Abstract
BACKGROUND AND PURPOSE
Spontaneous electrical activity, termed slow waves, drives rhythmic, propulsive contractions in the smooth muscle of the oviduct (myosalpinx). Myosalpinx contractions cause egg transport through the oviduct. Agents that disrupt slow wave pacemaker activity will therefore disrupt myosalpinx contractions and egg transport. Caffeine is commonly used as a ryanodine receptor agonist and has been previously associated with delayed conception. Here we assessed the effects of caffeine on pacemaker activity in the murine myosalpinx.
EXPERIMENTAL APPROACH
The effects of caffeine on electrical pacemaker activity were studied using intracellular microelectrode and isometric force measurements on intact oviduct muscle preparations. Responses to caffeine were compared with responses caused by 3-isobutyl-1-methylxanthine (IBMX) and forskolin.
KEY RESULTS
Caffeine caused hyperpolarization of membrane potential and inhibited slow wave generation and myosalpinx contractions. The effects of caffeine could be mimicked by the KATP channel agonist pinacidil and antagonized by the KATP channel antagonist glibenclamide. Caffeine is known to inhibit cyclic nucleotide phosphodiesterases (PDEs), leading to an increase in cytosolic cAMP and stimulation of downstream cAMP-dependent mechanisms. The effects of caffeine were mimicked by the PDE inhibitor, IBMX, and the adenylyl cyclase activator forskolin. These effects were also reversed by glibenclamide.
CONCLUSIONS AND IMPLICATIONS
These results suggest that caffeine activates KATP channels in oviduct myosalpinx. Since caffeine abolishes slow waves and associated contractions of the myosalpinx, it would have a negative effect on egg transport through the oviduct and may contribute to the documented delayed conception in women consuming caffeinated beverages.
Keywords: oviduct, female reproductive tract, smooth muscle, myosalpinx, caffeine, KATP, cAMP
Introduction
Caffeine has been reported to be ‘the most frequently ingested pharmacologically active substance in the world’ (Nawrot et al., 2003). Dietary sources of caffeine include coffee, tea, soft drinks and chocolate as well as several over-the-counter medications. A number of epidemiological studies have linked caffeine consumption to delayed conception (Hatch and Bracken, 1993; Stanton and Gray, 1995; Bolumar et al., 1997). A European multicentre study on women aged 25–44 years found that those who consumed ≥501 mg of caffeine daily while trying to conceive had an increase in the waiting time until their first pregnancy of 11% (Bolumar et al., 1997). A USA conducted study found that women who drank over 300 mg of caffeine a day had a 27% lower chance of conceiving for each cycle compared with women who did not consume caffeine (Hatch and Bracken, 1993). A statistically significant progressive decrease in fertility has also been reported with increasing caffeine consumption in nonsmoking women, suggesting a negative dose-dependent effect of caffeine on conception (Jensen et al., 1998). To provide some perspective, the caffeine content of brewed coffee is 56–100 mg·100 mL−1, instant coffee and tea contains 20–73 mg·100 mL−1 and cola contains 9–19 mg·100 mL−1 (Nawrot et al., 2003).
A factor linked to infertility is compromised transport of oocytes though the fallopian tubes (Croxatto, 2002). In the mouse, oviducts display spontaneous electrical activity termed slow waves (Dixon et al., 2009). This activity has been recorded from oviducts of several species including guinea pigs (Talo and Hodgson, 1978; Parkington, 1983), baboons (Talo and Hodgson, 1978) and humans (Kishikawa and Kuriyama, 1981). Recently, we demonstrated that slow waves drive the rhythmic contractions of the oviduct smooth muscle termed the myosalpinx, which are essential for egg transport through the oviduct (Dixon et al., 2009). In the same study, we showed that slow waves are generated by a specialized population of pacemaker cells, referred to as oviduct interstitial cells of Cajal (ICC-OVI). Thus, any agent that reduces pacemaker activity can negatively affect contractions of the myosalpinx, delay egg transport through the oviduct and reduce fertility.
Caffeine is a widely used drug for studies of smooth muscle tissues and is known to have, at a minimum, the following three major effects: (i) stimulation of Ca2+ release from ryanodine receptor (RyR)–operated stores (Berridge, 1993); (ii) inhibition of IP3 receptors (Parker and Ivorra, 1991) and (iii) inhibition of cyclic nucleotide phosphodiesterases (PDEs), resulting in increased cAMP and enhanced downstream cAMP-dependent mechanisms (Butcher and Sutherland, 1962; Beavo, 1995). We have found that the effects of caffeine on oviduct muscles are unaffected by ryanodine, suggesting that these effects are not mainly due to the stimulant effects of the drug on Ca2+ release from RyR-operated stores (Dixon et al., unpubl. obs.). Furthermore, in contrast to the hyperpolarization caused by caffeine, the IP3 receptor antagonist 2-aminoethyl diphenylborinate (2-APB) caused depolarization (Dixon et al., unpubl. obs.). Thus, the effects of caffeine are unlikely to be due to inhibition of IP3 receptors. In the present study, we tested whether the effects of caffeine on the oviduct are consistent with its action as an inhibitor of PDEs in smooth muscles.
In several previous studies of visceral smooth muscles, increased cAMP resulted in hyperpolarization and inhibition of spontaneous electrical and mechanical activity (von der Weid, 1998; von der Weid and van Helden, 1996; Morales et al., 2004). The adenylyl cyclase activator forskolin and other substances known to elevate cAMP inhibit spontaneous contractions in canine colon and antral smooth muscle (Huizinga et al., 1991; Ozaki et al., 1992; Smith et al., 1993), hyperpolarize membrane potential and reduce frequency and amplitude of slow waves in murine small intestine (Koh et al., 2000). Similarly, the cAMP analogue 8-bromo-cAMP, forskolin, caffeine and cyclic nucleotide PDE inhibitors have all been shown to reduce slow wave frequency in guinea pig antrum (Tsugeno et al., 1995). Studies on individual smooth muscle cells from gastrointestinal muscles have shown that increased cAMP leads to activation of glibenclamide-sensitive KATP channels via cAMP-dependent protein kinase A (PKA) phosphorylation of the channel (Zhang et al., 1994a,b). We tested the hypothesis that caffeine might cause hyperpolarization of oviduct muscles and inhibit slow waves and contractions by activation of KATP channels.
