Abstract
BACKGROUND AND PURPOSE
Second-hand tobacco smoke (SHS) exposure in children increases the risk of asthma and sudden infant death syndrome. Epidemiological and experimental data have suggested SHS can alter neuroplasticity in the CNS, associated with substance P. We hypothesized that exposure to SHS in young primates changed the effect of substance P on the plasticity of neurons in the nucleus tractus solitarius (NTS), where airway sensory information is first processed in the CNS.
EXPERIMENTAL APPROACH
Thirteen-month-old rhesus monkeys were exposed to filtered air (FA, n = 5) or SHS (n = 5) for >6 months from 50 days of their fetal age. Whole-cell patch-clamp recordings were performed on NTS neurons in brainstem slices from these animals to record the intrinsic cell excitability in the absence or presence of the NK1 receptor antagonist, SR140333 (3 µM).
KEY RESULTS
Neurons were electrophysiologically classified based on their spiking onset from a hyperpolarized membrane potential into two phenotypes: rapid-onset spiking (RS) and delayed-onset spiking (DS) types. In RS neurons, SR140333 reduced the spiking response, similarly in both FA- and SHS-exposed animals. In DS neurons, SR140333 almost abolished the spiking response in FA-exposed animals, but had no effect in SHS-exposed animals.
CONCLUSIONS AND IMPLICATIONS
The contribution of NK1 receptors to cell excitability depended on firing phenotype of primate NTS neurons and was disrupted by SHS exposure, specifically in DS neurons. Our findings reveal a novel NK1 receptor function in the primate brainstem and support the hypothesis that chronic exposure to SHS in children causes tachykinin-related neuroplastic changes in the CNS.
Keywords: air pollutants, neurokinin 1 receptor, substance P, intrinsic cell excitability, nucleus tractus solitarius, primates
Introduction
Chronic second-hand tobacco smoke (SHS) exposure in children exacerbates respiratory symptoms and has been implicated in increased risk of sudden infant death syndrome (SIDS) as well as an increased rate and earlier onset of asthma (Weitzman et al., 1990; DiFranza et al. 2004). Previous animal studies, investigating the effects of SHS on the respiratory system, suggest such incidents are due to a sensitization of the peripheral airway sensory receptors that induce defensive lung-CNS reflexes (Mutoh et al. 1999; Mutoh et al., 2000). It has also been reported that children exposed to SHS have a higher risk for behavioural and neurocognitive problems (DiFranza et al., 2004). SHS exposure may alter central components of reflex pathways or regulatory mechanisms but there has been very little study of the central mechanisms involved in these reflexes induced by SHS exposure.
The composition of SHS is complex, including thousands of chemical compounds and more than 50 carcinogens (U.S. Department of Health and Human Services, 2006), along with large amounts of particulates which can be taken up by the lung and systemically circulated and may cause an inflammatory response (Zhang et al., 2002; Campbell et al. 2005). As ultra-fine (nanometre range) particles in the systemic circulation could cause damage to the blood brain barrier (Oberdorster et al., 2004), the nucleus tractus solitarius (NTS), which is located in medulla oblongata and incompletely protected by the blood brain barrier (Gross et al., 1990), could be easily affected by exposure to those particulates and mediators. Furthermore, SHS can directly stimulate airway sensory C-fibres (Lee et al., 1989) and increase signal trafficking into the NTS (Mutoh et al., 1999). Therefore, SHS exposure could result in NTS neurons being exposed to excessive afferent signals and direct chemical mediators and cytokines.
The NTS receives airway sensory information via first central synapses from the vagus, where the neuropeptide, substance P is contained within the C- and Aδ-fibres and can be released peripherally through local axon reflexes (Barnes, 1986). In a previous study, substance P immunoreactivities in the nodose, neurofilament positive, neurons and at NTS lung afferent synaptic boutons were markedly increased after SHS exposure in young guinea pigs (Sekizawa et al., 2008). The data suggested that substance P synthesized in the vagal ganglia was transported centrally and released in the NTS to change intrinsic cell excitability and/or modulate synaptic transmission following SHS exposure.
Here, we tested the hypothesis that, in the young rhesus monkey, chronic SHS exposure changes the substance P- NK1 receptor mechanism in the NTS (receptor nomenclature follows Alexander et al., 2009). We performed whole-cell patch-clamp experiments using brainstem slices from 13-month-old rhesus monkeys which had been exposed to filtered air (FA) or SHS and recorded intrinsic cell excitability of NTS neurons in the presence or absence of the NK1 receptor antagonist (SR140333, 3 µM). The antagonist effects were compared in both exposure groups as well as within firing phenotype of neurons, which were previously reported as rapid-onset spiking (≤20 ms, RS) and delayed-onset spiking (≥100 ms, DS) types in response to depolarizing current injection from hyperpolarized membrane potential (Sekizawa et al., 2010). Neurons of these phenotypes have been proposed to receive inputs from capsaicin-resistant (large myelinated fibres, i.e. slowly adapting receptors) and capsaicin-sensitive (Aδ- and C-fibres) vagal afferents respectively (Bailey et al., 2002). Therefore, these neuronal phenotypes may be involved in transducing the signals of primary afferent fibres (e.g. C-fibres vs. myelinated fibres) in the NTS.
Methods
This study was conducted according to the principles expressed in the Declaration of Helsinki. Care and housing of animals before, during and after treatment complied with the provisions of the Institute of Laboratory Animal Resources and conforms to practices established by the American Association for Accreditation of Laboratory Animal Care. All experimental protocols in this work were reviewed and approved by the Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Exposure to second-hand tobacco smoke (SHS)
All 10 young rhesus monkeys used for this study were California Regional Primate Research Center colony-born rhesus macaques (Macaca mulatta). Pregnant monkeys were exposed to either FA (control, n = 5) or sidestream tobacco smoke (n = 2) for 6 h·day−1, 5 days·week−1 starting on day 50 of gestation through parturition. Sidestream tobacco smoke was the surrogate for SHS from paternal smoking, and thus the fetuses were exposed to SHS in utero. After each dam gave birth, each offspring was continued on the same exposure protocol until 13 months of age. In another group, pregnant monkeys (n = 3) were exposed to FA beginning on day 50 of gestation through parturition. After each dam gave birth, each offspring was exposed to FA from birth to 7 months of age, and then switched to SHS exposure until 13 months of age (for a total of 6 months of SHS exposure). Although these animals were not exposed to SHS during the prenatal or early postnatal period (first 7 months), the data of intrinsic cell excitability as well as synaptic transmission were not statistically different from the animals exposed to SHS from gestation day 50 (Sekizawa et al., 2010). Therefore, data from these two groups were pooled for analysis as one group (SHS group, n = 5).
