Abstract
BACKGROUND AND PURPOSE
Erectile dysfunction correlates with cardiovascular disease and its common risk factors due to the development of endothelial dysfunction. Positive effects on endothelial and erectile function have been described for substances inhibiting the renin-angiotensin-system. Here, we investigated in an atherosclerosis model, whether telmisartan (angiotensin receptor blocker) and ramipril (angiotensin converting enzyme inhibitor) are equivalent or the combination of both is superior in improving endothelial function in the aorta and the corpus cavernosum and in reducing atherosclerosis.
EXPERIMENTAL APPROACH
Wild-type (WT, C57/B6) and apolipoprotein-E-deficient (ApoE−/−) mice were treated with a cholesterol-rich diet for 8 weeks. ApoE−/− mice were supplemented with either telmisartan (20 mg·kg−1·day−1), ramipril (2.5 mg·kg−1·day−1) or the combination thereof.
KEY RESULTS
Systolic blood pressure significantly decreased in treatment groups (P < 0.001), with significantly smaller reduction under ramipril monotherapy (P < 0.05). Endothelial function (assessed by pharmacological stimulation of aortic rings and corpus cavernosum in organ bath chambers) was impaired in ApoE−/− mice compared to WT animals, which was improved by all three treatments to a comparable extent (P < 0.05). Atherosclerotic lesion size in the ascending aorta and aortic sinus (P < 0.001), the amount of lipid peroxides in cavernosal and aortic tissue (P < 0.05) and free radical load (dihydroethidium-stain) (P < 0.05) were enhanced in untreated ApoE−/− mice in comparison to WT animals and were significantly reduced by either treatment. In penile tissue, expression of eNOS could be restored by renin-angiotensin-aldosterone system blockade.
CONCLUSIONS AND IMPLICATIONS
Telmisartan and ramipril significantly improved endothelial function of aortic and cavernosal tissues in ApoE−/− via reduction of oxidative stress. Combination of both agents does not enhance beneficial effects significantly.
Keywords: telmisartan, ramipril, endothelial function, erectile function
Introduction
Erectile dysfunction (ED), defined as the inability to attain and/or maintain an erection sufficient for satisfactory sexual intercourse for a period of at least 6 months, does not only represent a highly prevalent health problem of considerable socioeconomic impact, but is considered an early end-organ manifestation of atherosclerosis as well (NIH, 1993; Feldman et al., 2000; Kirby et al., 2001). Therefore, diagnosis of ED, especially in younger patients gains greater importance, as it should prompt investigation of cardiovascular risk factors (Thompson et al., 2005). Hypertension, dyslipidemic conditions, diabetes and further cardiovascular risk factors correlate strongly with presence and extent of ED (Esposito et al., 2004). These conditions are associated with development of endothelial dysfunction, that is, impaired release of nitric oxide from endothelial cells leading to decreased vasomotor responses (Harrison, 1997). Because of the small diameter of the helical arteries and a relatively high content of endothelium and smooth muscle within the corpora cavernosa, erectile tissue is prone to early endothelial dysfunction caused by oxidative stress and reduced bioavailability of nitric oxide, which occurs early in the process of atherosclerosis (Yavuzgil et al., 2005; Billups et al., 2008).
Angiotensin II (Ang II) and expression, as well as activity of the angiotensin (AT)1 receptor (receptor nomenclature follows Alexander et al., 2009) mediates vascular oxidative stress and inflammatory processes and finally endothelial dysfunction, while genetic disruption of the AT1 receptor leads to inhibition of vascular oxidative stress, endothelial dysfunction and formation of atherosclerotic lesions (Griendling et al., 1994; Rajagopalan et al., 1996; Wassmann et al., 2004).
