Abstract
The principles obtained from studies on molecular chaperones have provided explanations for the assisted protein folding in vivo. However, the majority of proteins can fold without the assistance of the known molecular chaperones, and little attention has been paid to the potential chaperoning roles of other macromolecules. During protein biogenesis and folding, newly synthesized polypeptide chains interact with a variety of macromolecules, including ribosomes, RNAs, cytoskeleton, lipid bilayer, proteolytic system, etc. In general, the hydrophobic interactions between molecular chaperones and their substrates have been widely believed to be mainly responsible for the substrate stabilization against aggregation. Emerging evidence now indicates that other features of macromolecules such as their surface charges, probably resulting in electrostatic repulsions, and steric hindrance, could play a key role in the stabilization of their linked proteins against aggregation. Such stabilizing mechanisms are expected to give new insights into our understanding of the chaperoning functions for de novo protein folding. In this review, we will discuss the possible chaperoning roles of these macromolecules in de novo folding, based on their charge and steric features.
Keywords: molecular chaperones, macromolecules, hydrophobic interactions, stabilization, aggregation, surface charges, steric hindrance, de novo folding
1. Introduction
Proteins frequently encounter misfolding and aggregation during their biogenesis and their life cycles in a cellular environment crowded by macromolecules [1,2], although the amino acid sequences of proteins generally encode the information necessary for their native structures [3]. Moreover, a substantial fraction of proteins have been known to be intrinsically disordered proteins (IDPs) or have intrinsically disordered regions (IDRs) under the physiological conditions [4–6]. The amyloid fibrils and oligomers of many proteins, most of which are IDPs and the proteins with long IDRs (e.g., amyloid-ß, α-synucein, tau, prion protein, and huntingtin) are closely associated with many neurodegenerative diseases [7–9]. Therefore, the understanding of protein aggregation in vivo with respect to chaperoning function is of paramount importance in modern biology.
Studies of the representative molecular chaperones such as hsp60 (e.g., GroEL) and hsp70 first introduced the concept of the “assisted” de novo folding in vivo [10,11]. As a general rule, these chaperones assist protein folding by preventing aggregation (a passive role) in most cases and/or misfolding (an active role, that is, an enhancement of folding rate by inducing global or local conformational changes) in limited cases, via transient binding to the exposed hydrophobic regions of nonnative conformers of substrate proteins [1,2,12–15]. It is evident that the aggregation tendency of proteins strictly depends on their conformational states, thus current studies on the intrinsic or extrinsic factors affecting the protein aggregation have been understood in the context of conformation. Nevertheless, it is the passive role of chaperones, independent of conformational changes, that is mainly responsible for their chaperoning functions. Although the chaperone functions driven by the hydrophobic interaction-mediated substrate recognition and stabilization against aggregation have been the underlying framework for our understanding of the “assisted” protein folding in vivo, it still remains unknown what features of the chaperones are important for their substrate stabilization. Recent biochemical and genetic studies have shown that the majority of newly synthesized proteins can fold without assistance of the known chaperones [16–21]. The folding of about ∼3% of E. coli proteins was predicted to be significantly dependent on GroEL [19]. Consistently, GroEL depletion using the tightly controlled system was reported to have little effect on de novo folding of the majority of E. coli proteins [20]. It should be also noted that the GroEL gene is absent or non-essential in some eubacteria [21]. In addition to de novo folding, chaperones play crucial roles in the aggregation inhibition of damaged proteins, disaggregation, protein translocation, proteolysis, protein maturation, and signal transduction [11,22–26].
Here we suggest that there might be other chaperone types and mechanisms operating in de novo folding in vivo, basically distinct from the classical chaperones and their known mechanisms. So far, the chaperoning functions in vivo have been understood mainly in terms of conformational changes and intermolecular hydrophobic interactions. Both factors can be described by a bimolecular interaction system. Protein aggregation is a multimolecular and even specific process [27,28]. Especially in multimolecular assembly processes, the intrinsic properties of macromolecules, such as their surface charges and steric hindrance by excluded volume repulsion, might play an important role in stabilizing the interacting aggregation-prone polypeptides. Indeed, newly synthesized polypeptides interact directly or indirectly with a variety of macromolecules in vivo. Based on the above mentioned charge and steric factors, we will discuss the potential chaperoning roles of interacting macromolecules in de novo protein folding in vivo.