Sidestream smoke was generated by a modified ADL/II smoke-exposed system (Little, Cambridge, MA) from conditioned 1R4F research cigarettes from the University of Kentucky Tobacco and Health Research Institute (Lexington, KY). Two to three cigarettes at a time were smoked under Federal Trade Commission conditions in a staggered fashion at rate of 1 puff·min−1 (35 mL, 2 s duration). The sidestream smoke was diluted with FA in a mixing chamber, and then passed into the stainless steel-and-glass Hinners-type 4.2 m3 exposure chamber. The exposure chamber was characterized by the following parameters (means ± SD): relative humidity of 39.0 ± 9.7%, temperature of 24.5 ± 1.9°C, total suspended particulate concentration of 0.99 ± 0.11 mg·m−3, carbon monoxide concentration of 5.3 ± 0.66 p.p.m., and nicotine concentration of 259 ± 76 µg·m−3. Relative humidity and temperature were sampled continuously. Nicotine was sampled daily for 15 min twice during each 6 h exposure period. The total suspended particle concentration was sampled with the piezobalance technique for 2 min every 30 min, per chamber.
The target total suspended particulate concentration of 1.00 mg·m−3 used in this study was in the high range of that to which humans are exposed (U.S. Department of Health and Human Services, 2006). Since exposure increases as the distance to the source (the end of a smouldering cigarette) decreases, infants and young children are likely exposed to these high relevant concentrations while cared for by a smoking adult. We have reported that short-term exposure to this concentration of SHS can induce an acute systemic inflammatory response in young primates, and long-term exposure to the same concentration of SHS can significantly alter immune effectors, enhancing a local Th2 immunity by impairing normal pulmonary Th1 immune maturation (Wang et al. 2007; Wang et al., 2008). In addition, we performed pulmonary function test using methacholine inhalation in these rhesus monkeys at 12 months of age. Baseline airway resistance (cmH2O·mL−1·s−1; 0.062 ± 0.008 for FA and 0.092 ± 0.038 for SHS, unpaired t-test, P = 0.232) and dynamic compliance (mL·cmH2O−1; 2.84 ± 0.49 for FA and 2.52 ± 0.40 for SHS; unpaired t-test, P = 0.314) were not altered by SHS exposure. Changes in airway resistance and dynamic compliance after methacholine challenges (0.5 mg·mL−1) were similar between FA- and SHS-exposed animals (airway resistance: 79 ± 32 and 60 ± 23% increases respectively; unpaired t-test, P = 0.327) (dynamic compliance: 27 ± 5.4 and 30 ± 9.9% decreases respectively; unpaired t-test, P = 0.383). Based on these results, SHS exposure in non-human primates might generate an asthma-prone condition but not an asthma model.
Brainstem slice preparation and electrophysiology
After at least 16 h from the final exposure, the monkeys were anesthetized with ketamine (10 mg·kg−1 i.m.), and then given a lethal dose of pentobarbital (Fatal-Plus, >44 mg·kg−1). After decapitation, the brains were rapidly exposed and submerged in ice-cold (<4°C) high-sucrose artificial cerebrospinal fluid (aCSF) that contained (in mM) 3 KCl, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 220 sucrose and 2 CaCl2 (300 mOsm), pH 7.4 when continuously bubbled with 95% O2–5% CO2. Brain stem coronal slices (300 µm thickness) were cut with the Vibratome 1000 (Technical Products International, St. Louis, MO). After incubation for 45 min at 37°C in high-sucrose aCSF, the slices were placed in 37°C normal aCSF that contained (in mM) 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose and 2 CaCl2 (300 mOsm), pH 7.4 when continuously bubbled with 95% O2–5% CO2. During the experiments, a single slice was transferred to the recording chamber, held in place with a silk meshed platinum ring, and continuously perfused with oxygenated aCSF at a rate of 3–4 mL·min−1. All experiments were performed at 33–34°C.
Each slice was viewed by using a fixed-stage upright microscope equipped with infrared differential interference contrast for visualizing the neurons. Borosilicate glass electrodes [2.5–5 MΩ, 4.0 ± 0.7 MΩ (mean ± SD)] were filled with a potassium gluconate solution containing in (mM): 130 K gluconate, 1 NaCl, 1 MgCl2, 2 K-ATP, 0.3 Na-GTP, 10 EGTA and 10 HEPES (300 mOsm); pH was adjusted to 7.4 with KOH and whole-cell patch recordings in NTS neurons were made with the Axoclamp 1D patch-clamp amplifier (Axon Instruments, Foster City, CA). Currents and voltages were filtered at 2 kHz digitized at 10 kHz with the DigiData1322A interface (Axon Instruments) and stored in an IBM-compatible computer. The seal resistance was always greater than 1 GΩ, and the series resistance was <22 MΩ. Data were analysed off-line using the pCLAMP9 software (Axon Instruments) and Mini Analysis program (Synaptosoft, Leonia, NJ). The resting membrane potential was measured immediately after the whole-cell configuration.
All experiments were performed on neurons located in the caudomedial NTS that displayed stable resting membrane potentials and showed distinct excitatory postsynaptic currents in response to electrical stimulation of the tractus solitarius (TS) where bipolar tungsten electrodes (1 µm tips separated by 80 µm; FHC, Bowdoin, ME) were placed and square-wave 0.1 ms pulses were delivered through them. Membrane potential of neurons was appropriately held at −50 mV, or unless otherwise stated, for current-clamp recordings and was clamped at −60 mV for voltage-clamp recordings.