Moreover, besides being subject to systemic changes in the activity of the renin-angiotensin-aldosterone system (RAAS), the local angiotensin homeostasis of erectile tissue has to be taken into account, as the corpus cavernosum produces and secretes physiologically relevant amounts of Ang II which contribute to the development of detumescence (Kifor et al., 1997). Previously, we observed reduction of vascular oxidative stress and endothelial dysfunction with subsequent improvement of endothelial function of penile tissue in apolipoprotein-E-deficient (ApoE−/−) treated with the AT1 receptor antagonist irbesartan, which is in line with clinical findings of advantageous effects of AT1 receptor blockade in hypertensive patients (Fogari et al., 2001; Dusing, 2003; Baumhakel et al., 2008). For reduction of Ang II formation via angiotensin converting enzyme (ACE) inhibitors, comparable effects have been described (Dorrance et al., 2002; Speel et al., 2005).
However, local Ang II formation in the vasculature, as well as in erectile tissue is supposed to play a major role in promoting oxidative stress and inflammatory processes, independent of ACE functionality (Hirono et al., 2007). On the other hand, blockade of AT1 receptors without ACE-inhibition leads to accumulation of Ang II, stimulating the AT2 receptor, while degradation of bradykinin through ACE reduces bradykinin B2 receptor-mediated stimulation of endothelial nitric oxide synthesis in erectile tissue (Becker et al., 2001). Thus, the combination of both modes of action, ACE inhibition and AT1 receptor blockade, could lead to synergistic effects, greater than the results achievable with monotherapy using either class of drug.
The aim of this study was to determine the effect of the AT1 receptor antagonist telmisartan, the ACE inhibitor ramipril and the combination of both substances on vascular and cavernosal endothelial function as well as on oxidative stress and lipid peroxidation in aortic tissue and corpora cavernosa of cholesterol fed ApoE−/− mice.
Methods
Animals and procedures
All animal care and experimental procedures were in accordance with institutional guidelines, the German animal protection law and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). Male C57BL/6J mice (wild-type, WT) and ApoE−/− mice (C57BL/6J, genetic background, Charles River, Sulzfeld, Germany), previously demonstrated to be an appropriate model for the assessment of cavernosal endothelial function in atherosclerotic mice, were used in this study (Xie et al., 2007). The animals were maintained at 22°C with a 12 h light/dark cycle. All mice were fed a high-fat, cholesterol-rich diet (21% fat, 19.5% casein and 1.25% cholesterol, Ssniff, Soest, Germany).
Twenty ApoE−/− mice were used as a positive, and 20 WT mice as a negative control respectively. These animals received the atherogenic diet throughout 8 weeks, while three groups, each consisting of 20 ApoE−/− mice were fed the same diet, supplemented orally with either telmisartan in drinking water (20 mg·kg−1·day−1), ramipril in chow-pellets (2.5 mg·kg−1·day−1) or the combination thereof at the same doses throughout the treatment period.
Systolic blood pressure and heart rate were measured non-invasively via the tail-cuff method in conscious mice (BP-2000, Visitech-Systems, Apex, NC, USA). After the treatment period, mice were killed and tissue and blood samples were collected immediately. Plasma lipid concentrations were measured with low density lipoprotein (LDL)-cholesterol being calculated with the Friedewald formula. Chemicals were obtained from Sigma-Aldrich, Taufkirchen, Germany. All chemicals were dissolved in distilled water.