2. Macromolecule-Mediated Chaperone Type Based on Their Surface Charges and Steric Hindrance
2.1. Accumulating Evidence for Charge and Steric Hindrance as Important Stabilizing Factors
Hydrophobic interactions have long been widely accepted to be major driving forces for protein folding and protein aggregation in the aqueous environment [29,30]. Thus, the direct masking of the exposed hydrophobic regions by intermolecular hydrophobic interactions has been widely believed to be a major factor responsible for stabilizing aggregation-prone polypeptides. However, there are other well-known stabilizing factors, distinct from the hydrophobic masking. First, the charge effects on protein solubility are obvious, as evidenced by the following observations. Charged residues interrupt continuous hydrophobic residues in protein sequences as “structural gatekeepers” [31]. A close correlation of net charge with protein solubility has been well documented [32–36]. Strikingly, relatively high net charge observed in IDPs compared to the classical globular proteins serves to maintain their solubility under the physiological conditions [32,34], highlighting the charge effect on protein solubility. Moreover, many IDPs can act as chaperones, and the unstructured regions of chaperones are important for their actions [26,37]. Mechanistically, these unstructured regions were suggested to exert a solubilizing effect on nonnative substrates due to their highly hydrophilic property and entropic exclusion of other molecules [37]. The fusion of a small charged tag to the N- or C-terminus can improve the solubility of the tagged proteins in some cases [38–40]. Mechanistically, intermolecular electrostatic repulsions by charged residues are widely believed to be important for protein solubility [33,35,36]. Second, large polymers such as glycan and PEG were suggested to inhibit the aggregation of their linked proteins due to their steric hindrance [41,42]. Given that protein aggregation is a self assembly process as mentioned above, the inhibition of protein aggregation by the steric hindrance of the bound bulky macromolecules appears to be highly reasonable. In the colloidal aggregation that has long been studied, both electrostatic repulsions of surface charges and steric hindrance of the polymers absorbed on colloidal surfaces have been known to be major factors for stabilizing colloids against aggregation [43,44]. The supposed action mechanisms of aggregation inhibition by surface charges and steric hindrance are different from those by conformational changes and hydrophobic masking, which will be discussed in more detail.
2.2. N-Terminal Domains as Solubility Enhancers for Their Linked Domains
Empirically, the linkage of aggregation-prone proteins to soluble carriers has been known to be an effective way to stabilize proteins against aggregation, although the molecular mechanisms remain unknown [45]. Indeed, the fusion of soluble protein to the N-terminus of aggregation-prone protein is currently the most efficient tool to overcome the aggregation of heterologous proteins expressed in the E. coli cytoplasm [46,47], whereas the coexpression of chaperones has been successful for soluble expression of target proteins only in limited cases [48]. Despite the popularity of this fusion technology, artificial “tagging” has been considered to be biologically irrelevant. It could be argued, however, that the fusion proteins mimic multidomain proteins in which the N-terminal domain acts as a solubility enhancer for the downstream domains, prompting us to speculate that this chaperoning type in an artificial construct could be employed in de novo folding of native multidomain proteins in vivo. Indeed, the N-terminal domains of native multidomain proteins have the ability to solubilize their C-terminally fused various heterologous proteins in vivo, which suggests that the native N-terminal domains have the potential to assist de novo folding of their authentic downstream domains in vivo by acting as solubility enhancers [49]. Traditionally, multidomain proteins, because of their high propensity to aggregation, were thought to require assistance of chaperones [2,50]. In contrast, these results provided a possible chaperoning role of the cotranslationally or independently folded domains for their linked domains, contributing to the autonomous folding of multidomain proteins in vivo.