Classification of action potential firing phenotypes in NTS neurons
The NTS neurons were classified as two firing phenotypes, RS and DS types as reported previously (Sekizawa et al., 2010). Briefly, under the current clamp condition neurons were first injected with a hyperpolarizing current for 500 ms to reach −80 mV and then given step depolarizing current injections (+100 and +120 pA); RS types responded immediately (onset ≤ 20 ms), and DS types responded with significant delay (onset ≥ 100 ms) (Figure 1). To further confirm RS or DS type, neurons were also injected with depolarizing current (100 and 120 pA) without a hyperpolarizing pre-pulse, and then the difference in spiking onset in the absence or presence of hyperpolarizing pre-pulse was calculated. A ≤10 ms onset for RS type or ≥100 ms onset for DS type were also used for classification.
Figure 1.
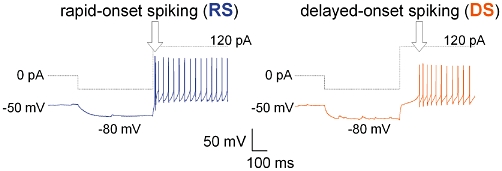
Typical traces of rapid-onset spiking (RS) and delayed-onset spiking (DS) phenotypes of nucleus tractus solitarius neurons. Dotted traces are the command current steps and immediately below are the simultaneous recordings of the membrane potential and spiking. Recordings were made from nucleus tractus solitarius neurons in filtered air-exposed rhesus monkeys. Neurons were initially held at −50 mV and then a hyperpolarizing current was injected for 0.5 s in order to reach a membrane potential of −80 mV, followed by a depolarizing current injection (+100 pA). The RS phenotype displayed a spiking onset of ≤20 ms and the DS phenotype displayed a spiking onset of ≥100 ms.
Endogenous substance P contribution to passive membrane properties, neuronal excitability and action potential characteristics
The input resistance, cell membrane capacitance and intrinsic cell excitability were measured under the current clamp condition. Neuronal excitability was tested at −50 mV of membrane potential by injecting 2 s intracellular depolarizing current pulses in 20 pA steps from 20 to 120 pA at 0.125 Hz to measure the total number of spikes evoked during current injection. First evoked action potential (AP) shapes were characterized by threshold, width, peak amplitude and after-hyperpolarization (AHP). Threshold was measured as the potential at which the AP rose steeply (Nakajima and Onodera, 1969), and width was measured at the level of threshold potential. The effects of SHS exposure on specific substance P (NK1) receptor mechanisms in NTS neuronal excitability were investigated using the NK1 receptor antagonist, SR140333 (3 µM). SR140333 perfusion was performed for at least 3 min before running any protocols and continued until all the tests were finished. Spiking response, input resistance and AP characteristics under the current-clamp condition were compared before and during SR140333 perfusion. A total of 48 RS and DS neurons, 22 neurons from FA-exposed animals and 26 neurons from SHS-exposed animals were recorded in this study; however, 23 neurons (10 and 13 neurons respectively) were not completed with the NK1 receptor antagonist and were excluded from this study.
Statistical analysis
Data were analysed within each firing phenotype of neurons and are expressed as means ± SEM unless otherwise stated. Significance was set at P < 0.05. NK1 receptor antagonist effects were tested with paired t-test except spiking response which was evaluated with two-way repeated measures (RM) analysis of variance (anova). SHS exposure effects on NK1 receptor mechanisms were evaluated by comparing (Δ) changes in each parameter between exposure groups using t-test. Other data comparisons were made using Student's t-test.
Materials
Ketamine and Fatal-Plus were obtained from Bioniche Pharma (Lake Forest, IL) and Vortech Pharmaceuticals (Dearborn, MI) respectively. K-gluconate, Mg-ATP, Na-ATP, EGTA, HEPES and CaCl2 were obtained from Sigma (St. Louis, MO). The NK1 receptor antagonist (SR140333) was a generous gift of Sanofi Recherche (Montpellier, France). All other chemicals were obtained from Fisher (Fair Lawn, NJ). All solutions were freshly prepared and SR140333 stock solution (5 mM) made with dimethyl sulphoxide (DMSO) was dissolved in aCSF just before application.
Results
A total of 25 neurons were included in this study: 12 neurons from FA-exposed animals and 13 neurons from SHS-exposed animals. Averaged input resistance (Rin) of each FA or SHS exposure group was 573 ± 75 or 533 ± 105 MΩ (unpaired t-test, P = 0.407) respectively, and cell membrane capacitance (Cm) was 62 ± 7.4 or 88 ± 20 pF (unpaired t-test, P = 0.132) respectively.
NK1 receptor antagonism on intrinsic cell excitability in RS phenotype neurons
As shown in example traces of Figure 2A, the NK1 receptor antagonist reduced spiking response to depolarizing current injection in RS phenotype neurons from both FA- and SHS-exposed animals. Analysis of group data (n = 7 from five FA-exposed animals and n = 9 from four SHS-exposed animals) confirmed a significant antagonist effect [Figure 2C; two-way RM anova interaction, FA: F(6,36)= 2.679, *P = 0.030; SHS: F(6,48)= 5.452, *P < 0.001]. The NK1 antagonist did not make a significant difference in input resistance in either exposure group (Figure 2B; paired t-test, FA: P = 0.092; SHS: P = 0.091). There was no significant interaction between the effects of the NK1 antagonist and SHS exposure in input resistance (unpaired t-test, P = 0.307) or in spiking response [Figure 2D; two-way RM anova interaction, F(6,84)= 0.450, P = 0.843].
Figure 2.