Preparation of corpus cavernosum strips and aortic rings and tension recording
Penises were removed and immersed in chilled Tyrode-buffer containing in mM: NaCl 118.0, CaCl2 2.5, KCl 4.73, MgCl2 1.2, KH2PO4 1.2, NaHCO3 25.0, EDTA 0.026, D(+)glucose 5.5 (pH 7.4). The glans penis and urethra were excised and adherent tissue was removed, keeping the tunica albuginea intact. Strips of corpora cavernosal strips (CCS) were separated, cleaned and suspended in organ baths chambers filled with the Tyrode solution described above (37°C, aerated with 95% O2 and 5% CO2). CCS were attached to a force transducer recording isometric tension and subsequently equilibrated at a resting tension of 3 mN, which was maintained throughout the experiment. Following equilibration, CCS were precontracted with the α-adrenoceptor agonist (R)-(-)-phenylephrine-HCl (5 µM). After a steady state of contraction had stabilized, drugs were added in increasing concentrations to obtain cumulative concentration-response curves for carbachol (representing an endothelium-dependent relaxing agent, 1 nM–1 µM) and glyceryl trinitrate (as an nitric oxide-donor, 100 nM–100 µM). Drugs were washed out before adding the next substance. The relaxing effect of carbachol was abolished by adding Nω-nitro-L-arginine methyl ester (L-NAME, 1 µM). CCS without any response to carbachol (relaxation < 10%) were excluded from statistical analysis due to a presumable damage of the endothelium. Relaxation of cavernous smooth muscle as a key step in haemodynamics of erection is strongly dependent on nitric-oxide production by the epithelial lining of the cavernosal sinusoids, subsequent to nervous and endocrine stimuli. A deterioration of endothelium-dependent relaxation to carbachol indicates dysfunction of the cavernosal endothelium (Buyukafsar and Un, 2003).
After excision, the descending thoracic aorta was immersed in Tyrode solution (as described above) immediately and adventitial tissue was carefully removed. Three-millimetre rings were mounted in organ bath chambers and attached to a force transducer as described above. Aortic rings were equilibrated at a resting tension of 10 mN, which was maintained throughout the experiment. Contraction of aortic rings was induced by adding (R)-(-)-phenylephrine-HCl (10 µM). Cumulative concentration-response curves for carbachol (1 nM–100 µM) and glyceryl trinitrate (100 nM–100 µM) were obtained as described above, abolishing the relaxing effect of carbachol by adding L-NAME (1 µM).
Staining procedures
Corpora cavernosa and hearts including the ascending aorta were snap-frozen at −80°C and sectioned on a Leica cryostat, obtaining transverse sections of 10 µm thickness. At least five consecutive sections per animal and per staining were used for analysis.
For assessment of atherosclerotic lesion size, sections of the aortic sinus as well as the ascending aorta were stained with Oil-Red O, as described before (Laufs et al., 2005). Morphometric analysis of microscopic images was performed by using Image-J software 1.37v (National Institutes of Health, USA) to determine the relation of lipid-stained plaque area to vessel diameter.
Fluorescence produced by the oxidation of dihydroethidium (DHE) by superoxide anions, was used to quantify superoxide production in situ for both tissues. Krebs-HEPES buffer containing DHE (2 µM) was topically applied to each tissue section and subsequently incubated in a dark humidified chamber at 37°C for 30 min. Aortic tissue and CCS from each treatment group were processed in parallel and images were immediately acquired and digitally stored, using fluorescent microscopy with acquisition parameters kept identical at all times. Intensity of fluorescence was subsequently quantified using Image-J software 1.37v. Prior to measurement, images were converted to greyscale and the epithelial regions of vessels and cavernae of erectile tissue were outlined. Each pixel within the outlined area was digitally allocated a numeric value (0 = black, 255 = white) according to its brightness which were subsequently averaged.
Measurement of lipid peroxidation
Corpus cavernosal as well as abdominal aortic tissue was homogenized in distilled water. Oxidative degradation of cell membrane lipids, dependent on the equilibrium of cellular oxidative load and antioxidant potential was assessed via redox reactions with ferric ions performed in deoxygenated chloroform-methanol, as in the instructions of the Lipid Peroxidation Assay Kit II (Calbiochem, Darmstadt, Germany). Subsequent to protein assessment, hydroperoxide concentrations were expressed as nmol·mg−1 protein (Laufs et al., 2005).