We also showed that the solubilizing ability of the N-terminal domains, including the solubility enhancers used in the fusion protein technology, strongly correlates with their net charge and size. Based on these observations, we proposed a model of how folded N-terminal domains could solubilize their linked domains, as illustrated in Figure 1. The electrostatic repulsions and steric hindrance of folded N-terminal domains could prevent the oligomerization driven by the C-terminal aggregation-prone domains, shifting the oligomeric states toward the monomeric states, and thus keeping the C-terminal domains in a folding-competent state. In particular, the folded domains could exert a chaperoning activity on their linked domains even without direct contact to the aggregation-prone regions and even without native interdomain interactions, potentially enabling this chaperoning type to be applied to a broad range of multidomain proteins. This model well explains why the linkage of aggregation-prone proteins to large soluble carriers generally improves protein solubility.
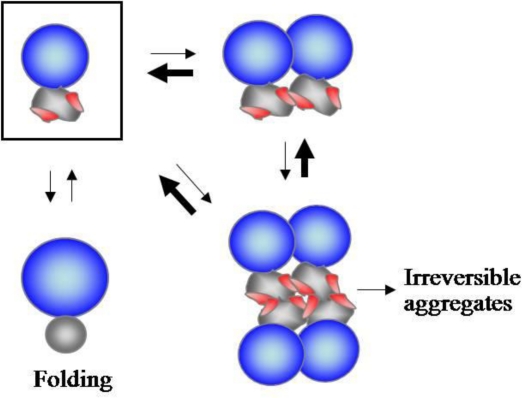
Figure 1.
A model for how N-terminal domains solubilize their linked domains. The blue, gray, and wrinkled spheres represent the folded N- and C-terminal domains, and incompletely folded C-terminal domains, respectively. The red spots on wrinkled spheres indicate the exposed regions involved in the intermolecular interactions. Thick arrows represent the shift from the oligomeric state to the monomeric state (boxed) of proteins driven by the electrostatic repulsions and steric hindrance of folded N-terminal domains. (Reproduced from Reference [49]).
2.3. Substrate Stabilizing Factors of DnaK
Historically, the hydrophobic interaction-mediated chaperoning mechanism originated from Pelham’s speculations on the action mechanism of hsp70 [11]; “during heat shock, proteins become partially denatured, exposing hydrophobic regions which then interact to form insoluble aggregates. By binding tightly to hydrophobic surfaces, hsp70 limits such interactions and promote disaggregation.” Consistent with this prediction, hsp70 as well as other chaperones generally recognize their substrates largely via hydrophobic interactions [2,51,52]. DnaK (an E. coli hsp70 homolog) recognizes short linear peptides with 2–4 contiguous hydrophobic residues flanked by basic residues (e.g., NRLLLTG) [51,53]. Notably, DnaK binds a tiny fraction of hydrophobic regions of its substrates. In contrast, BiP, an hsp70 homolog in the endoplasmic reticulum (ER), can recognize the hydrophilic peptides without hydrophobic residues [54]. Even hsp60 and TF can recognize their substrates by electrostatic interactions [55,56]. The major chaperones in ER, calnexin and calrecticulin, recognize their substrates by binding to the glycan moiety of substrates [57]. These observations raise fundamental questions as to whether the intermolecular hydrophobic interaction is a major substrate-stabilizing factor of the chaperones or a tool for the recognition of nonnative substrates or other regulatory functions such as protein translocation and quality control.
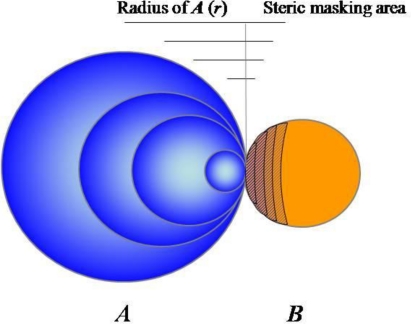
Would DnaK have an intrinsic and additional stabilizing ability to substrate proteins irrespective of its hydrophobic interactions with substrates? To address this issue, the aggregation-prone proteins were fused to the C-termini of DnaK and its variants with a point mutation in the residue critical for the substrate recognition or deletion of the C-terminal substrate-binding domain [58]. Here, the assumption was that the covalent linkage can mimic the noncovalent association between DnaK and its substrate. There was no significant difference in the cis-acting solubilizing ability between DnaK and its variants in vivo, indicating that DnaK has an intrinsic substrate-stabilizing ability, irrespective of its hydrophobic masking by direct contacts. Based on these results, we proposed a simplified model to explain what factors of macromolecules, including DnaK, can stabilize their linked substrates (Figure 2). In this oversimplified model, a soluble macromolecule (sphere A) with varying radius (r) but constant surface charge density is associated with an aggregation-prone protein (sphere B) via limited hydrophobic contact. As radius (r) of sphere A increases, its surface net charge (related to electrostatic repulsion) and excluded volume (related to steric hindrance) are proportional to r2 and r3, respectively, whereas the hydrophobic contact area is constant. This suggests that both surface charges and steric hindrance of large soluble macromolecules, including chaperones, would provide dominant stabilizing factors as relative to hydrophobic interactions. An important implication of this model is that soluble macromolecules could have the intrinsic ability to stabilize their linked aggregation-prone polypeptide chains against aggregation, independent of the nature of linkage between them.