Methods
Ethical approval
An established in-house colony of BALB/c mice, originally set up using breeder pairs purchased from the Jackson Laboratory (Bar Harbor, MN, USA) was used for the present study. Female mice, 8–16 weeks old, were selected from the colony and humanely killed using isoflurane (Baxter, Deerfield, IL, USA) anaesthetic inhalation prior to cervical dislocation. The animals were maintained, and experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The Institutional Animal Use and Care Committee at the University of Nevada approved all procedures used.
Tissue preparation
Immediately after cervical dislocation, the abdomen was cut open, and the oviducts were removed and placed in Krebs-Ringer bicarbonate solution (KRB). The oviducts were then uncoiled by sharp microdissection and cut free of the ovaries. Care was taken to dissect the intramural segment free of the uterine horn. The final preparations used for electrophysiology experiments were intact (infundibulum to uterotubal junction), uncoiled oviducts.
For reverse transcription PCR (RT-PCR), the above procedure was performed, and uncoiled oviducts were then cut open along their length to reveal the mucosal surface. The mucosa was removed and discarded. The remaining oviduct myosalpinx was then cut into three pieces, which were considered to approximate (i) the infundibulum and ampulla, (ii) the isthmus and (iii) the intramural segment. This approximation was necessary as the ampullary isthmic junction (AIJ) cannot be structurally distinguished in mice. Oviduct myosalpinx segments were then frozen in liquid nitrogen and stored at −80°C until needed.
Drugs and solutions
Electrophysiology recording chambers were constantly perfused (at a rate of 3 mL·min−1) with oxygenated KRB containing (mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; and glucose, 11.5. KRB was bubbled with 97% O2-3% CO2 at 37 ± 0.5°C to maintain pH at 7.3–7.4. Glibenclamide, caffeine, pinacidil and IBMX (3-isobutyl-1-methylxanthine) were purchased from Sigma-Aldrich (St Louis, MO, USA). Forskolin was purchased from Calbiochem (San Diego, CA, USA). To make stock solutions, drugs were dissolved in H2O, ethanol or dimethyl sulphoxide as per manufacturer's recommendations. To achieve the desired final concentration, appropriate volumes of stock solution were diluted in KRB before being perfused at a rate of 3 mL·min−1.
Electrophysiological and isometric force measurements
For electrophysiology experiments, oviducts were pinned to the Sylgard-elastomer (Dow Corning, Midland, MI, USA) lined base of a recording chamber with fine tungsten pins (120 µm diameter) and left to equilibrate for at least 1 h prior to commencement of experiments. Pinning facilitated intracellular impalements as it stabilized the tissue and minimized movements of the muscle. Impalements of smooth muscle cells were made using sharp glass microelectrodes filled with 3 M KCl with tip resistances of 80–120 MΩ. All impalements were made at locations ∼50% along the oviduct length (isthmic segment). Transmembrane potentials were recorded with a high impedance electrometer (Axoclamp 2B, Axon Instruments/Molecular Devices, Sunnyvale, CA, USA). Data were recorded onto a PC via a Digidata 1322A system and Axoscope 9.2 (Axon Instruments). In other experiments, the myosalpinx was isolated using tungsten pins, and the opposite end of the oviduct was attached to an isometric force transducer (Gould-Statham UC3, Gould-Statham, Oxnard, CA, USA) for the simultaneous measurement of intracellular electrical activity and/or phasic contractile activity
Statistical analysis
Data analysis and measurements of resting membrane potential (RMP), slow wave amplitude, frequency, ½ maximal duration and rate of rise (dV/dt max) were performed using Clampfit 9.0 (Axon Instruments). Statistical analyses and data summary were performed using SigmaStat and SigmaPlot (Systat Software Inc., Chicago, IL, USA) software. Student's paired t-tests or one-way anovas with post hoc Tukey tests were used to calculate statistical significance. P-values < 0.05 were considered significant. In the results section, all data are expressed as means ± SEM, and n numbers refer to the number of oviducts on which experiments were performed, with each oviduct from a different animal. Figures were constructed in Corel Draw 13.0 (Corel Inc., Mountain View, CA, USA).
RT-PCR
Total RNA from BALB/c oviduct and brain was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and the RNA was treated with 1 U·µL−1 DNase I (Promega, Madison, WI, USA). First-strand cDNA was prepared from 1 µg RNA using SuperScript IIΣ reverse transcriptase (Invitrogen) in a 20 µL reaction containing 25 ng oligo dT (12–18) primer, 1 µL 10 mM dNTP, 5X first-strand buffer (250 mM Tris-HCl, pH 8.3, 375 mM KCl, 15 mM MgCl2) and 0.1 M dithiothreitol. PCR was performed with specific primers for Kcnj8 and Kcnj11 encoding Kir 6.1 and Kir 6.2 potassium channels and Abcc9 encoding the modulatory subunits for the sulphonylurea receptor (SUR) 2A and 2B, on 2 µL cDNA using AmpliTaq Gold® PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The PCR primers used are described in Table 1. Primers were designed to span intronic sequences to eliminate amplification of contaminating genomic DNA in the source RNA. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for cDNA integrity. No-template PCR reactions served as controls for primer contamination. PCR reactions were performed in a GeneAmp 2700 thermal cycler (PE Applied Biosystems). The amplification profile was: 95°C for 10 min to activate the AmpliTaq polymerase, then 35 cycles of 95°C for 15 s, 60°C for 20 s and 72°C for 30 s, followed by a final step of 72°C for 5 min. RT-PCR amplification fragments were analysed by size analysis on 2% agarose gels alongside a 100-base pair (bp) marker.
Table 1.