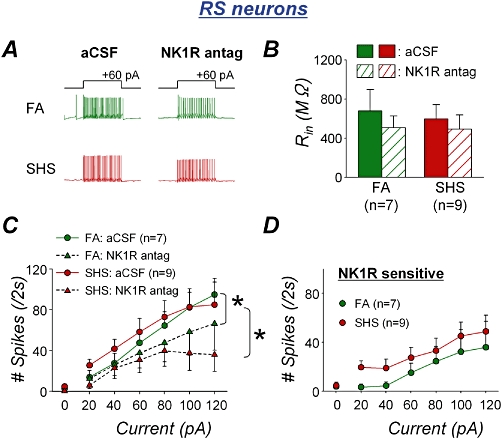
Second-hand tobacco smoke (SHS) exposure effects on endogenous substance P contribution to spiking response in rapid-onset spiking (RS) neurons. A. Typical spiking traces of RS neurons in the absence and presence of the NK1 receptor antagonist (NK1R antag; SR140333, 3 µM) from both filtered air (FA)- and SHS-exposed animals. The antagonist slightly reduced the number of action potentials in response to 2 s depolarizing current injection in both FA- and SHS-exposed animals. B. The NK1 receptor antagonist had a slight reduction in input resistance in both exposure groups. C. Group data confirmed that spiking response was significantly attenuated by NK1 receptor antagonism in both FA (anova interaction, *P = 0.030) and SHS (anova interaction, *P < 0.001) exposure groups. D. NK1 receptor sensitive spiking response to depolarizing current injection. The antagonist effects on spiking responses were similar in between FA and SHS exposure groups (anova interaction, P = 0.843), confirming no SHS exposure effect in RS neurons. aCSF, artificial cerebrospinal fluid.
NK1 receptor antagonism on intrinsic cell excitability in DS phenotype neurons
In comparison, the NK1 receptor antagonist dramatically reduced the spiking response in DS phenotype neurons from FA-exposed animals [Figure 3A,C, n = 5 from three animals; two-way RM anova interaction: F(6,24)= 24.038,*P < 0.001], while no change in spiking response was detected in DS neurons from SHS-exposed animals [Figure 3A,C, n = 4 from four animals; two-way RM anova interaction: F(6,18)= 0.448, P = 0.837]. Thus, there was a significant interaction between effects of the NK1 antagonist and SHS exposure [Figure 3D; two-way RM anova interaction, F(6,42)= 18.940, *P < 0.001]. The NK1 receptor antagonist did not change the input resistance in DS neurons from FA-exposed animals, but increased it 32% in DS neurons from SHS-exposed animals (Figure 3B; paired t-test, FA: P = 0.425; SHS, *P = 0.034). There was no exposure effect on input resistance in DS neurons (unpaired t-test, P = 0.215).
Figure 3.
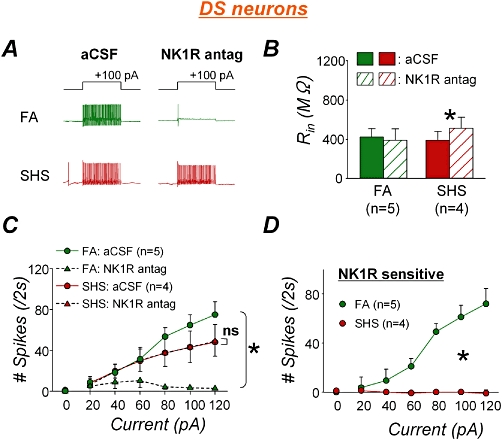
Second-hand tobacco smoke (SHS) exposure effects on endogenous substance P contribution to spiking response in delayed-onset spiking (DS) neurons. A. Example spiking traces of DS neurons in the absence and presence of NK1 receptor antagonist from both filtered air (FA)- and SHS-exposed animals. In FA-exposed animals, the NK1 receptor antagonist left only one action potential in response to 100 pA depolarizing current injection which could evoke 65 action potentials per 2 s in the absence of antagonist. However, the antagonist hardly changed spiking response in SHS-exposed animal. B. The antagonism did not change the input resistance in FA-exposed animals (P = 0.425) but did increase that in SHS-exposed animals (*P = 0.034). C. Group data confirmed that spiking response was markedly reduced by NK1 receptor antagonist in FA-exposed animals (*P < 0.001), but the antagonism did not change spiking response in SHS exposure group (P = 0.837). Note that spiking responses in the absence or presence of NK1 receptor antagonist in SHS exposure group were closely overlapped each other. D. NK1 receptor sensitive spiking response to depolarizing current injection. The antagonist effects on spiking responses were significantly different between FA and SHS exposure groups (anova interaction, *P < 0.001). aCSF, artificial cerebrospinal fluid.
Note that from Figures 2D and 3D, the contribution of the NK1 receptor to the spiking responses was greater in RS neurons from SHS exposure group than in DS neurons from FA exposure group. Therefore, when data were pooled together regardless of neuronal phenotypes, NK1 receptor antagonist effects remained clearly decrease in both exposure groups while the interaction between effects of the antagonist and SHS exposure was masked (Figure S1), demonstrating the importance of neuronal phenotype classification when the cell excitability is evaluated.
NK1 receptor antagonism on action potential characteristics in RS phenotype neurons
Figure 4 shows the effects of the NK1 receptor antagonist on AP characteristics in RS neurons from animals from both FA and SHS exposure groups as in Figure 2, when AP thresholds, AHP, peak amplitude and AP width between exposure groups were assessed. The NK1 receptor antagonist significantly increased AP threshold in RS neurons exposed to SHS but not in those exposed only to FA (Figure 4A left; paired t-test, P = 0.103 and P = 0.004 for FA and SHS exposure groups respectively) while it did not change AHP (Figure 4B left; paired t-test, P = 0.138 in FA and P = 0.479 in SHS). The NK1 receptor antagonist significantly reduced AP peak amplitude (>8 mV) in RS neurons exposed to SHS but not in those exposed to FA (Figure 4C left; paired t-test, P = 0.078 in FA and P = 0.036 in SHS) and prolonged AP width by >0.2 ms in RS neurons exposed to SHS but not in FA-exposed neurons (Figure 4D left; paired t-test, P = 0.103 in FA and P = 0.011 in SHS). There was no significant exposure effect on the antagonist-induced changes seen in the AP characteristics of RS neurons (Figure 4 right; unpaired t-test, P > 0.05).
Figure 4.