Figure 2.
A schematic illustration of substrate-stabilizing factors of macromolecules and their correlation with the size of the macromolecule. Here, an example of a soluble macromolecule, DnaK, with varying radius r and constant surface charge density and its bound aggregation-prone protein are represented as sphere A and B, respectively. The potential factors of sphere A such as electrostatic repulsions, steric hindrance, and hydrophobic shielding are considered as a function of the radius r of sphere A. The hatched area represents the surfaces inaccessible to other B by the steric masking of the corresponding A. (Adapted from Reference [58]).
The hsp70 can actively unfold its substrates by inducing local conformational changes through ATP hydrolysis. Seemingly, our model does not include this mechanism. An entropic pulling mechanism was proposed to underlie the functions of hsp70 as diverse as inhibition of aggregation, unfolding, disaggregation, and membrane translocation [23]. In this model, the hsp70 has the tendency to move away from protein aggregates or membrane surfaces for more freedom, generating an entropic pulling force; in the closer proximity to the surfaces, hsp70 has less freedom due to its excluded volume. Interestingly, the entropic pulling force and steric hindrance in our model come from a common origin or the excluded volume of hsp70.
2.4. RNA-Mediated Chaperone Type
Nascent polypeptides emerging from ribosomes, prior to the formation of stable structure, were thought to be highly aggregation-prone due to the increased effective concentration by close proximity of identical chains on the polysomes and the macromolecular crowding effect in the cytosol [2,59,60]. The aggregation problems of nascent chains on ribosomes have provided a rationale for the existence of the ribosome-associated chaperones such as trigger factor [61,62]. However, their contribution to de novo folding on ribosomes still remains unknown [62]. Rather, these factors were recently reported to play an important role in ribosome assembly [56,63,64]. Therefore, it still remains an outstanding issue how the aggregation of the nascent chains on the ribosomes is prevented in vivo.
In terms of this issue, the effects of the physical linkage to ribosome on the aggregation behavior of the nascent chains have not been given due consideration. The ribosome is a gigantic RNP complex (its size is approximately 2.6 × 106 dalton in E. coli) in which RNAs (polyanionic macromolecules) provide basic structural frames. From the viewpoint of charge and steric factors, in Figure 2, ribosome and large RNAs are ideal chaperoning macromolecules, implying that the RNA-mediated chaperoning functions might be ubiquitous in vivo. Indeed, the ribosome, its 23S rRNA, and the V domain of 23S rRNA have been known to function as molecular chaperones in vitro [65,66]. Here, the substrate recognition was mediated by the peptidyl transferase center (PTC). However, during protein synthesis, the PTC is expected to be difficult for the nascent chains on the exit sites of ribosomes to physically access.
We previously showed that large RNAs can increase the solubility and folding of their linked proteins, as shown in Figure 3 [67]. When an RNA-binding domain (RBD) is used as a soluble carrier, the RNA binding to RBD (RNP complex) further promoted the solubility of whole proteins and the proper folding of C-terminal proteins. The similarity between the RNP-linked aggregation-prone proteins and the ribosome-linked nascent chains made us speculate that ribosomes might contribute to the solubility enhancement of their linked nascent chains in a cis-acting manner. Indeed, the ribosome displays technology has been known to be very effective for promoting the solubility and folding of highly aggregation-prone proteins [68,69]. By combining these observations with the model in Figure 2, we proposed that the aggregation-prone nascent chains on ribosomes might gain aggregation-resistance due to the gigantic size and overall negative surface charges of ribosomes [70]. This cis-acting chaperoning role of ribosomes has the potential to alleviate the aggregation problems of nascent chains on them. In addition, the three-dimensional organization of bacterial polysomes showed that the polypeptide exit sites are positioned to maximize the distance between them for reducing intermolecular interactions of nascent chains [71]. Thus, the aggregation problems of nascent chains on ribosomes should be understood in the ribosome linkage context.
