Abstract
Energy absorbtion from particles and photons moving at relativistic speeds has been a fundamental part of life on earth and wherever else life might exist. Heat and visible light have deeply influenced the course of human evolution, affecting habitat and nutrition. The photons of ionizing radiation that over time can possibly affect evolution, contribute to the more immediate problem of morbidity and mortality of cancer.
This review addresses our radiative energy absorbtion, from both natural and manmade sources, and its relationship with disease and death. Educational Public Health efforts to offset the dangers of solar ultraviolet overexposure are presented, together with data on the significant mortality of metastatic melanoma.
Ever since the generation of radio waves in a laboratory experiment by Hertz in 1888, in which he related the radio waves to light, the effects on biologic systems of the entire range of electromagnetic radiation have been of intense research interest.
In our daily activities we often forget how completely we are, even in our bodily composition, linked to cosmic energy and matter, and how our biologic future hangs on the stability of a few physical constants in our biosphere, and probably, as individuals, our lifetime atomic particle and photon exposure.
E=Mc^2. This familiar expression has much more of a role in our lives than just the devastating reality of the atom bomb. The transfer of energy throughout the entire electromagnetic spectrum involves the interaction between the magnetic and electric fields described by Maxwell in 1873. Enormous levels of kinetic energy can be transferred through microscopic to cosmic distances by photons that travel at the speed of light, carrying a relativistic mass/energy. This energy, when photons collide with atoms and molecules of living systems can cause serious damage or death. The energy imparted to target tissues rises linearly with the radiation frequency, and the more photons that arrive per second from any source, measured as flux, the greater the potential for damage.
The radiation frequencies in all these categories of electromagnetic output range from a few thousand Hertz in the radio spectrum to 10^21 Hertz in the gamma ray spectrum.
Energies range proportionally from a harmless billionth of a volt to a trillion electron volts at the highest frequencies. At the gamma end, and through X-ray and UV above 10 electron volts, destructive ionization in cells and tissues occurs, DNA is altered or destroyed, and in the infrared and microwave range, enough heat can be generated by the movement of water molecules to fatally “cook” tissues. Most radar operates in the “X-band” (microwave radio region, 8–12 GHz). Very close exposure to radar sources can cause serious heat injury to tissues. Standards for safe human exposure will be found in the ANSI/IEEE C95.1 1991 publications.
With relatively long radio wavelengths, no significant biologic interaction is known. This is important since there has been, for years, a great furor over possible human injury from exposure to the electromagnetic fields produced by 60 Hz AC electric power distribution systems. After some forty million dollars worth of careful research at the Electric Power Research Institute in Palo Alto and many other sites, no biologic effects were found.
Photon energy is miniscule at 60 Hz, the usual power line frequency radiation with a wavelength of 3107 miles. Relatively little energy is transferred and no reaction appears to occur with the atoms and molecules of living forms. The same is true for broadcasting station, TV, and computer equipment radiation. In the seventies and eighties, the late Dr. Stanley Batkin and I studied the effects of various non-ionizing electromagnetic fields on bacteria, cell cultures, mice, and rats at the Cancer Center of Hawaii, and in 1990 and 1998, on osteoporotic- prone and arthritic patients. No ill effects were found in laboratory animals or patients from field exposures at low radio frequencies, which concurs with the valid research literature.1,2
Robert K. Adair, in 1998 and 1999,3,4 held that any possible photon energy absorbed at the low frequencies and long wavelengths is far less than the mean energy from thermal agitation, kT (where k=Boltzmann's Constant and T=temperature), present in living systems. Random power line frequency electromagnetic energy absorbtion at five milligauss exposure (an uncommonly high environmental level) would be roughly one ten thousandths of kT, producing no conceivable effect. This is only true of low frequency, long wave photons—but the story changes dramatically as the oscillation frequency rises, resulting in biologically devastating energy transfer.