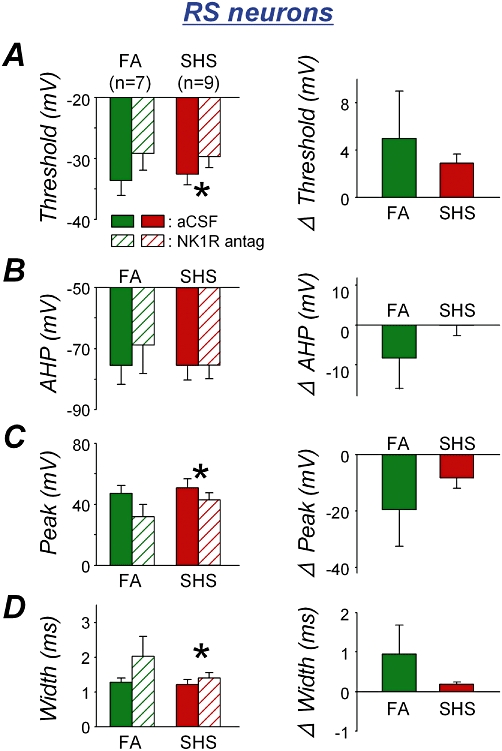
NK1 receptor antagonist effects (left panel) and their comparison between exposure groups (right panel) on action potential characteristics in rapid-onset spiking (RS) neurons. A. Action potential (AP) threshold. The NK1 receptor antagonist significantly increased AP threshold in second-hand tobacco smoke (SHS) exposure group (*P = 0.008) but not in filtered air (FA) exposure group (P = 0.205) (left). The antagonist effects between exposure groups were not different (P = 0.279) (right). B. Afterhyperpolarization (AHP). The antagonist did not change AHP in either exposure group (P > 0.05) (left). The antagonist effects between exposure groups were not different (P = 0.140) (right). C. AP peak amplitude. The antagonist significantly reduced the AP peak amplitude in SHS but not in the FA exposure group (*P = 0.036 and P = 0.078 respectively) (left). The antagonist effects between exposure groups were not significantly different (P = 0.177) (right). D. AP width. The antagonist significantly prolonged AP width in SHS exposure group (*P = 0.011) but not in FA exposure group (P = 0.103) (left). The antagonist effects between exposure groups were not significantly different (P = 0.115) (right). aCSF, artificial cerebrospinal fluid.
NK1 receptor antagonism on action potential characteristics in DS phenotype neurons
Similarly, a significant increase in AP threshold was observed when DS neurons, from SHS-exposed monkeys, were treated with the NK1 receptor antagonist (Figure 5A left; paired t-test, P = 0.226 in FA and P = 0.014 in SHS). The antagonist did not significantly change AHP in DS neurons from either FA- or SHS-exposed animals (Figure 5B left; paired t-test, P = 0.155 and P = 0.063 respectively). But there was a significant exposure effect (unpaired t-test, P = 0.038) in the NK1 receptor antagonist-induced changes in AHP (Figure 5B right). The NK1 antagonist did not significantly change the AP peak amplitude in the DS neurons (Figure 5C left; paired t-test, P = 0.064 and P = 0.085 respectively), nor did the type of exposure significantly affect the antagonist effect (Figure 5C right; unpaired t-test, P = 0.163). The NK1 receptor antagonist did not significantly increase AP width in the FA- or the SHS-exposed group (Figure 5D left, paired t-test, P = 0.095 and P = 0.418 respectively). The antagonist effects between exposure groups were not different (Figure 5D right, unpaired t-test, P = 0.100).
Figure 5.
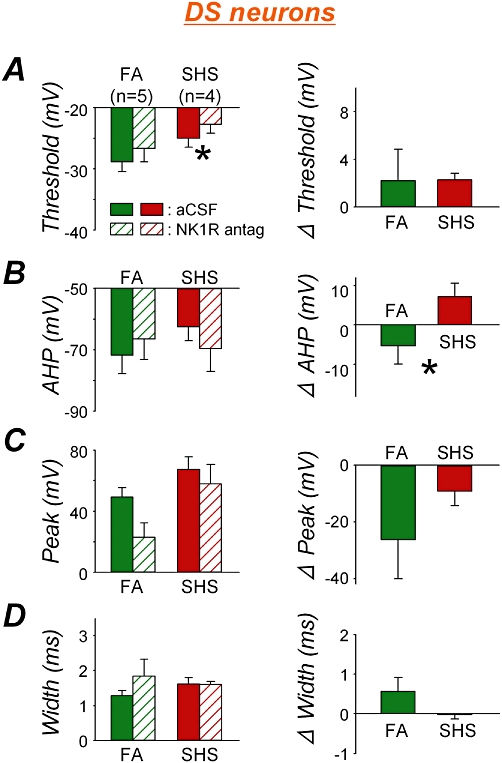
NK1 receptor antagonist effects (left panel) and their comparison between exposure groups (right panel) on action potential characteristics in delayed-onset spiking (DS) neurons. A. Action potential (AP) threshold. The NK1 antagonist increased AP threshold in DS neurons from second-hand tobacco smoke (SHS) exposure group (*P = 0.014) but not from filtered air (FA) exposure group (P = 0.226) (left). The antagonist effects between exposure groups were not different (P = 0.445) (right). B. Afterhyperpolarization (AHP). The NK1 receptor antagonist did not change AHP in either exposure group (P > 0.05) (left). The antagonist effects between exposure groups, however, were significantly different (*P = 0.038) (right). C. AP peak amplitude. The antagonist had a tendency to decrease the AP peak amplitude in both FA and SHS exposure groups (P = 0.064 and P = 0.085 respectively) (left). The antagonist effects between exposure groups were not different (P = 0.163) (right). D. AP width. The antagonist did not change AP width in either exposure group (FA, P = 0.095; SHS, P = 0.418) (left). The antagonist effects between exposure groups were not different (P = 0.100) (right). aCSF, artificial cerebrospinal fluid.
Discussion
The aims of this study were to clarify the role of endogenous tachykinin, especially substance P in NTS intrinsic cell excitability in young non-human primates and to investigate SHS exposure effects. Firstly, we found a primate-specific unique feature of NK1 receptor function which spontaneously modulated neuronal excitability and has not been observed in rats nor guinea pigs (Takeda et al. 2005; Sugiura-Tomita et al., 2006; Sekizawa et al., 2008). Secondly, we found the effect of NK1 receptor antagonist was phenotype-specific in intrinsic cell excitability in control (FA-exposed) animals. NK1 receptor blockade in RS neurons induced a slight but significant reduction in cell excitability while the reduction in DS neurons was dramatic (Figures 2 and 3). The data indicate firing phenotypes can be characterized by pharmacological receptors and/or physiological function as well. Thirdly, we found SHS exposure effects only in DS neurons despite the fact that it is still unknown what kinds of factors (i.e. chemicals) from the SHS and/or possibly what kinds of substrates, metabolites and/or resultant immunomodulating agents could be responsible for the effect. We have previously shown that SHS exposure significantly reduced spiking response to depolarizing current injection in DS neurons of 13-month-old rhesus monkeys (Sekizawa et al., 2010), but NK1 receptor antagonist application was not able to reinstate this SHS exposure effects in the current study, suggesting non-NK1 receptor-mediated mechanism(s), likely different kinds of exposure-related factors were causing exposure effects. Thus, SHS exposure might alter NTS signal processing in non-human primates probably in a variety of ways.