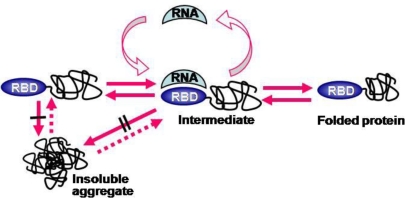
Figure 3.
A model for RNA binding-mediated protein folding. Both the folded RNA-binding domain (RBD) at the N-terminal position and bound RNA prevent inter-molecular interactions among folding intermediates, leading to soluble expression and favoring kinetic network into productive folding. The number of black bars (| and ||) represents the extent of aggregation inhibition. (Reproduced from Reference [67]).
3. Perspectives
Here we discussed that macromolecule-mediated chaperoning types and mechanisms might exist in de novo protein folding inside cells. In particular, two intrinsic properties, charge and steric hindrance, of soluble macromolecules were emphasized as to having an important role in stabilizing their linked proteins against aggregation. Given that a variety of soluble macromolecules are linked to aggregation-prone polypeptides in vivo, the chaperoning roles of these macromolecules presented here could give new insights into de novo protein folding in vivo.
The above chaperoning types and mechanisms might be applied to multimolecular assemblies such as amorphous aggregation, ordered aggregation, nonnative or native oligomerization. For example, the members of the hsp70 family are involved in diverse multimolecular associations such as amorphous aggregation, ordered aggregation, oligomerization, and the assembly/disassembly of clathrin and virus particles [11,72,73]. The chaperoning mechanisms mediated by charge and steric factors as discussed in this review are not mutually exclusive with those exerted by conformational changes and hydrophobic interactions. Thus, the idea of a combination of these factors would advance our understanding of the roles of interacting macromolecules in the multimolecular assembly processes.
Acknowledgments
The work was supported by the National Strategic Research Grant from the Ministry of Knowledge Economy of the Korean Government (10031969), the National Research Foundation of Korea Grant from the Korean Government (MEST) (2010-0001932), and the Korea Healthcare Technology R&D Project, Ministry of Health&Welfare, Republic of Korea (A085105).
References
- 1.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 2.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 3.Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 4.Dunker AK, Oldfield CJ, Meng J, Romero P, Yang JY, Chen JW, Vacic V, Obradovic Z, Uversky VN. The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics. 2008;16:S1. doi: 10.1186/1471-2164-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim. Biophys. Acta. 2010;1804:1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue B, Williams RW, Oldfield CJ, Dunker AK, Uversky VN. Archaic chaos: intrinsically disordered proteins in Archaea. BMC Syst. Biol. 2010;4:S1. doi: 10.1186/1752-0509-4-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 8.Prusiner SB. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uversky VN, Oldfield CJ, Midic U, Xie H, Xue B, Vucetic S, Iakoucheva LM, Obradovic Z, Dunker AK. Unfoldomics of human diseases: linking protein intrinsic disorder with diseases. BMC Genomics. 2009;10:S7. doi: 10.1186/1471-2164-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, Hendrix RW, Ellis RJ. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 11.Pelham HR. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986;46:959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- 12.Ellis RJ. Protein folding: importance of the Anfinsen cage. Curr. Biol. 2003;13:R881–R883. doi: 10.1016/j.cub.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 13.Brinker A, Pfeifer G, Kerner MJ, Naylor DJ, Hartl FU, Hayer-Hartl M. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 2001;107:223–233. doi: 10.1016/s0092-8674(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 14.Apetri AC, Horwich AL. Chaperonin chamber accelerates protein folding through passive action of preventing aggregation. Proc. Natl. Acad. Sci. USA. 2008;105:17351–17355. doi: 10.1073/pnas.0809794105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty K, Chatila M, Sinha J, Shi Q, Poschner BC, Sikor M, Jiang G, Lamb DC, Hartl FU, Hayer-Hartl M. Chaperonin-catalyzed rescue of kinetically trapped states in protein folding. Cell. 2010;142:112–122. doi: 10.1016/j.cell.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Deuerling E, Schulze-Specking A, Tomoyasu T, Mogk A, Bukau B. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature. 1999;400:693–696. doi: 10.1038/23301. [DOI] [PubMed] [Google Scholar]