RT-PCR primers used in this study
| Details of primers used for RT-PCR | |||
|---|---|---|---|
| Target transcript | Accession number | Oligonucleotide sequences (5′-3′) (+): sense (−): antisense | Fragment length (bp) |
| Gapdh | NM_008084 | GTCTTCACCACCATGGAGA (+) | 190 |
| AAGCAGTTGGTGGTGCAG (−) | |||
| Kcnj8 (Kir6.1) | NM_008428 | CTCTTCCCTCATGGTGCCCAAGGTG (+) | 190 |
| CGGAACTCAATGAACTGTCTCCGGA (−) | |||
| Kcnj11 (Kir6.2) | NM_010602 | TCTGCTTTCAGCTCCCATGGGCAG (+) | 200 |
| TGGAGTTCTGGGCAAGGACACCACA (−) | |||
| Abcc9 (SUR2A) | NM_021041 | CCAGCGTGATCTGCTCCGACAAGTG (+) | 167 |
| CTGCACGGACAAACGAGGCAAACAC (−) | |||
| Abcc9 (SUR2B) | NM_021041 | AGTCATGACAGCCTTTGCGGATCGC (+) | 172 |
| TGCACGGACAAACGAGGCAAACACT (−) | |||
All primers were designed against Mus musculus GenBank sequences. Gapdh (glyceraldehyde-3-phosphate dehydrogenase).
Results
Effects of caffeine on slow wave activity and associated myosalpinx contractions
Application of caffeine (10 µM–1 mM) to the myosalpinx caused a dose-dependent membrane hyperpolarization and a reduction in oviduct myosalpinx pacemaker frequency (Figure 1). For example, 300 µM and 1 mM caffeine caused membrane hyperpolarization from an average of −58 ± 2 mV under control conditions (n = 13) to −69 ± 4 mV (n = 6; 300 µM) and to −74 ± 4 mV (n = 5, 1 mM; one-way anova, P = 0.025). Slow wave frequency decreased from 9.9 ± 1 under control conditions to 5.9 ± 1.7 cycles·min−1 in 300 µM and to 1.5 ± 0.8 cycles·min−1 in 1 mM caffeine (n = 5; P < 0.001). At concentrations higher than 1 mM (i.e. 5 mM), caffeine caused membrane hyperpolarization from −55 ± 3 mV to −68 ± 2 mV (n = 7; P < 0.001) and completely inhibited pacemaker activity. We therefore chose concentrations of 1 and 5 mM to test the mechanisms underlying the caffeine inhibition of myosalpinx slow waves in additional experiments. The effects of caffeine on pacemaker activity were completely reversible upon washout of the drug. Caffeine also caused dose-dependent inhibition of phasic myosalpinx contractions (Figure 2A, n = 5); these effects are summarized in Figure 2B. Simultaneous intracellular microelectrode and isometric force recordings revealed that reduction and inhibition in electrical slow waves produced by caffeine caused the reduction and inhibition in phasic contractile activity (Figure 2C–E).
Figure 1.
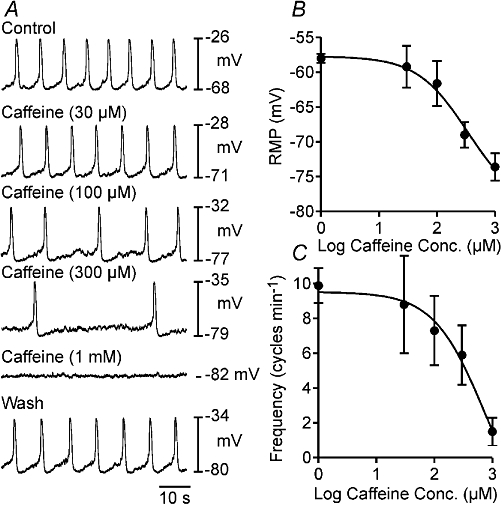
Effects of caffeine on myosalpinx pacemaker activity. (A) Shows the dose-dependent effects of caffeine on oviduct slow wave activity. Caffeine at concentrations between 30 and 300 µM hyperpolarized RMP and reduced the frequency of slow waves. Application of 1 mM caffeine produced further hyperpolarization of membrane potential and abolished slow wave activity. Upon washout of caffeine, slow wave activity returned. (B and C) A summary of data on the effects of caffeine on RMP (B) and slow wave frequency (C) as a function of caffeine concentration (n = 5). The EC50 for caffeine on oviduct RMP and slow wave frequency was 337 and 713 µM respectively. RMP, resting membrane potential.
Figure 2.
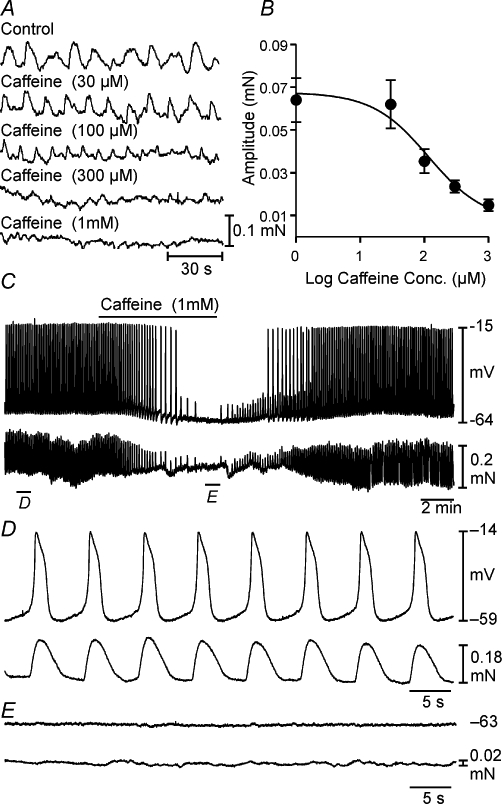
Effects of caffeine on myosalpinx contractions. (A) The dose-dependent effects of caffeine on oviduct phasic contractions. (B) A summary of the effects of caffeine on contraction amplitude as a function of caffeine concentration (n = 5). The EC50 for caffeine on phasic contractile amplitude was 116 µM. (C) The intracellular microelectrode (top trace) and myosalpinx contractions in the bottom trace before, during and after the application of caffeine (1 mM). (D) Slow waves were coupled on a 1:1 basis with myosalpinx contractions. Caffeine (1 mM) slowed slow wave frequency and hyperpolarized membrane potential (C; top trace). The reduction in slow wave frequency was accompanied by an associated reduction in the frequency of myosalpinx contractions (C; bottom trace). (E) After several minutes of exposure to caffeine, slow wave and contractile activity was completely abolished and returned upon washout.