Immuno-staining for endothelial nitric oxide synthase (eNOS)
Corpora cavernosa segments were sectioned as described above. Immediately prior to staining, sections were air-dried and bordered with water-repellent bromopropane/dipentene-pen. Slides were then incubated in 4%-paraformaldehyde for 2 min and subsequently rinsed in phosphate-buffered saline (PBS) for 5 min. Following which, slides were treated twice with a 0.5% solution of tert-octylphenoxypolyethanol (Igepal, Sigma Aldrich, Deisenhofen, Germany) in PBS for 5 min before being incubated in a 0.5% solution of goat serum (Sigma Aldrich, Deisenhofen, Germany) in PBS. The primary antibody (eNOS/NOS type III rabbit-anti-mouse, isotype IgG, Becton Dickinson, Heidelberg, Germany) was diluted 1:100 in 0.5% goat serum in PBS and 100 µL applied to each section. Slides were incubated for 90 min in a humid chamber and were then rinsed in PBS three times before being incubated with the secondary antibody using the same amount (TRITC-conjugated goat-anti-rabbit, affinity-isolated and adsorbed with human IgG, Sigma Aldrich, Deisenhofen, Germany, dilution 1:100 in 0.5% goat serum in PBS) for 45 min in a humid chamber. Slides were subsequently rinsed in PBS three times for 10 min and embedded in mounting medium for fluorescence microscopy (Dako Deutschland GmbH, Hamburg, Germany). Digital images of the sections were acquired immediately afterwards (fluorescence microscopy, G-2A-fluorescence filter, excitation 510–560 nm, 100-fold magnification). Exposure of the TRITC-conjugated secondary antibody to artificial light or daylight was avoided at all times. Images were subsequently converted to grey-scale and Image-J software was used to assess the fluorescence intensity by outlining cavernosal tissue and measuring brightness in pixel values (black = 0, white = 255).
Statistical analysis
All data are expressed as mean ± SEM. Statistical significance was assumed when P < 0.05. In organ bath chamber experiments, the arithmetic mean of the response of all aortic rings and CCS was calculated for each animal. Those mean values were averaged for the treatment group. Intergroup differences were assessed with the anova test followed by Newman–Keuls post hoc analysis (GraphPad Prism 4.03, GraphPad, San Diego, CA, USA). Quantification and analysis of all assays was performed without knowledge of the treatments.
Results
Vital parameters
Systolic blood pressure significantly decreased in all treatment groups in comparison to untreated ApoE−/− and WT animals (Table 1). Treatment with telmisartan alone or in combination with ramipril decreased systolic blood pressure to a greater extent than ramipril monotherapy. Heart rate was significantly lower in untreated ApoE−/− mice than in all other groups. Irrespective of intervention, total cholesterol and LDL cholesterol levels of all ApoE−/− groups were higher than those measured in WT animals (Table 1).
Table 1.
Cardiovascular parameters and lipid values of experimental groups
| Systolic blood pressure (mmHg) | Heart rate (bpm) | Total cholesterol (mg·L−1) | LDL cholesterol (mg·L−1) | HDL cholesterol (mg·L−1) | Triglycerides (mg·L−1) | |
|---|---|---|---|---|---|---|
| WT | 111.7 ± 2.5 | 640.8 ± 9.9+ | 21.2 ± 1.9 | 5.3 ± 1.7 | 14.6 ± 2.7 | 7.9 ± 0.8 |
| ApoE−/− | 105.6 ± 2.3 | 593.4 ± 10.9 | 117.8 ± 17.1** | 90.6 ± 14.1** | 26.0 ± 4.2 | 10.8 ± 3.1 |
| ApoE−/−+ ramipril (2.5 mg·kg−1·day−1) | 91.1 ± 2.5**,++ | 635.8 ± 6.2+ | 114.6 ± 18.3** | 85.2 ± 14.8** | 32.6 ± 4.7 | 13.2 ± 3.3 |
| ApoE−/−+ telmisartan (20 mg·kg−1·day−1) | 79.8 ± 3.3**,++,# | 621.2 ± 4.3+ | 121.3 ± 14.6** | 90.7 ± 12.4** | 28.5 ± 5.0 | 11.6 ± 2.5 |
| ApoE−/−+ telmisartan/ramipril (20 mg·kg−1·day−1; 2.5 mg·kg−1·day−1) | 79.7 ± 2.7**,++,# | 639.1 ± 10.2+ | 80.3 ± 16.2* | 68.8 ± 11.3* | 21.5 ± 9.4 | 12.2 ± 3.2 |
Systolic blood pressure and heart rate were determined in all animals. Lipid levels were measured in five to nine mice per group. Data shown are means ± SEM.