In our universe, we live in an incredible soup of radiation—the electromagnetic energy engulfing us from many directions and sources at widely varying frequencies. The degree of interaction of these energy waves with life is dependent on the nature of the absorbing entity, the intensity of exposure (number of impinging photons/tissue mass), and the length of time of the exposure. Here are some examples of the photon energy range, which is remarkably wide.
Now just where do all these electromagnetic waves really only light of vastly different colors and energies most of which we cannot see come from? First, earth is bathed in natural or background radiation. This arrives from cosmic sources that include the infrared radiation from the yet unexplained Big Bang, gamma, X-ray, and radio frequency radiation from distant quasars, stars and galaxies.
Nearer sources of background radiation include unstable elements in the earth and its atmosphere, such as uranium and radon gas. Although the total photon energies of these very high frequency sources from far and near are enormous, their biologic effect, ordinarily, is insignificant when compared with that of the sun, a close 93 million miles away. As radiation power drops with the square of the distance from the source, the great distances of cosmic sources plus atmospheric filtration insure our survival. Still, a gamma ray burst of an imploding star, supernova, or fusion of two neutron stars, at even a few thousand light years away from us could be lethal through destruction of our ozone layer.5 Fortunately, such explosive events, known from recorded history and their clearly visible telescopic remains, are far enough away to have been harmless to us. The light from one such stellar explosion reached us on 4 July 1054. Here is today's image of that cataclysm, 6500 light years away.
Two such exploding stars during at least ten billion years produced all the material in our solar system, including the elements of earth,and ourselves. Our body composition, of star forged atoms, is indeed ancient, since our sun is clearly a third generation star involving billions of years of history, with our heaviest elements having been formed in the earlier stars.6 Fortunately, we have inherited the materials, without radiation damage from these enormously distant early cosmic events. In our menu of photons that threaten us, we must include exposure to diagnostic and treatment radiation of all types, all highly energetic and able to destroy life. Although these are usually from well-designed machines or the energy release from radioactive elements, that are well understood and controlled, cumulative doses can be carcinogenic, the result of multiple diagnostic procedures done in unrelated outpatient settings.7
Political toying with radioactive bombs may well constitute our greatest risk of widespread extinction. Tailored nuclear reactions now include enhanced neutron sources that are highly and quickly lethal without significant blast damage. Neutrons are relatively low energy uncharged particles that deeply penetrate the body, damaging blood-forming organs. Neutron radiation is about 20 percent of the radiation received at the International Space Station.
A new photon source to which we are exposed is the ubiquitous cell phone with its roughly 900MHz, 1/2 watt output right against our skulls, raising questions about brain and parotid cancer. Several studies of cell phone use have investigated the risk of developing three types of brain tumors, namely glioma, meningioma, and acoustic neuroma. Results from the majority of these studies have found no association between hand-held cellular telephone use and the risk of brain cancer; however, some, but not all, long-term studies have suggested slightly increased risks for certain types of brain tumors. Further evaluation of long-term exposures of at least 10 years is needed.
Three other studies reported relative risk rates in phone users of 1.6, 1.2, and 4—the first study found these elevated rates in parotid tumors, the second glioma, and the third in acoustic neuroma.8,9 The FCC has recommended that users not place phone antennas close to the head. The effectiveness of shielding is minimal.
NCI has clear recommendations for cell phone use, based on present knowledge of risk of use.10
From a public health standpoint, the most important electromagnetic energy ordinarily reaching us is radiation from the sun, which includes outputs from gamma rays through the entire spectrum to radio, the very longest waves.
Hydrogen atoms fusing to become Helium lose mass at 4 million tons a second, releasing photon energy primarily as gamma rays that take up to a million years to break out of the sun's surface, reaching the earth in about eight minutes as various wavelengths of light. Some of these photons reach ground level. Important biologic interactions involve heat, photosynthesis, vision, and ultraviolet effects, all of which underlie major elements of our evolution. Biologically, we are remarkably tuned to the specific outputs of the solar spectrum. Several components of sunlight are essential to us—and two are detrimental, their effects dependent on exposure (cumulative photon interaction, involving intensity and time). Two essential parts of the spectrum are the infrared that warms our entire world and ourselves, and the two ends of the visible spectrum, the red and the blue, that are critical in photosynthesis. These two narrow bands of light drive the production of almost all of our food. A critical third band is the ultraviolet with its production of Vit. D3 during skin exposure and its major role in DNA damage.