Neuronal phenotype-dependent NK1 receptor antagonist effects on spiking response
In DS neurons from FA-exposed animals, the NK1 receptor antagonist-induced ‘significant’ reduction in spiking response was not associated with any changes in AP characteristics, indicating the reduction was not due to changes in ion channel conductance. This suggests that NK1 receptors in DS neurons may link with gating properties of ion channels, such as recovery from inactivation stage, which can also regulate repetitive firings (or adaptation), regardless of channel conductance. In addition, there is a possibility that DS neurons have a higher density of NK1 receptors as well as substance P containing nerve innervations compared to RS neurons, which may also explain the different NK1 receptor antagonist effects on spiking response in FA-exposed animals. This DS neuron-specific NK1 receptor mechanism may be targeted and eliminated by SHS exposure, with the repetitive firing mechanism becoming independent from NK1 receptors and being compensated by any other mechanism(s), resulting in ablation of these antagonist effects as shown in Figure 3C and D. The lack of change in spiking response of DS neurons exposed to SHS may be caused by NK1 receptor malfunction, resulting from receptor internalization (Marvizón et al., 1997; Marvizón et al. 2003; Chen et al., 2009), which can be induced by robust release of substance P possibly during SHS exposure. On the other hand, SHS exposure may disrupt a mechanism(s) on the intracellular signalling pathways between NK1 receptors and ion channels, resulting in the lack of NK1 receptor antagonist effects on spiking response in DS neurons. NK1 receptors could be an important modulatory mechanism on cell excitability in the NTS. The present data indicate that SHS exposure may be specifically targeting this mechanism in the DS type subset of neurons, thus disabling tachykinin-dependent regulation.
On the other hand, in RS neurons SHS exposure had no effect on the contribution of endogenous substance P to intrinsic cell excitability since NK1 receptor antagonism-induced reduction in spiking response was not significantly different between FA and SHS exposure groups as shown in Figure 2D. Reduced spiking responses in RS neurons were accompanied by a trend of increased AP threshold, decreased AP peak amplitude and prolonged AP width (Figure 4), all of which might contribute to this reduction.
Unique function of NK1 receptor mechanism in non-human primates
NK1 receptors are coupled with G-protein (Gq/11) which can activate intracellular signalling including activation of protein kinase C, phospholipase C, etc. One of the electrophysiological responses from the receptor activation is cell membrane depolarization due to an inhibition of K+ currents (Yasuda et al., 2001) or an induction of non-specific cationic inward currents (Pena and Ramirez, 2004). The present study showed that NK1 receptor activation could modulate AP shape and the repetitive firing mechanism. This NK1 receptor function is a novel mechanism for excitable membranes possibly unique to primates, and could potentially be a mechanism for neuroplastic changes, especially in DS neurons, induced by various factors including SHS exposure.
A comparative study of different tachykinin receptor distribution has demonstrated that the NK1 receptors are the dominant tachykinin receptors in primate brain compared to other tachykinin receptors, NK2 and NK3 receptors (Rigby et al., 2005). Although the specific ligands of NK1, NK2 and NK3 receptors are substance P, neurokinin A and neurokinin B respectively, the selectivity is relatively poor and thus the NK1 receptors may also be activated by neurokinin A and/or neurokinin B. Additionally, substance P immunoreactivity in the NTS has been investigated in our lab. We found that substance P terminal staining in the NTS, under the same immunohistochemical and optical conditions, was strikingly increased in the primate as compared to the rodent (Figure S2). This suggests that substance P is spontaneously released from terminals in the in vitro brainstem slice preparation even if no active afferent signal was transmitted to NTS neurons. Our previous study using guinea pigs (Sekizawa et al., 2008) showed that substance P is also located at the respiratory afferent terminals in the NTS. Therefore, substance P located at the afferent terminals could be released more intensely at the synapses and modulate transmission more dynamically, when the TS was stimulated in order to evoke excitatory postsynaptic currents. In fact, NK1 receptor antagonists had an inhibitory effect on TS-evoked synaptic transmission in FA-exposed monkeys (Figure S3) but had no effect on synaptic transmission in FA-exposed guinea pigs (Sekizawa et al., 2008). This suggests that the tachykinin–, probably substance P–NK1 receptor mechanism in the primate NTS, plays an important role in signal transmission even under normal, healthy conditions, and thus neuroplastic changes in this mechanism could have a significant impact on respiratory reflex pathways.
NK1 receptor antagonism on NTS neuronal excitability in primates exposed to other environmental pollutants
In our previous study, the intrinsic cell excitability of NTS neurons was investigated in 6-month-old rhesus monkeys, which were chronically exposed to ozone or FA for 5 months of postnatal period (Chen et al., 2003). The excitability was significantly attenuated by NK1 receptor antagonist to a greater extent in ozone-exposed animals than that seen in FA-exposed animals. In the current study, using 13-month-old rhesus monkeys, NK1 receptor antagonist similarly reduced the spiking response in RS neurons in both FA and SHS exposure groups. The reduction in spiking responses in DS neurons was much greater in FA-exposed animals but not observed in SHS-exposed animals. These two studies differ in terms of the age of the animals and exposure particles, which may explain different NK1 receptor antagonist effects. However, since the current study demonstrated that the tachykinin–NK1 receptor antagonist effects on cell excitability are different depending on neuronal phenotypes, classification of cell types are crucial to detect underlying mechanisms.
