation, made self-control more difficult for smokers in this study. Smokers randomized to food and nicotine deprivation in this study were more likely to smoke at least once during the self-administration period. This finding has clinical importance given results from clinical studies in which smoking a single cigarette typically leads to relapse among those attempting to quit (Brandon et al. 1990; Garvey et al. 1992; Kenford et al. 1994). Those in the food + nicotine deprivation condition smoked slightly more cigarettes in the lab than those who were only nicotine deprived, however this difference was not significant. These results follow clinical findings suggesting that simultaneous restriction of food and cigarettes makes resisting cigarettes more difficult (Perkins et al., 2001).
There were minimal findings linking assessments obtained at initial screening with laboratory session outcomes. Nicotine dependence severity was inversely related to length of delay but only among those who were food and nicotine deprived. In a prior study using this model of smoking lapse behavior, nicotine dependence severity was related to decreased ability to resist smoking after alcohol, as compared to placebo consumption (McKee et al., 2006). This prior finding and the present finding suggest dependence severity may work synergistically with other risk factors (e.g., alcohol use, food restriction) to make resisting cigarettes more difficult.
Regarding assessments administered during the laboratory session, tobacco craving was predictive of outcome in both conditions. Craving in response to negative reinforcement was inversely related to length of delay in both conditions and craving in response to positive reinforcement was positively associated with number of cigarettes smoked in the nicotine deprivation alone condition. Unlike Alsene et al. (2003), we did not find tobacco craving levels to be particularly strong with combined food and nicotine deprivation. The present results follow clinical findings linking craving with smoking and specifically, with smoking cessation relapse (Killen et al. 2006; Shiffman et al. 1997).
The food craving priming task utilized in this study was a valid manipulation as self-reported craving for the snacks and hunger both increased with the task. As expected, food craving and hunger were higher in the food + nicotine deprivation condition. Pre-to-post delay period and across the self-administration period, hunger decreased to a greater extent in the food + nicotine deprivation condition than in the nicotine deprived alone condition.
This study had several limitations. While the study was powered to detect large differences between the two conditions on the primary outcome variables, it was not as well powered to detect changes in self-report measures during the laboratory session, nor was it well powered to find significant predictors of the primary outcomes by condition. While the results of this study suggest some mechanisms underlying difficulty to resist the first cigarette and heavier smoking, studies with larger sample sizes would likely reveal more about these mechanisms.
There are several future directions for this line of research. The present findings suggest that food deprivation impairs smokers’ ability to resist the first cigarette and increases the likelihood that they will smoke. Behavioral and/or pharmacologic interventions could be tested for their efficacy in reducing these associations. As an initial study in a line of research, we believed that testing the effects of complete food deprivation would be the best way to begin, in order to demonstrate that food restriction could have an impact on smoking lapse behavior in the laboratory. A possibility for future research is to test restricted diets rather than outright food deprivation in combination with nicotine deprivation for their impact on smoking lapse behavior. While some smokers will likely fast for periods of time to prevent weight gain during smoking cessation, food restriction may be even more common, thus the impact of food restriction on ability to resist the first cigarette has clinical implications. Given findings that concerns about weight gain may increase risk of smoking relapse (e.g., Meyers et al. 1997), weight concerns could be examined in future research as potential predictors of smoking lapse behavior in the laboratory and/or as moderators of the relationship between food deprivation/restriction and smoking behavior.
Acknowledgments
Funding was provided by K05 AA014715, K23 DK071646, P50 AA015632, RL1 DA024857, T32 DA07238, the Connecticut Department of Mental Health and Addiction Services and CTSA grant UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors thank Vanessa Costa-Massimo and Joseph Palmer for assistance with data collection, Dr. Julia Shi for her role as study physician, Dr. Ned Cooney for advice about procedures, and Dr. Peter Jatlow and Haleh Nadim for conducting the nicotine assays.