ATP-sensitive K+ channel and sulphonylurea receptor subunit transcriptional expression in oviduct myosalpinx
The hyperpolarization observed upon application of caffeine suggested that either a depolarizing conductance (inward current) was inhibited or a hyperpolarizing conductance (outward current) was activated. We hypothesized that the inhibitory effects of caffeine might activate KATP channels because these channels are known to be activated by cAMP-dependent mechanisms in a variety of smooth muscles (Quayle et al., 1994; Zhang et al., 1994a; Wellman et al., 1998).
RT-PCR was performed on oviduct myosalpinx to examine the transcriptional expression of Kcnj8, Kcnj11 and Abcc9, which encode KATP channels Kir6.1, Kir6.2 and sulphonylurea receptors (SUR; Figure 3). Transcripts encoding Kir6.1, Kir6.2 and SUR2B were found in all segments of the oviducts, whereas SUR2A was only present in the isthmus.
Figure 3.
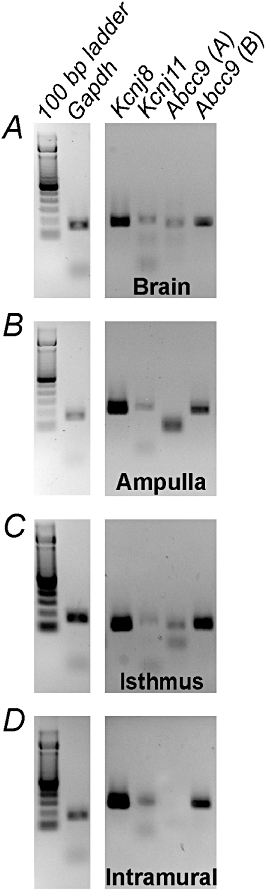
Transcriptional expression of ATP-sensitive K+ channels and sulfonylurea receptor subunits in the oviduct myosalpinx. RT-PCR showing transcriptional expression of Kcnj8 and Kcnj11 encoding the KATP channels Kir6.1, 6.2 and Abcc9, which encodes the sulphonylurea receptor (SUR) subunits SUR2A and 2B. Brain was used as a positive control for each channel transcript and Gapdh was used as a loading control. The gel was part of a complete analysis of potassium channel expression within the myosalpinx. Other potassium channels not relevant to this study have been excluded. NB. The large band in the Abcc9 (A) lane of the ampulla gel was judged to be nonspecific as it was lower than the predicted fragment length of 167 bp.
Effects of KATP channel blockade on slow waves
Glibenclamide (10 µM) was used to selectively block KATP channels in oviduct preparations (n = 15; Figure 4; Sanborn, 1995; Kubo et al., 2005). Glibenclamide significantly depolarized myosalpinx membrane potential from −60 ± 1 mV under control conditions to −55 ± 2 mV in glibenclamide (P < 0.05; Figure 4A–C). Frequency of slow waves increased from 8.4 ± 1.0 cycles·min−1 under control conditions to 10.3 ± 0.9 cycles·min−1 in glibenclamide; however, this did not reach statistical significance. Slow wave amplitude was significantly affected by glibenclamide, decreasing from 41 ± 1 mV in control to 35 ± 2 mV (P < 0.05). The half-maximal duration of slow waves was unaltered (1.7 ± 0.2 s in control, 2.1 ± 0.4 s in glibenclamide). The rate of rise of the upstroke was slightly reduced from 175 ± 17 mV·s−1 to 143 ± 11 mV·s−1 in glibenclamide, but this did not reach statistical significance (P = 0.12). These results suggest that KATP channels probably contribute to the maintenance of RMP and may influence the frequency of slow waves in the oviduct myosalpinx.
Figure 4.
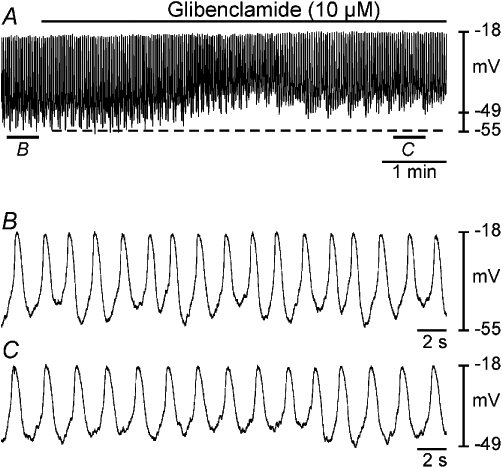
Effects of glibenclamide on slow wave activity. Intracellular microelectrode recordings (A–C) show the effects of the KATP channel antagonist glibenclamide (10 µM) on oviduct slow wave activity. (A) A period of control slow wave activity followed by application of glibenclamide (black bar). (B and C) Expanded time scales of the sections indicated in (A). Glibenclamide caused membrane depolarization (dashed line), increased slow wave frequency, decreased slow wave amplitude and reduced rate of rise of the upstroke phase of slow waves.
Inhibitory effects of caffeine were antagonized by glibenclamide
We further tested whether the effects of caffeine (1 and 5 mM) could be antagonized by glibenclamide (10 µM). As above, glibenclamide (10 µM) caused membrane depolarization from control averaging −60 ± 1 to −58 ± 2 mV (n = 10; Figure 5A–C). In the continued presence of glibenclamide, caffeine still produced a transient hyperpolarization in membrane potential and reduction in slow wave activity. However, unlike the direct exposure to caffeine, in the presence of glibenclamide, membrane hyperpolarization and inhibition of myosalpinx slow waves often recovered within 5–10 min of exposure to caffeine (1 or 5 mM). For example, in the presence of glibenclamide, caffeine (1 mM) caused a similar membrane hyperpolarization as caffeine alone (i.e. −68 ± 2 mV (1 mM; n = 10; P < 0.002 compared with glibenclamide alone; Figure 5A, D). At 5 mM, caffeine produced an initial transient hyperpolarization to −67 ± 2 mV (P < 0.05 compared with glibenclamide) and loss of slow waves (Figure 5A, E), but this was always followed by membrane depolarization to a membrane potential similar to glibenclamide alone (i.e. to −59 ± 2 mV; n = 10; P = 0. 776; Figure 5A, F). In the presence of glibenclamide, caffeine reduced the frequency of slow waves from 10 ± 1 to 7 ± 2 cycles·min−1 (1 mM; n = 5; P < 0.05) but did not inhibit them (Figure 5A, D). The reduction in frequency was less than with caffeine alone (i.e. 1.5 ± 0.8 cycles·min−1). Caffeine (5 mM) caused an initial inhibition (Figure 5A, E), but slow wave frequency recovered to 5 ± 3 cycles·min−1 in the continued presence of caffeine (Figure 5A, F) compared with caffeine alone (n = 4; P < 0.05 compared with glibenclamide alone). Slow wave amplitude recovered to control levels upon washout of caffeine and glibenclamide (Figure 5A, G).