Although the sun's energy output has been fairly stable throughout earth's geologic history, the study of sunspot numbers has shown occasional correlation with wide swings in earth temperatures such as the seventy year chill during the Maunder Minimum of 1650 to 1710 that devastated agriculture in Europe.11 Numbers of sunspots normally vary in approximately 12 year cycles, causing small changes in radiation—less than one percent. The long-term role of the Sun's energy output on earth's temperature is poorly understood.
In addition to the sun's fairly stable long-term photon radiation, there are occasional solar flares on the sun's surface up to 100 000 miles high that, unlike sunspots, put out enormous blasts of alpha particles, protons, neutrons and electrons.
These particles arrive much more slowly than radiation as huge clouds of material at about 300 miles a second taking 4–5 days for the storm to reach the earth. Fortunately, our earth's magnetic field deflects most of these clouds of material, but enough often gets through to completely disable electrical transmission lines and radio communication. Such storms can also injure satellites, and by their neutron content, be a major radiation danger to astronauts out in space. These winds are unpredictable, and fortunately have no effect on persons on earth. Cosmic rays are another source of neutrons that comprise twenty percent of the ionizing radiation that bombards the International Space Station. Without adequate shielding, the long-term exposure of proposed interplanetary travel can be fatal.
These sporadic onslaughts of energy, dramatic as they may be, are of little importance to human health compared with the effect the ultraviolet irradiation that steadily penetrates our atmosphere, with partial but important blockage by our ozone layer.
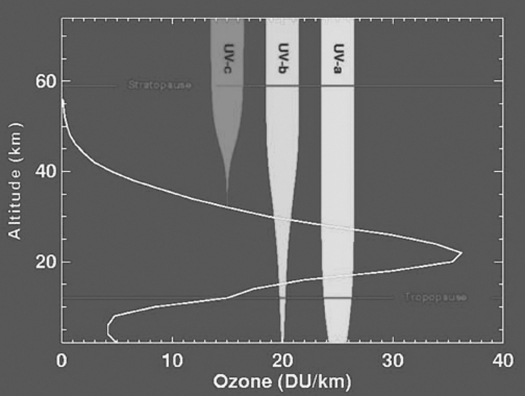
Here is a diagram of the blocking of UV at various Ozone levels. UV is characterized by its wavelength as C, B, and A—two of these, A and B, are most important. C barely reaches us because of atmospheric blockage. B gets through, and is our main concern. Some B exposure is essential for production of vitamin D3, unless it is provided in the diet. Ultraviolet A, although less energetic than B, is significant in producing solar damage to collagen in the skin. For all these wavelengths taken together, for one who is not tanned one MED (minimum erythemal dose) equals roughly ten minutes of bright sunlight exposure.
For most of us, other than those who have unusual exposure to microwave energies, xray or radioactive materials, our greatest risk of tissue damage is to skin from sun exposure and from artificial ultraviolet light sources such as tanning lamps. There is a large literature explaining the biologic lesions induced by UVA and UVB, primarily DNA damage. The damage is described in detail12 and in a review article by Griffiths et al., 1998.
UVB is a complete carcinogen, absorbed by DNA, causing damage by formation of cyclobutane pyrimidine dimers. If repair mechanisms fail, mutations occur and persist through further cell divisions. UVB can also induce the formation of singlet oxygen species that can cause DNA damage. UVA causes damage indirectly by causing the formation of reactive oxygen in other cellular structures, leading to delayed mutations with p53 protein changes. In addition, UV is known to fuse the thymine molecules in DNA, disabling its repair functions.