- 17.Ullers RS, Luirink J, Harms N, Schwager F, Georgopoulos C, Genevaux P. SecB is a bona fide generalized chaperone in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2004;101:7583–7588. doi: 10.1073/pnas.0402398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vorderwülbecke S, Kramer G, Merz F, Kurz TA, Rauch T, Zachmann-Brand B, Bukau B, Deuerling E. Low temperature of GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Lett. 2004;559:181–187. doi: 10.1016/S0014-5793(04)00052-3. [DOI] [PubMed] [Google Scholar]
- 19.Kerner MJ, Naylor DJ, Ishihama Y, Maier T, Chang HC, Stines AP, Georgopoulos C, Frishman D, Hayer-Hartl M, Mann M, Hartl FU. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell. 2005;122:209–220. doi: 10.1016/j.cell.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Masters M, Blakely G, Coulson A, McLennan N, Yerko V, Acord J. Protein folding in Escherichia coli: the chaperonin GroE and its substrates. Res. Microbiol. 2009;160:267–277. doi: 10.1016/j.resmic.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Wong P, Houry WA. Chaperone networks in bacteria: analysis of protein homeostasis in minimal cells. J. Struct. Biol. 2004;146:79–89. doi: 10.1016/j.jsb.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Parsell DA, Kowal AS, Singer MA, Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 23.De Los Rios P, Ben-Zvi A, Slutsky O, Azem A, Goloubinoff P. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc. Natl. Acad. Sci. USA. 2006;103:6166–6171. doi: 10.1073/pnas.0510496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandror O, Busconi L, Sherman M, Goldberg AL. Rapid degradation of an abnormal protein in Escherichia coli involves the chaperones GroEL and GroES. J. Biol. Chem. 1994;269:23575–23582. [PubMed] [Google Scholar]
- 25.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 26.Tompa P, Kovacs D. Intrinsically disordered chaperones in plants and animals. Biochem. Cell Biol. 2010;88:167–174. doi: 10.1139/o09-163. [DOI] [PubMed] [Google Scholar]
- 27.Speed MA, Wang DI, King J. Specific aggregation of partially folded polypeptide chains: the molecular basis of inclusion body composition. Nat. Biotechnol. 1996;14:1283–1287. doi: 10.1038/nbt1096-1283. [DOI] [PubMed] [Google Scholar]
- 28.Wright CF, Teichmann SA, Clarke J, Dobson CM. The importance of sequence diversity in the aggregation and evolution of proteins. Nature. 2005;438:878–881. doi: 10.1038/nature04195. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin RL. Energetics of protein folding. J. Mol. Biol. 2007;10:283–301. doi: 10.1016/j.jmb.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 30.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 31.Otzen DE, Kristensen O, Oliveberg M. Designed protein tetramer zipped together with a hydrophobic Alzheimer homology: a structural clue to amyloid assembly. Proc. Natl. Acad. Sci. USA. 2000;97:9907–9912. doi: 10.1073/pnas.160086297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uversky VN, Gillespie JR, Fink AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins. 2000;41:415–427. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Chiti F, Calamai M, Taddei N, Stefani M, Ramponi G, Dobson CM. Studies of the aggregation of mutant proteins in vitro provide insights into the genetics of amyloid diseases. Proc. Natl. Acad. Sci. USA. 2002;99:16419–16426. doi: 10.1073/pnas.212527999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dosztányi Z, Csizmók V, Tompa P, Simon I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J. Mol. Biol. 2005;347:827–839. doi: 10.1016/j.jmb.