Figure 5.
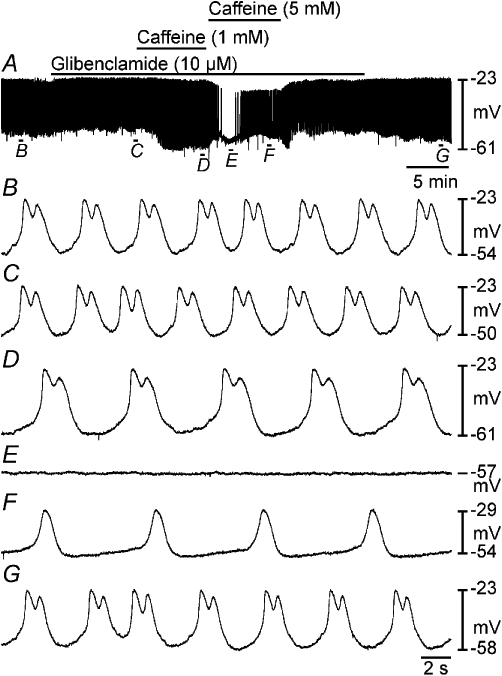
Glibenclamide partially antagonized the effects of caffeine on myosalpinx slow waves. (A) A period of control slow waves followed by application of glibenclamide (10 µM, black bar), which produced membrane depolarization and reduced slow wave amplitude. In the continued presence of glibenclamide, caffeine 1 and 5 mM were exogenously applied (as indicated by black bars). Caffeine (1 mM) caused membrane hyperpolarization and a reduction in slow wave frequency but did not reduce the amplitude of slow waves. Caffeine (5 mM) caused an initial transient hyperpolarization and loss of slow waves, but slow wave activity recovered in the continued presence of caffeine. Following washout of caffeine (5 mM), slow wave frequency recovered, and washout of glibenclamide membrane potential returned to control levels. (B–G) Expanded time scales of the sections indicated in (A).
In a separate series of experiments, oviduct myosalpinx was initially exposed to caffeine, and then glibenclamide was added in the continued presence of caffeine. Caffeine (5 mM) caused myosalpinx hyperpolarization from −57 ± 3 mV to −68 ± 2 mV; (n = 11; P < 0.002; Figure 6), and spontaneous slow wave activity was abolished. Application of glibenclamide (10 µM) in the continued presence of caffeine caused repolarization to −55 ± 2 mV (not statistically different from control, P = 0.45) and restored myosalpinx slow wave activity (Figure 6). These data suggest that the caffeine-induced membrane hyperpolarization and inhibition of slow waves was at least in part due to activation of KATP channels.
Figure 6.
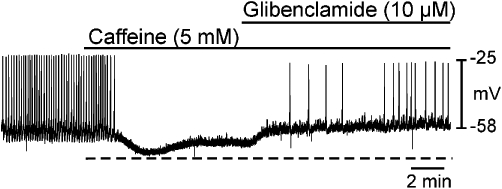
Glibenclamide reversed the inhibitory effects of caffeine on slow wave activity. Intracellular microelectrode recording showing the effects of caffeine and caffeine in the presence of glibenclamide on myosalpinx slow waves. Application of caffeine (5 mM; black bar) caused membrane hyperpolarization and abolished slow wave activity. In the continued presence of caffeine, application of glibenclamide (10 µM; black bar) caused membrane depolarization and resumption of slow wave activity.
Effects of pinacidil on electrical slow waves
Since caffeine appeared to hyperpolarize the membrane by activating KATP channels, we examined the effects of the KATP channel opener pinacidil to see whether this would mimic the effect of caffeine. Pinacidil (10 µM) hyperpolarized the membrane potential from a mean control level of −59 ± 1.5 mV to −71 ± 2.8 mV (n = 5; P < 0.01; Figure 7A–C) and abolished slow wave activity in all tissues (Figure 7A, C). The effect of pinacidil was very similar to that of caffeine (−55 ± 3 mV in control to −68 ± 2 mV in 5 mM caffeine; i.e. 12 mV vs. 13 mV hyperpolarization).
Figure 7.
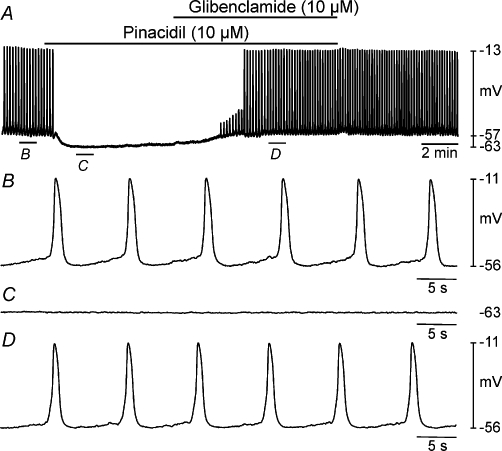
Inhibitory effects of pinacidil on slow waves were antagonized by glibenclamide. (A) A microelectrode recording showing the effects of pinacidil (10 µM) on pacemaker activity and in the continued presence of pinacidil application of glibenclamide (10 µM). (B–D) Faster sweep speed traces of the regions indicated in (A). Pinacidil caused membrane hyperpolarization and abolished slow waves (C). Upon addition of glibenclamide in the continued presence of pinacidil, membrane potential depolarized towards resting levels and slow wave activity resumed (D).
If opening of KATP channels causes membrane hyperpolarization and abolishes slow wave activity, then closing them again should depolarize the membrane and slow wave generation should resume. To test this, we added the KATP channel antagonist glibenclamide (10 µM) in the continued presence of pinacidil (10 µM). Coapplication of pinacidil and glibenclamide caused the membrane potential to depolarize to −61 ± 1.4 mV (not statistically different from control RMP, P = 0.32), and slow wave activity resumed (n = 5; Figure 7A, D).