UV also damages collagen that leads to the thinning and destructive changes that often occur in aging- thinning of the skin and capillary fragility. Although lifelong protection from sun damage to the skin by the use of protective clothing, shelter, and sunscreens may reduce the incidence of skin cancer, lifestyle, fashion, and the popularity of tanning surely offset cancer prevention efforts.
Excess sunlight exposure is thought to be one cause of a million new cases a year of basal and squamous cell malignancy, and over sixty thousand cases of melanoma that causes 75% of all skin cancer deaths. The incidence of malignant melanoma in the United States has more than doubled between 1973 and 2004 (5.5/100,000 to 13.9/100,000) (Ref. 13), varying inversely with latitude and skin pigmentation.
Limiting exposure to lifelong repeated erythema-producing doses of UV may reduce the incidence of skin cancer. Although education of health workers and particularly the public about exposure risks may change attitudes toward sun exposure, social and cosmetic norms are likely to minimize any significant behavioral changes affecting morbidity. Despite this, and with some hope of success, CDC has attacked the problem of chronic overexposure with the following:
CDC's National Leadership Efforts
CDC's skin cancer prevention and education efforts are designed to reduce illness and death, and help achieve the Healthy People 2010 skin cancer prevention goal: Increase to at least 75% the proportion of adults who regularly use at least one sun protection option, limit sun exposure, and use sunscreen. To help achieve this goal, CDC supports the following activities to prevent skin cancer:
Collecting and Applying Vital Information — CDC develops epidemiological research and monitoring systems to determine national trends in sun protection behaviors and attitudes about sun exposure. Findings are used to better target and evaluate skin cancer prevention efforts. CDC and other federal agencies are also helping the independent Task Force on Community Preventive Services review studies of population-based interventions to prevent skin cancer. Recommended interventions will be published in the Guide to Community Preventive Services. This guide will help communities make better use of available scientific information as they plan and implement interventions to prevent skin cancer.
CDC's Guidelines for School Programs to Prevent Skin Cancer — released April 26, 2002, in the Morbidity & Mortality Weekly Report. Overall, the guidelines emphasize the following:
Skin cancer is the most common type of cancer, and new cases and deaths from its deadliest form have been increasing dramatically;
Exposure to the sun during childhood and adolescence typically plays a critical role in developing skin cancer;
To be most effective and efficient, school-based approaches to skin cancer prevention should be implemented as part of a coordinated school health program because no single strategy in isolation can solve the problem;
Schools can do a variety of things to prevent skin cancer such as creating supportive, caring environments that make skin cancer prevention a priority.
CDC urges teens and young adults to play it safe when outdoors and protect their skin from the sun's harmful UV rays. — Campaign messages are delivered through upbeat radio and television public service announcements (PSAs) that are geared to teens and young adults — two groups that spend hours in the sun and are among the least likely to protect themselves. The campaign emphasizes that young people can protect their skin while still having fun outdoors by choosing five sun protection options: Seek shade, especially during midday when UV rays are strongest and do most damage; cover up with clothing to protect exposed skin; get a hat with a wide brim to shade the face, head, ears, and neck; grab shades that wrap around and block as close to 100 percent of both UVA and UVB rays as possible; and rub on sunscreen with SPF 30 or higher, with both UVA and UVB protection.14
An excellent CDC summary of skin cancer will be found at http://www.cdc.gov/cancer/skin/pdf/0809_skin_fs.pdf
Conclusions
Ever since the discovery of ionizing radiation from many sources, efforts have been made to identify and measure their effects on life. As the electromagnetic spectrum was better understood to include all radiated energy from gamma to radio wavelengths, questions were raised about the threat of non-ionizing radiation that floods our communities at the common 60 Hz power line frequency. This risk appears to have been laid at rest; but on the basis of recent studies, cell phone output may be a problem. Conclusions about this await time and further data. Safety with commercial and military exposure to radar and near infrared sources lies in well-established standards of caution.