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J. Am. Chem. Soc. 2007;129:10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 37.Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004;18:1169–1175. doi: 10.1096/fj.04-1584rev. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Skehel JJ, Wiley DC. A polar octapeptide fused to the N-terminal fusion peptide solubilizes the influenza virus HA2 subunit ectodomain. Biochemistry. 1998;37:13643–13649. doi: 10.1021/bi981098l. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YB, Howitt J, McCorkle S, Lawrence P, Springer K, Freimuth P. Protein aggregation during overexpression limited by peptide extensions with large net negative charge. Protein Expr. Purif. 2004;36:207–216. doi: 10.1016/j.pep.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Kvam E, Sierks MR, Shoemaker CB, Messer A. Physico-chemical determinants of soluble intrabody expression in mammalian cell cytoplasm. Protein Eng. Des. Sel. 2010;23:489–498. doi: 10.1093/protein/gzq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Høiberg-Nielsen R, Fuglsang CC, Arleth L, Westh P. Interrelationships of glycosylation and aggregation kinetics for Peniophora lycii phytase. Biochemistry. 2006;45:5057–5066. doi: 10.1021/bi0522955. [DOI] [PubMed] [Google Scholar]
- 42.Rajan RS, Li T, Aras M, Sloey C, Sutherland W, Arai H, Briddell R, Kinstler O, Lueras AM, Zhang Y, Yeghnazar H, Treuheit M, Brems DN. Modulation of protein aggregation by polyethylene glycol conjugation: GCSF as a case study. Protein Sci. 2006;15:1063–1075. doi: 10.1110/ps.052004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen SN, Andersen KB, Randolph TW, Carpenter JF, Westh P. Role of electrostatic repulsion on colloidal stability of Bacillus halmapalus alpha-amylase. Biochim. Biophys. Acta. 2009;1794:1058–1065. doi: 10.1016/j.bbapap.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Cho YH, Decker EA, McClements DJ. Competitive adsorption of mixed anionic polysaccharides at the surfaces of protein-coated lipid droplets. Langmuir. 2009;25:2654–2660. doi: 10.1021/la8033287. [DOI] [PubMed] [Google Scholar]
- 45.Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8:1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braun P, LaBaer J. High throughput protein production for functional proteomics. Trends Biotechnol. 2003;21:383–388. doi: 10.1016/S0167-7799(03)00189-6. [DOI] [PubMed] [Google Scholar]
- 47.Esposito D, Chatterjee DK. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 2006;17:353–358. doi: 10.1016/j.copbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Wall JG, Plückthun A. Effects of overexpressing folding modulators on the in vivo folding of heterologous proteins in Escherichia coli. Curr. Opin. Biotechnol. 1995;6:507–516. doi: 10.1016/0958-1669(95)80084-0. [DOI] [PubMed] [Google Scholar]
- 49.Kim CW, Han KS, Ryu KS, Kim BH, Kim KH, Choi SI, Seong BL. N-terminal domains of native multidomain proteins have the potential to assist de novo folding of their downstream domains in vivo by acting as solubility enhancers. Protein Sci. 2007;16:635–643. doi: 10.1110/ps.062330907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mogk A, Tomoyasu T, Goloubinoff P, Rüdiger S, Röder D, Langen H, Bukau B. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999;18:6934–6949. doi: 10.1093/emboj/18.24.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenton WA, Kashi Y, Furtak K, Horwich AL. Residues in chaperonin GroEL required for polypeptide binding and release. Nature. 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misselwitz B, Staeck O, Rapoport TA. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol. Cell. 1998;2:593–603. doi: 10.1016/s1097-2765(00)80158-6. [DOI] [PubMed] [Google Scholar]