The depolarized membrane potential observed in experiments in which glibenclamide antagonized the effects of caffeine (i.e. RMP =−68 ± 2 mV in caffeine, RMP in caffeine and glibenclamide =−58 ± 2 mV, i.e. 10 mV depolarization) was compared with the membrane potential observed when glibenclamide antagonized the effects of pinacidil (i.e. RMP in pinacidil =−71 ± 2.8 mV, RMP in glibenclamide and pinacidil =−61 ± 1.4 mV, i.e. 10 mV depolarization). A Student's t-test revealed that the difference between these groups was not statistically significant.
Effects of the PDE inhibitor IBMX on slow wave activity
Caffeine and other xanthines inhibit cyclic nucleotide PDEs that mute or terminate cellular signalling mediated by cAMP (Butcher & Sutherland, 1962; Beavo, 1995). Inhibition of PDEs causes accumulation of cAMP and enhanced PKA activity. We hypothesized that caffeine may act via this mechanism because it is known that KATP channels are activated by PKA phosphorylation in visceral smooth muscles. We compared the effects of caffeine with the general PDE inhibitor, IBMX (10 µM; n = 9; Figure 8A). IBMX hyperpolarized cells from −66 ± 2 mV to −72 ± 1 mV (P < 0.001) and reduced slow wave frequency from control averaging 9.1 ± 1.0 cycles·min−1 to 1.7 ± 0.5 cycles·min−1 (P < 0.001). Slow wave generation was totally abolished by IBMX in three of nine preparations (Figure 8A, C). These effects are the same qualitatively as those observed in response to caffeine and were reversible upon washout of the drug (Figure 8A).
Figure 8.
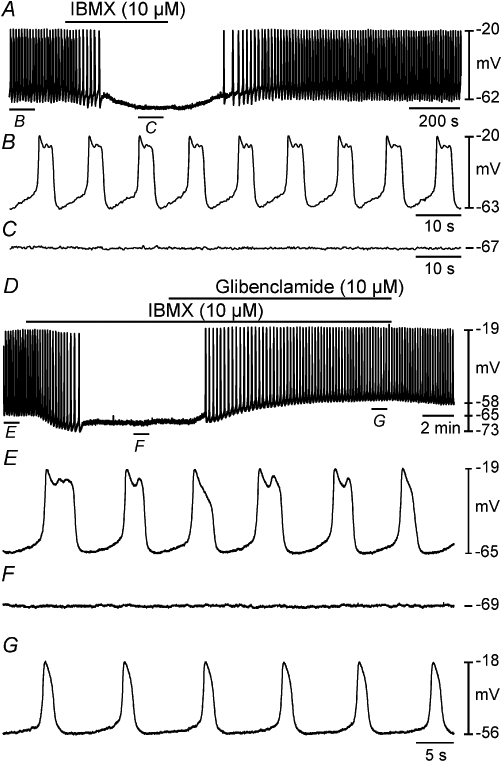
Inhibition of myosalpinx slow waves by IBMX was antagonized by glibenclamide. (A) An intracellular microelectrode recording showing the effects of the PDE inhibitor IBMX (10 µM) on slow wave activity. Application of IBMX caused membrane hyperpolarization and inhibited slow wave activity. (B and C) Indicate sections of the trace in (A) at a faster sweep speed. (D) Intracellular recording showing the effects of IBMX and coapplication with glibenclamide on slow waves. Application of IBMX (10 µM) caused significant membrane hyperpolarization and abolished slow waves. In the continued presence of IBMX, application of glibenclamide (10 µM) caused membrane depolarization and resumption of slow wave activity. (E, F, G) Indicate sections of the trace in (D) at a faster sweep speed. IBMX, 3-isobutyl-1-methylxanthine.
Inhibitory effects of IBMX were antagonized by glibenclamide
In the next series of experiments, we tested the effects of glibenclamide (10 µM) on responses to IBMX. In the continued presence of IBMX, glibenclamide depolarized cells to −60 ± 2 mV, and slow waves were restored to a frequency not statistically different from control (e.g. 7.7 ± 1.4 cycles·min−1 in control, 6.1 ± 2.1 cycles·min−1 in IBMX and glibenclamide; n = 4; P = 0.724 one-way anova; Figure 8D–G).
Effects of the adenylyl cyclase stimulator forskolin on slow wave activity
We studied the effects of forskolin on electrical activity as a more direct test of the role of cAMP in the responses to caffeine. Forskolin (100 nM) hyperpolarized cells from −64 ± 2 mV to −74 ± 1 mV (P = 0.012) and reduced slow wave frequency from 8.9 ± 1.1 cycles·min−1 to 0.4 ± 0.4 cycles·min−1 (n = 5; P = 0.004; slow waves were completely abolished in 4 out of 5 preparations; Figure 9A–C). Glibenclamide (10 µM), added in the continued presence of forskolin (100 nM), repolarized cells to −65 ± 3 mV and restored slow waves (n = 4; Figure 9D–G).
Figure 9.
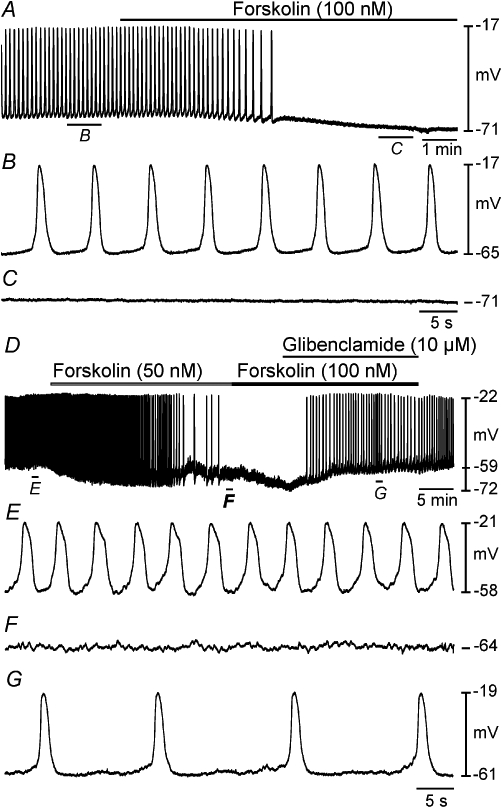
Inhibitory effects of the adenylyl cyclase activator forskolin on slow wave activity was blocked by glibenclamide. (A) Intracellular microelectrode recording showing the effects of the forskolin (100 nM) on slow wave activity. Indicated sections of the trace in (A) are shown at a faster sweep speed in (B and C). Application of forskolin caused membrane hyperpolarization and abolished slow waves. (D) An intracellular recording showing the effects of forskolin and coapplication with glibenclamide on myosalpinx slow waves. Indicated sections of the trace in (D) are shown at a faster sweep speed in (E–G). Application of forskolin (50 nM) hyperpolarized membrane potential and reduced slow wave frequency. At a higher concentration, forskolin (100 nM) caused further membrane hyperpolarization and abolished slow wave generation (D). In the continued presence of forskolin (100 nM), application of glibenclamide (10 µM) caused membrane depolarization and restoration of slow wave activity (D, G).