Plans for space travel must address the difficulty of shielding against exposure to extremely high-energy particles, gamma ray, and neutron exposure.
The role of UV light in the induction of skin cancer with its melanoma mortality is an important public health problem, which has triggered the appropriate attention of CDC and Australian workers. Simple efforts increasing periodic examination of the skin by primary care providers may help to reduce the metastatic melanoma death rate of about 9000 patients a year in the USA.
Postscript
Following acceptance of this paper, Michael Green CTR, of the Cancer Center of Hawai‘i provided these additional data from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program under contract NO 1-PC-35137, reporting melanoma incidence in Hawai‘i in years 2000 through 2005, by ethnic groups and gender.
| Group | Total cases | Rate |
| Caucasian | ||
| M | 791 | 69.8 |
| F | 450 | 41.7 |
| Chinese | ||
| M | 3 | 1.2 |
| F | 6 | 2.0 |
| Filipino | ||
| M | 5 | 0.98 |
| F | 17 | 2.8 |
| Japanese | ||
| M | 33 | 3.7 |
| F | 22 | 2.0 |
| Native Hawaiian | ||
| M | 29 | 6.4 |
| F | 16 | 2.7 |
Considering the similarity of skin color (pigmentation) in Caucasians and Japanese, the nearly twenty-fold disparity in melanoma rates between these two groups in Hawai‘i is remarkable. Since the history of outdoor exposure without protective covering appears to be similar in each group, it is likely that additional factors may be involved.
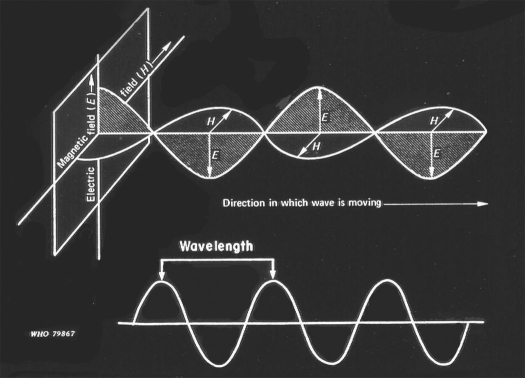
Figure 1.
Electromagnetic waves (ie. light, xray, infrared, and gamma rays. Waves consist of electrical and magnetic forces moving in consistent wave-like patterns at right angles to one another.
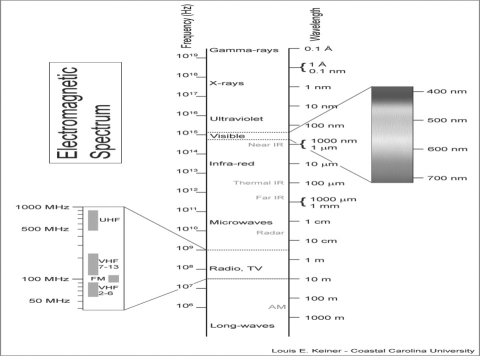
Figure 2.
This is the electromagnetic spectrum - note the narrow band of visible light.
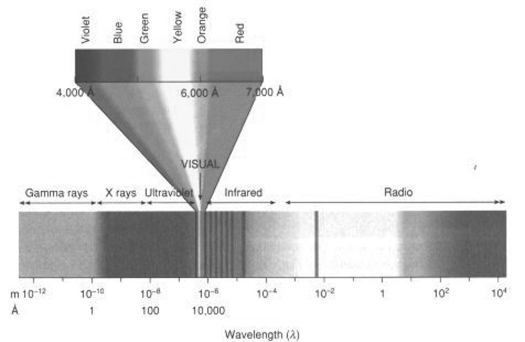
Figure 3.
Some important photon energy ranges. Note the extreme difference in energy carried by gamma rays and 60 Hz power line radiation.