Physiological relevance for findings
Biondo et al. (2004) reported that the NTS of SIDS victims showed a significantly higher substance P expression in comparison with non-SIDS victims, suggesting that the substance P–NK1 receptor mechanism might be linked with the incidence of sudden death. Excessive substance P release can internalize NK1 receptors to cause a non-functioning of the substance P–NK1 receptors, which were coincidentally observed in DS neurons from SHS-exposed primates in this study. Since SHS exposure can increase the risk of SIDS (U.S. Department of Health and Human Services, 2006), SHS-induced dysregulation of signal transmission may be critical to prolonged sleep apnoea and sudden death in the worst case.
Chronic exposure to SHS in children has been known as a factor in increasing airway reactivity, risk of asthma, lower airway disease and/or SIDS (Wang et al. 1994; Klonoff-Cohen et al. 1995; Strachan and Cook, 1997; DiFranza et al., 2004). Although effects of SHS exposure have been established by these epidemiologic studies, mechanisms especially in the CNS are poorly understood. Our present study shows that SHS exposure changes NK1 receptor contribution to intrinsic cell excitability, specifically in DS neurons while not affecting RS neurons. Considering the possibility that DS neurons are synaptically driven by capsaicin-sensitive unmyelinated fibres (Bailey et al., 2002), which are responding to noxious stimuli, SHS exposure may not change the regular breathing pattern but may modulate activity- or stimulus-dependent airway defence reflexes, such as observed in citric acid-induced coughs in guinea pigs (Joad et al., 2004), by eliminating the substance P-dependent mechanism. Confirming the physiological function of the firing phenotypes of NTS neurons and their pharmacological characteristics may suggest a therapeutic strategy for preventing adverse respiratory symptoms in children and even adults.
Stahl (2003) suggested that the NK1 receptor mechanism in the NTS is involved in gastrointestinal reflex response, particularly for nausea and emesis. Although the gastrointestinal region of NTS is slightly removed from the caudal NTS containing area postrema in the current study, the NK1 receptor mechanism may be similar between respiratory- and gustatory-related neural circuits. In addition, the caudal NTS lacks a complete blood brain barrier in rats (Gross et al., 1990), indicating the NTS has a different permeability compared to other regions of the brain.
The findings of the present study support the hypothesis that chronic exposure to SHS in children may worsen various respiratory symptoms through the tachykinin–, probably substance P–NK1 receptor mechanism in the NTS. Regulating or modulating this mechanism may reduce the risks of respiratory diseases even in non-SHS-exposed children.
Acknowledgments
This study was supported by funds from the California Tobacco-Related Disease Research Program, grant 9RT-0010, 13RT-0004 and a Health Services Research Award from the University of California Davis Medical Center. This research has also been funded in part by the United States Environmental Protection Agency through STAR grant RD832414 to the University of California at Davis. It has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors gratefully acknowledge the contributions of the Respiratory Diseases Unit at the California Regional Primate Research Center, the support of Primate Services at the California Regional Primate Research Center for animal handling, care, and coordination and veterinary care. The authors are also grateful to Rick Tham for excellent technical assistance for immunohistochemistry, to John M. Bric for performing and providing data of pulmonary function test, and to Drs Kayleen S. Kott and Andrea G. Bechtold for helpful criticisms in preparing the manuscript. The NK1 receptor antagonist, SR 140333, was a generous gift of Sanofi-Synthelabo Recherche, Montpellier, France.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AHP
after-hyperpolarization
- AP
action potential
- Cm
cell membrane capacitance
- DMSO
dimethyl sulphoxide
- DS
delayed-onset spiking
- FA
filtered air
- NK
neurokinin
- NTS
nucleus tractus solitarius
- Rin
input resistance
- RM
repeated measures
- RS
rapid-onset spiking
- SHS
second-hand tobacco smoke
- SIDS
sudden infant death syndrome
- TS
tractus solitarius
Conflicts of interest
None of the authors has a conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Second-hand tobacco smoke (SHS) exposure effects on endogenous substance P contribution to spiking response. A. Group data show that spiking response was significantly attenuated by NK1 receptor antagonism in both filtered air (FA) and SHS exposure groups [two-way repeated measures (RM) anova interaction, F(6,66) = 12.606, P < 0.001 and F(6,72) = 4.447, P < 0.001 respectively]. B. NK1 receptor sensitive spiking response to depolarizing current injection. The antagonist effects on spiking responses were not significantly different between FA and SHS exposure groups (two-way RM anova interaction, F(6,138) = 1.612, P = 0.148), masking SHS exposure effect as observed especially in delayed-onset spiking neurons.
Figure S2 Intense substance P immunoreactivity in primate nucleus tractus solitarius (see Appendix S1 for method). Photographs show examples of substance P immunoreactivity in the nucleus tractus solitarius from each one rhesus monkey and one guinea pig. These photographs were obtained under the same settings of microscope, laser and fluorescent detection, as well as the same number of slices used for Z-stack reconstruction between two animal species. Green colour indicates substance P immunoreactivity and blue indicates neuronal specific nuclear protein immunoreactivity. Substance P immunoreactivity is more intense in the rhesus monkey (left) than the guinea pig (middle), confirmed by group data (right; unpaired t-test, *P = 0.022). The number in the parentheses indicates the number of animals.
Figure S3 NK1 receptor antagonist depressed tractus solitarius-evoked excitatory postsynaptic current (EPSC) amplitudes in nucleus tractus solitarius neurons. A. Example traces of tractus solitarius-evoked EPSCs (eEPSCs) from a filtered air (FA)-exposed animal in the absence (gray) or presence (black) of NK1 receptor antagonist (SR140333, 3 µM) under the voltage clamp condition at −60 mV. Dots (•) indicate stimulus artefacts with 20 ms intervals. B. Group data for the first eEPSC amplitude in the absence (solid) and presence (hatched) of NK1 receptor antagonist. NK1 receptor antagonist significantly decreased first eEPSC amplitudes in both FA and Second-hand tobacco smoke (SHS) exposure groups (paired t-test, *P = 0.006 and *P = 0.037 respectively). The effect of the antagonist was not different between exposure groups (unpaired t-test, P = 0.222) (right graph). C. Group data of paired pulse ratio (PPR) in the absence (solid) and presence (hatched) of NK1 receptor antagonist. PPR was calculated by dividing first eEPSC peak amplitudes into second eEPSC peak amplitudes. As the second eEPSC amplitudes were also decreased by NK1 receptor antagonist (paired t-test, P = 0.019 for FA and P = 0.013 for SHS), the paired pulse ratio was not changed by the antagonist in either exposure group (paired t-test, FA: P = 0.135; SHS: P = 0.375) (left graph). The antagonist effects between exposure groups were not different (unpaired t-test, P = 0.151) (right graph).