Discussion
Data presented in the current study indicate that oviduct KATP channels are stimulated by caffeine in a cAMP-dependent manner. Caffeine hyperpolarized membrane potential, abolished slow waves and inhibited myosalpinx contractions. These effects were mimicked by the KATP channel opener pinacidil. Furthermore, the effects of caffeine could be reversed by the KATP channel inhibitor glibenclamide. The effects of the PDE inhibitor IBMX and the adenylyl cyclase activator forskolin mirrored those of caffeine and were also reversed by glibenclamide. Caffeine is best known as an agent which activates ryanodine receptors (Berridge, 1993), but it has also been reported to inhibit IP3 receptors (Parker and Ivorra, 1991) and PDEs (Butcher and Sutherland, 1962; Beavo, 1995). The effects of caffeine are not altered by coapplication of ryanodine and are not mirrored by the IP3 receptor blocker 2-APB (Dixon et al., unpubl. obs.) and based on the results of the present study, the mechanism of action of caffeine on the oviduct myosalpinx must involve its known inhibitory action on PDEs rather than its action on ryanodine receptors.
Inhibition of PDEs leads to accumulation of cyclic nucleotides including cAMP, while activation of adenylyl cyclase catalyses the conversion of ATP to cAMP. In the present study, caffeine was found to inhibit slow waves and associated myosalpinx contractions; this may be the result of elevated levels of cytosolic cAMP, consequential to caffeine's inhibitory effect on PDEs. Similarly, the PDE inhibitor IBMX also inhibited slow waves, as did the adenylyl cyclase activator forskolin, again resulting in elevated cAMP. Elevation in cytosolic cAMP can lead to activation of PKA which can phosphorylate phospholamban, preventing it from inhibiting the SERCA pump (Eggermont et al., 1988). Enhanced store refilling leads to increased frequency of calcium release events which can stimulate Ca2+-activated K+ channels (KCa) and promote membrane hyperpolarization and smooth muscle relaxation (Kim et al., 2005). PKA has been reported to directly stimulate KCa channels (Meera et al., 1995; Sanborn et al. 1998) and can also phosphorylate myosin light chain kinase (MLCK), which decreases its affinity for the calcium–calmodulin complex and inhibits smooth muscle contraction (Price and Bernal, 2001). Finally, in vascular smooth muscle tissues, PKA has been reported to phosphorylate specific serine and threonine residues on KATP channels, activating them (Quayle et al., 1994; Wellman et al., 1998; Quinn et al., 2004; Shi et al., 2007; 2008). The resultant hyperpolarization caused by the activation of KATP channels, shifts membrane potential out of the activation range for voltage-dependent Ca2+ channels and promotes relaxation. Since the effects of caffeine, IBMX and forskolin were mirrored by pinacidil and could all be reversed by the KATP channel inhibitor glibenclamide, this suggests that these agents all activate KATP channels and promote relaxation. This mechanism must be dependent on cAMP since all of these substances produce elevations in cytosolic cAMP. It remains to be determined whether cAMP stimulates KATP channels directly or whether its stimulation of PKA leads to KATP channel phosphorylation and subsequent activation of these channels. It is possible that other K+ channels, as well as KATP, may be activated by caffeine although additional conductances were not investigated in the present study.
It has been previously reported that slow waves provide the electrical basis for rhythmic contractions of the myosalpinx (Dixon et al., 2009). In the present study, this concept has been proven again with an intracellular microelectrode recording and simultaneous isometric force measurement. Slow waves were coupled to phasic myosalpinx contractions on a 1:1 basis. Myosalpinx contractions have been found to be essential effectors of egg transport (Dixon et al., 2009). The loss of slow wave activity and associated loss of contractions observed with caffeine suggests that this agent has the potential to impede egg transport along the oviduct. Several studies have reported a correlation between high consumption of caffeinated beverages and a delay in conception (Hatch and Bracken, 1993; Stanton and Gray, 1995; Bolumar et al., 1997; Jensen et al., 1998). The present study provides a possible explanation as to why elevated caffeine consumption could lead to delayed conception or infertility.
In conclusion, oviduct KATP channels are activated upon application of caffeine. This effect is mirrored by IBMX and forskolin, suggesting that it is cAMP-dependent. The hyperpolarization caused by the activation of KATP channels leads to inhibition of spontaneous myosalpinx contractions that are essential to egg transport along the oviduct, providing an intriguing explanation as to why women with high caffeine consumption often take longer to conceive than women who do not consume caffeine.
Acknowledgments
Supported by NIH NIDDK 057236 (SMW). FCB was partially supported by NIH HL091238 and KMS by NIH 41315. The authors wish to express their sincere appreciation to Lauren Peri and Christina M. Rollings for excellent technical assistance.
Glossary
Abbreviations
- 2-APB
2-aminoethyl diphenylborinate
- IBMX
3-isobutyl-1-methylxanthine
- ICC-OVI
oviduct interstitial cells of Cajal
- IP3
inositol 1,4,5 trisphosphate
- KATP
ATP-sensitive potassium channel
- Kir
inward rectifier potassium channel
- KRB
Krebs-Ringer bicarbonate
- PDE
phosphodiesterase
- PKA
cAMP dependent protein kinase
- RMP
resting membrane potential
- RyR
ryanodine receptor
- SUR2A/2B
sulphonylurea receptor 2A/2B
Conflicts of Interest
None.