- 55.Aoki K, Taguchi H, Shindo Y, Yoshida M, Ogasahara K, Yutani K, Tanaka N. Calorimetric observation of a GroEL-protein binding reaction with little contribution of hydrophobic interaction. J. Biol. Chem. 1997;272:32158–32162. doi: 10.1074/jbc.272.51.32158. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Hackert E, Hendrickson WA. Promiscuous substrate recognition in folding and assembly activities of the trigger factor chaperone. Cell. 2009;138:923–934. doi: 10.1016/j.cell.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 58.Ryu K, Kim CW, Kim BH, Han KS, Kim KH, Choi SI, Seong BL. Assessment of substrate-stabilizing factors for DnaK on the folding of aggregation-prone proteins. Biochem. Biophys. Res. Commun. 2008;373:74–79. doi: 10.1016/j.bbrc.2008.05.186. [DOI] [PubMed] [Google Scholar]
- 59.Ellis RJ, Hartl FU. Principles of protein folding in the cellular environment. Curr. Opin. Struct. Biol. 1999;9:102–110. doi: 10.1016/s0959-440x(99)80013-x. [DOI] [PubMed] [Google Scholar]
- 60.Feldman DE, Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr. Opin. Struct. Biol. 2000;10:26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 61.Ferbitz L, Maier T, Patzelt H, Bukau B, Deuerling E, Ban N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature. 2004;431:590–596. doi: 10.1038/nature02899. [DOI] [PubMed] [Google Scholar]
- 62.Craig EA, Eisenman HC, Hundley HA. Ribosome-tethered molecular chaperones: the first line of defense against protein misfolding? Curr. Opin. Microbiol. 2003;6:157–162. doi: 10.1016/s1369-5274(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 63.Albanèse V, Reissmann S, Frydman J. A ribosome-anchored chaperone network that facilitates eukaryotic ribosome biogenesis. J. Cell. Biol. 2010;189:69–81. doi: 10.1083/jcb.201001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koplin A, Preissler S, Ilina Y, Koch M, Scior A, Erhardt M, Deuerling E. A dual function for chaperones SSB-RAC and the NAC nascent polypeptide-associated complex on ribosomes. J. Cell Biol. 2010;189:57–68. doi: 10.1083/jcb.200910074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das B, Chattopadhyay S, Bera AK, Dasgupta C. In vitro protein folding by ribosomes from Escherichia coli, wheat germ and rat liver: the role of the 50S particle and its 23S rRNA. Eur. J. Biochem. 1996;235:613–621. doi: 10.1111/j.1432-1033.1996.00613.x. [DOI] [PubMed] [Google Scholar]
- 66.Chattopadhyay S, Das B, Dasgupta C. Reactivation of denatured proteins by 23S ribosomal RNA: role of domain V. Proc. Natl. Acad. Sci. USA. 1996;93:8284–8287. doi: 10.1073/pnas.93.16.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi SI, Han KS, Kim CW, Ryu KS, Kim BH, Kim KH, Kim SI, Kang TH, Shin HC, Lim KH, Kim HK, Hyun JM, Seong BL. Protein solubility and folding enhancement by interaction with RNA. PLoS One. 2008;3:e2677. doi: 10.1371/journal.pone.0002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sørensen HP, Kristensen JE, Sperling-Petersen HU, Mortensen KK. Soluble expression of aggregating proteins by covalent coupling to the ribosome. Biochem. Biophys. Res. Commun. 2004;319:715–719. doi: 10.1016/j.bbrc.2004.05.081. [DOI] [PubMed] [Google Scholar]
- 69.Schimmele B, Gräfe N, Plückthun A. Ribosome display of mammalian receptor domains. Protein Eng. Des. Sel. 2005;18:285–94. doi: 10.1093/protein/gzi030. [DOI] [PubMed] [Google Scholar]
- 70.Choi SI, Ryu K, Seong BL. RNA-mediated chaperone type for de novo protein folding. RNA Biol. 2009;6:21–24. doi: 10.4161/rna.6.1.7441. [DOI] [PubMed] [Google Scholar]
- 71.Brandt F, Etchells SA, Ortiz JO, Elcock AH, Hartl FU, Baumeister W. The native 3D organization of bacterial polysomes. Cell. 2009;136:261–271. doi: 10.1016/j.cell.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem. 2005;280:14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 73.Ivanovic T, Agosto MA, Chandran K, Nibert ML. A role for molecular chaperone Hsc70 in reovirus outer capsid disassembly. J. Biol. Chem. 2007;282:12210–12219. doi: 10.1074/jbc.M610258200. [DOI] [PMC free article] [PubMed] [Google Scholar]