Appendix S1 Supplemental method.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Jin YH, Doyle MW, Andresen MC. Vanilloid-sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius. J Neurosci. 2002;22:8230–8237. doi: 10.1523/JNEUROSCI.22-18-08230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Asthma as an axon reflex. Lancet. 1986;1:242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Biondo B, Magagnin S, Bruni B, Cazzullo A, Tosi D, Matturri L. Glial and neuronal alterations in the nucleus tractus solitarii of sudden infant death syndrome victims. Acta Neuropathol. 2004;108:309–318. doi: 10.1007/s00401-004-0895-2. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bonham AC, Plopper CG, Joad JP. Neuroplasticity in nucleus tractus solitarius neurons after episodic ozone exposure in infant primates. J Appl Physiol. 2003;94:819–827. doi: 10.1152/japplphysiol.00552.2002. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bechtold AG, Tabor J, Bonham AC. Exercise reduces GABA synaptic input onto nucleus tractus solitarii baroreceptor second-order neurons via NK1 receptor internalization in spontaneously hypertensive rats. J Neurosci. 2009;29:2754–2761. doi: 10.1523/JNEUROSCI.4413-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, et al. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, Srinivasan IP, Kaegi D, Chang JC, et al. The Effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA. 1995;273:795–798. doi: 10.1001/jama.1995.03520340051035. [DOI] [PubMed] [Google Scholar]
- Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, et al. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol. 1989;66:2032–2038. doi: 10.1152/jappl.1989.66.5.2032. [DOI] [PubMed] [Google Scholar]
- Marvizón JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizón JCG, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance p release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Bonham AC, Kott KS, Joad JP. Chronic exposure to sidestream tobacco smoke augments lung C-fiber responsiveness in young guinea pigs. J Appl Physiol. 1999;87:757–768. doi: 10.1152/jappl.1999.87.2.757. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Joad JP, Bonham AC. Chronic passive cigarette smoke exposure augments bronchopulmonary C-fibre inputs to nucleus tractus solitarii neurones and reflex output in young guinea-pigs. J Physiol. 2000;523:223–233. doi: 10.1111/j.1469-7793.2000.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Onodera K. Membrane properties of the stretch receptor neurones of crayfish with particular reference to mechanisms of sensory adaptation. J Physiol. 1969;200:161–185. doi: 10.1113/jphysiol.1969.sp008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby M, O'Donnell R, Rupniak NM. Species differences in tachykinin receptor distribution: further evidence that the substance P (NK1) receptor predominates in human brain. J Comarat Neurol. 2005;490:335–353. doi: 10.1002/cne.20664. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Chen CY, Bechtold AG, Tabor JM, Bric JM, Pinkerton KE, et al. Extended secondhand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young guinea pigs. Eur J Neurosci. 2008;28:771–781. doi: 10.1111/j.1460-9568.2008.06378.x. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Joad JP, Pinkerton KE, Bonham AC. Secondhand tobacco smoke exposure differentially alters nucleus tractus solitarius neurons at two different ages in developing non-human primates. Toxicol Appl Pharmacol. 2010;242:199–208. doi: 10.1016/j.taap.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Stahl SM. The ups and downs of novel antiemetic drugs, part 1: substance P, 5-HT, and the neuropharmacology of vomiting. J Clin Psychiatry. 2003;64:498–499. doi: 10.4088/jcp.v64n0501. [DOI] [PubMed] [Google Scholar]
- Strachan DP, Cook DG. Health effects of passive smoking. 1. Parental smoking and lower respiratory illness in infancy and early childhood. Thorax. 1997;52:905–914. doi: 10.1136/thx.52.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura-Tomita M, Yasuda K, Mori R, Hasumi-Nakayama Y, Tomita I, Nakamura M, et al. NK1 receptor activation by geniohyoid primary afferents modulates parasympathetic postganglionic neuronal excitability in the rat. Brain Res. 2006;1112:106–113. doi: 10.1016/j.brainres.2006.06.120. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Nasu M, Ikeda M, Kadoi J, Matsumoto S. Activation of NK1 receptor of trigeminal root ganglion via substance P paracrine mechanism contributes to the mechanical allodynia in the temporomandibular joint inflammation in rats. Pain. 2005;116:375–385. doi: 10.1016/j.pain.2005.05.007. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke. 2006. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006. U.S. Printing Office: Washington, DC.
- Wang X, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG, et al. A longitudinal study of the effects of parental smoking on pulmonary function in children 6–18 years. Am J Respir Crit Care Med. 1994;149:1420–1425. doi: 10.1164/ajrccm.149.6.8004293. [DOI] [PubMed] [Google Scholar]
- Wang L, Joad JP, Abel K, Spinner A, Smiley-Jewell S, Liu H, et al. Effects of environmental tobacco smoke on the developing immune system of infant monkeys. J Allergy Clin Immunol. 2007;120:445–451. doi: 10.1016/j.jaci.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Wang L, Joad JP, Zhong C, Pinkerton KE. Effects of environmental tobacco smoke exposure on pulmonary immune response in infant monkeys. J Allergy Clin Immunol. 2008;122:400–406. doi: 10.1016/j.jaci.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Gortmaker S, Walker DK, Sobol A. Maternal smoking and childhood asthma. Pediatrics. 1990;85:505–511. [PubMed] [Google Scholar]
- Yasuda K, Robinson DM, Selvaratnam SR, Walsh CW, McMorland AJ, Funk GD. Modulation of hypoglossal motoneuron excitability by NK1 receptor activation in neonatal mice in vitro. J Physiol. 2001;534:447–464. doi: 10.1111/j.1469-7793.2001.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Y, Shi J, Larson DF, Watson RR. Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med. 2002;227:823–829. doi: 10.1177/153537020222700916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