References
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145:324–334. doi: 10.1093/oxfordjournals.aje.a009109. [DOI] [PubMed] [Google Scholar]
- Butcher RW, Sutherland EW. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J Biol Chem. 1962;237:1244–1250. [PubMed] [Google Scholar]
- Croxatto HB. Physiology of gamete and embryo transport through the fallopian tube. Reprod Biomed Online. 2002;4:160–169. doi: 10.1016/s1472-6483(10)61935-9. [DOI] [PubMed] [Google Scholar]
- Dixon RE, Hwang SJ, Hennig GW, Ramsey KH, Schripsema JH, Sanders KM, et al. Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct. Biol Reprod. 2009;80:665–673. doi: 10.1095/biolreprod.108.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JA, Vrolix M, Wuytack F, Raeymaekers L, Casteels R. The (Ca2+-Mg2+)-ATPases of the plasma membrane and of the endoplasmic reticulum in smooth muscle cells and their regulation. J Cardiovasc Pharmacol. 1988;12(Suppl 5):S51–S55. [PubMed] [Google Scholar]
- Hatch EE, Bracken MB. Association of delayed conception with caffeine consumption. Am J Epidemiol. 1993;138:1082–1092. doi: 10.1093/oxfordjournals.aje.a116826. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Farraway L, Den Hertog A. Effect of voltage and cyclic AMP on frequency of slow-wave-type action potentials in canine colon smooth muscle. J Physiol. 1991;442:31–45. doi: 10.1113/jphysiol.1991.sp018780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, et al. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reprod Toxicol. 1998;12:289–295. doi: 10.1016/s0890-6238(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Kim M, Cho SY, Han IS, Koh SD, Perrino BA. CaM kinase II and phospholamban contribute to caffeine-induced relaxation of murine gastric fundus smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1202–C1210. doi: 10.1152/ajpcell.00299.2004. [DOI] [PubMed] [Google Scholar]
- Kishikawa T, Kuriyama H. Electrical and mechanical activities recorded from smooth muscle cells of the human fallopian tube. Jpn J Physiol. 1981;31:417–422. doi: 10.2170/jjphysiol.31.417. [DOI] [PubMed] [Google Scholar]
- Koh SD, Kim TW, Jun JY, Glasgow NJ, Ward SM, Sanders KM. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol. 2000;527(Pt 1):149–162. doi: 10.1111/j.1469-7793.2000.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, et al. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev. 2005;57:509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- Meera P, Anwer K, Monga M, Oberti C, Stefani E, Toro L, et al. Relaxin stimulates myometrial calcium-activated potassium channel activity via protein kinase A. Am J Physiol. 1995;269:C312–C317. doi: 10.1152/ajpcell.1995.269.2.C312. [DOI] [PubMed] [Google Scholar]
- Morales S, Camello PJ, Mawe GM, Pozo MJ. Cyclic AMP-mediated inhibition of gallbladder contractility: role of K+ channel activation and Ca2+ signaling. Br J Pharmacol. 2004;143:994–1005. doi: 10.1038/sj.bjp.0706006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Blondfield DP, Hori M, Sanders KM, Publicover NG. Cyclic AMP-mediated regulation of excitation-contraction coupling in canine gastric smooth muscle. J Physiol. 1992;447:351–372. doi: 10.1113/jphysiol.1992.sp019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington HC. Intracellularly recorded electrical activity of smooth muscle of guinea pig oviduct. Am J Physiol. 1983;245:C357–C364. doi: 10.1152/ajpcell.1983.245.5.C357. [DOI] [PubMed] [Google Scholar]
- Price SA, Bernal AL. Uterine quiescence: the role of cyclic AMP. Exp Physiol. 2001;86:265–272. doi: 10.1113/eph8602182. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- Sanborn BM. Ion channels and the control of myometrial electrical activity. Semin Perinatol. 1995;19:31–40. doi: 10.1016/s0146-0005(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Sanborn BM, Yue C, Wang W, Dodge KL. G protein signalling pathways in myometrium: affecting the balance between contraction and relaxation. Rev Reprod. 1998;3:196–205. doi: 10.1530/ror.0.0030196. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, et al. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–R1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, et al. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem. 2008;283:7523–7530. doi: 10.1074/jbc.M709941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Ward SM, Zhang L, Buxton IL, Gerthoffer WT, Sanders KM, et al. Beta-adrenergic inhibition of electrical and mechanical activity in canine colon: role of cAMP. Am J Physiol. 1993;264:G708–G717. doi: 10.1152/ajpgi.1993.264.4.G708. [DOI] [PubMed] [Google Scholar]
- Stanton CK, Gray RH. Effects of caffeine consumption on delayed conception. Am J Epidemiol. 1995;142:1322–1329. doi: 10.1093/oxfordjournals.aje.a117600. [DOI] [PubMed] [Google Scholar]
- Talo A, Hodgson BJ. Electrical slow waves in oviductal smooth muscle of the guinea-pig, mouse and the immature baboon. Experientia. 1978;34:198–200. doi: 10.1007/BF01944675. [DOI] [PubMed] [Google Scholar]
- Tsugeno M, Huang SM, Pang YW, Chowdhury JU, Tomita T. Effects of phosphodiesterase inhibitors on spontaneous electrical activity (slow waves) in the guinea-pig gastric muscle. J Physiol. 1995;485(Pt 2):493–502. doi: 10.1113/jphysiol.1995.sp020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol. 1998;125:17–22. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, van Helden DF. Beta-adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. Am J Physiol. 1996;270:H1687–H1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(Pt 1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bonev AD, Mawe GM, Nelson MT. Protein kinase A mediates activation of ATP-sensitive K+ currents by CGRP in gallbladder smooth muscle. Am J Physiol. 1994a;267:G494–G499. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bonev AD, Nelson MT, Mawe GM. Activation of ATP-sensitive potassium currents in guinea-pig gall-bladder smooth muscle by the neuropeptide CGRP. J Physiol. 1994b;478(Pt 3):483–491. doi: 10.1113/jphysiol.1994.sp020267. [DOI] [PMC free article] [PubMed] [Google Scholar]