References
- Alsene KM, Li Y, Chaverneff F, de Wit H. Role of abstinence and visual cues on food and smoking craving. Behav Pharmacol. 2003;14:145–151. doi: 10.1097/00008877-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based smoking cessation program. Addict Behav. 1998;23:609–622. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Post cessation cigarette use: the process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology. 2005;179:430–436. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-Brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Bulik CM, Perkins KA, Caggiula AR, Rodefer J. Behavioral economic analysis of smoking: Money and food as alternatives. Pharmacol Biochem Behav. 1991;38:715–721. doi: 10.1016/0091-3057(91)90232-q. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- French SA, Jeffery RW, Pirie PL, McBride CM. Do weight concerns hinder smoking cessation efforts? Addict Behav. 1992;17:219–226. doi: 10.1016/0306-4603(92)90027-s. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Strycker LA, Eakin EG, Boles SM, Whitlock EP. Concern about weight gain associated with quitting smoking: Prevalence and association with the outcome in a sample of young female smokers. J Consult Clin Psych. 1999;67:1009–1011. doi: 10.1037//0022-006x.67.6.1009. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Greenwood MR, Collins F, Epstein LH, Hatsukami D, Niaura R, O'Connell K, Pomerleau OF, Ravussin E, Rolls BJ, Audrain J, Coday M. National working conference on smoking and body weight. Task Force 1: Mechanisms relevant to the relations between cigarette smoking and body weight; Health Psychol; 1992. pp. 4–9. [DOI] [PubMed] [Google Scholar]
- Hall SM, Ginsberg D, Jones RT. Smoking cessation and weight gain. J Consult Clin Psych. 1986;54:342–346. doi: 10.1037//0022-006x.54.3.342. [DOI] [PubMed] [Google Scholar]
- Hariharan M, VanNoord T, Greden JF. A high-performance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem. 1988;34:724–729. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Brit J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JF, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiat. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Boles SM, Strycker LA, Glasgow RE. Smoking-specific weight gain concerns and smoking cessation in a working population. Health Psychol. 1997;16:487–489. doi: 10.1037//0278-6133.16.5.487. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychol. 2000;19:242–246. doi: 10.1037//0278-6133.19.3.242. [DOI] [PubMed] [Google Scholar]
- Kendzor DE, Baillie LE, Adams CE, Stewart DW, Copeland AL. The effect of food deprivation on cigarette smoking in females. Addict Behav. 2008;33:1353–1359. doi: 10.1016/j.addbeh.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA-J Am Med Assoc. 1994;27:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Murphy GM, Hayward C, Arredondo C, Cromp D, Celio M, Abe L, Wang Y, Schatzberg AF. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psych. 2006;74:286–294. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA. Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol. 1988a;7:575–589. doi: 10.1037//0278-6133.7.6.575. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Somes G, Pascale RW, Klesges LM, Murphy M, Brown K, Williams E. Knowledge and beliefs regarding the consequences of cigarette smoking and their relationships to smoking status in a biracial sample. Health Psychol. 1988b;7:387–401. doi: 10.1037//0278-6133.7.5.387. [DOI] [PubMed] [Google Scholar]
- Lawson RH, Bulik CM, Rodefer JS, Scanlon W, Borger MD. The effect of a reduced energy diet and meal patterns on smoking and coffee drinking in women. Int J Eat Disorders. 1997;21:137–145. doi: 10.1002/(sici)1098-108x(199703)21:2<137::aid-eat4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Quiles ZN, Molinelli LA, Medaglia Terwal D, Nordstrom BL, Garvey AJ, Kinnunen T. Attrition in a multi-component smoking cessation study for females. Tob Induced Dis. 2006;3:59–71. doi: 10.1186/1617-9625-3-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psych. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: dose self-control resemble a muscle? Psychol Bull. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Fonte C, Mitchell SL, Grobe JE. Gender, dietary restraint, and smoking’s influence on hunger and reinforcing value of food. Physiol Behav. 1995;11:115–123. doi: 10.1016/0031-9384(94)00320-3. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller RL, Fernstrom MH, Sexton JE, Jacob RG. Perception and hedonics of sweet and fat taste in smokers and nonsmokers following nicotine intake. Pharmacol Biochem Behav. 1990;35:671–676. doi: 10.1016/0091-3057(90)90306-3. [DOI] [PubMed] [Google Scholar]
- Perkins KS, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, Ashcom J. Cognitive-Behavioral Therapy to Reduce Weight Concerns Improves Smoking Cessation Outcome in Weight-Concerned Women. J Consult Clin Psych. 2001;69:604–613. [PubMed] [Google Scholar]
- Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: results from a national survey of women smokers. Nicotine Tob Res. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. In: Assessing alcohol problems: A guide for clinicians & researchers. second edition. Allen P, Wilson VB, editors. Bethesda, Maryland: National Institute on Alcohol Abuse and Alcohol; 2003. pp. 75–99. [Google Scholar]
- Sobik L, Hutchison KE, Craighead L. Cue-elicited craving for food: a fresh, approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Weekley CK, Klesges RC, Reylea G. Smoking as a weight-control strategy and its relationship to smoking status. Addict Behav. 1992;17:259–271. doi: 10.1016/0306-4603(92)90031-p. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. The effects of a restricted feeding regimen on cigarette smoking in humans. Addict Behav. 1992;17:149–157. doi: 10.1016/0306-4603(92)90019-r. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. Effects of a 24-hour fast on cigarette smoking in humans. Brit J Addict. 1990;85:555–560. doi: 10.1111/j.1360-0443.1990.tb01676.x. [DOI] [PubMed] [Google Scholar]