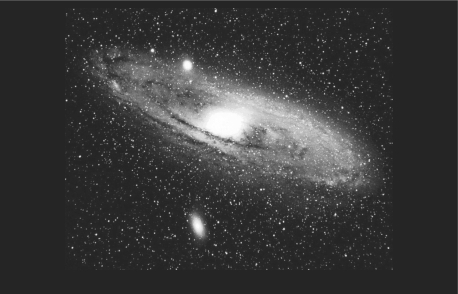
Figure 4.
This is M 31, a typical galaxy like ours, about 150 thousand light years in diameter, containing 100 billion stars, all radiating energy. In our galaxy, we and the sun lie close to the center. We are in one of 100 billion other galaxies within telescopic range. The active nuclei of galaxies emit prodigious amounts of radiation.
Figure 5.
The Crab Nebula, today's remains of the supernova explosion that became visible on earth in 1054 A.D. It is still radiating light and powerful X-rays which reach us from 6500 light years distance.
Figure 6.
The radiation output of the sun includes the entire electromagnetic spectrum.
Figure 7.
Sunspots. Sunspot cycles have correlated with solar radiation and significant swings in earth temperatures that have occurred since sunspot records have been kept, confusing the role of atmospheric gases in global warming.
Figure 8.
Solar flare.
Figure 9.
This graph shows the atmospheric blockage of UV light and the heights at which it is partially blocked by ozone. Note that some UVA and UVB gets through to the surface of the earth.
References
- 1.Tabrah FL. Bone Density Changes in Osteoporosis-prone Women Exposed to Pulsed Electromagnetic Fields (PEMFs) Journal of Bone Miner Res. 1990 May;5(5):437–442. doi: 10.1002/jbmr.5650050504. [DOI] [PubMed] [Google Scholar]
- 2.Tabrah FL, Ross P, Hoffmeier M, Gilbert F., Jr Clinical report on long-term bone density after short-term EMF application. Bioelectromagnetics. :75–78. doi: 10.1002/(sici)1521-186x(1998)19:2<75::aid-bem3>3.0.co;2-0. Published on line 6 Dec 98. [DOI] [PubMed] [Google Scholar]
- 3.Adair RK. Extremely low frequency electromagnetic fields do not interact directly with DNA. Bioelectromagnetics. 1998;19:136–137. doi: 10.1002/(sici)1521-186x(1998)19:2<136::aid-bem14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Adair RK. The fear of Weak Electromagnetic Fields SRAM Home. 1993;3 http:www.sram.org/0301/electromagnetic-fifields.html. [Google Scholar]
- 5.Thomas BC, Jackman CH, et al. Terrestrial Ozone Depletion Due to a Milky Way Gamma-Ray Burst. The Astrophysical Journal. 2005 Apr 1;622:L153–L156. [Google Scholar]
- 6. http://www.nasa.gov/home/index.html.
- 7.Fazel R, Krumholz HM, et al. Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures. NEJM. 2009 Aug 27; doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoemaker MJ, Swerdlow AJ, Ahlbom A, et al. Mobile phone use and risk of acoustic neuroma: Results of the Interphone case-control study in five North European countries. British Journal of Cancer. 2005;93(7):842–848. doi: 10.1038/sj.bjc.6602764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hours M, Bernard M, Montestrucq L, et al. [Cell phones and risk of brain and acoustic nerve tumours: The French INTERPHONE case-control study] Revue d'Epidemiologie et de Sante Publique. 2007:55. doi: 10.1016/j.respe.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10. http://www.cancer.gov/cancertopics/factsheet/risk/cellphones.
- 11. http://science.jrank.org/pages/4184/Maunder-Minimum.html.
- 12.Griffeths HR, Mistry P, et al. Critical Reviews in Clinical Laboratory Sciences. 1998;35(3):189–237. doi: 10.1080/10408369891234192. [DOI] [PubMed] [Google Scholar]
- 13. http.//www.cdc.gov/HealthyYouth/Skincancer/facts.htm.
- 14. http://www.cdc.gov/chooseyourcover.
- 15. General reference for public health issues in lifetime ionization effect http://monographs.iarc.fr/ENG/Monographs/vol75/mono75-5.pdf.