P < 0.05 versus WT
P < 0.001 versus WT
P < 0.001 versus ApoE−/−
P < 0.05 versus ApoE−/−
P < 0.05 versus ApoE−/−+ ramipril.
ApoE−/−, apolipoprotein-E-deficient; HDL, high density lipoprotein; LDL, low density lipoprotein; WT, wild-type.
Atherosclerotic lesion size
Ascending aortae, as well as the aortic sinus of untreated ApoE−/− mice exhibited a greater extent of atherosclerotic lesion formation, as shown by Oil Red-O staining than WT-animals. All interventions reduced plaque formation to a similar extent, compared with ApoE−/− mice without treatment (Figure 1).
Figure 1.
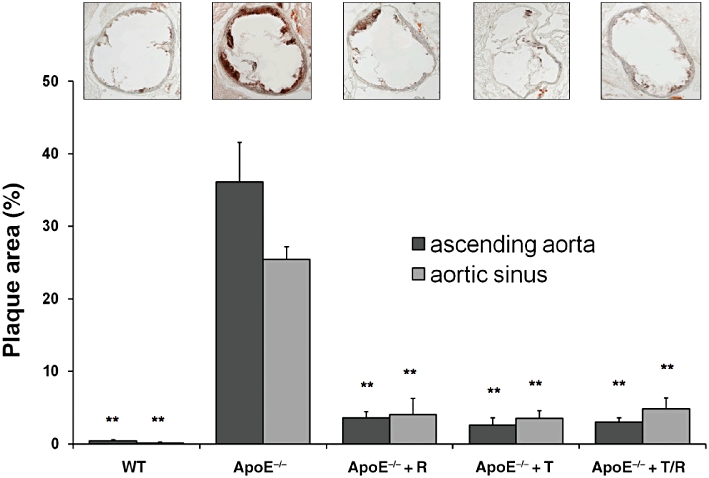
Atherosclerotic lesion size. Extent of atherosclerotic lesions was measured in the aortic sinus (representative images shown) and the ascending aorta. Mice were treated with ramipril (R), or telmisartan (T) or in combination (T/R) for 8 weeks. Data shown are means ± SEM from five animals per group. **P < 0.001, different from values for ApoE−/− mice. ApoE−/−, apolipoprotein-E-deficient; WT, wild-type.
Endothelial function in CCS
Contractile responses to phenylephrine of samples of both tissues, corpora cavernosa and aorta, were not different between all treatment groups (Table 2). CCS from untreated ApoE−/− animals showed a reduced relaxation to carbachol in comparison to WT animals which was significant at all concentrations above 0.03 µM carbachol. All treatment groups showed an improvement of endothelium-dependent relaxations to carbachol with no differences between treatment groups (Figure 2). Additionally, the potency of carbachol to induce endothelium dependent relaxation (as pD2) was impaired in ApoE−/− animals, with improvement in all treatment groups (Table 3). Neither the maximum effect nor the potency to induce endothelium-independent relaxation to glyceryl trinitrate differed at any concentration, between groups (Table 3).
Table 2.
Phenylephrine-induced contraction of corpora cavernosal strips and aortic rings
| % tension at baseline | Wild-type | ApoE−/− | ApoE−/−+ ramipril | ApoE−/−+ telmisartan | ApoE−/−+ telmisartan/ramipril |
|---|---|---|---|---|---|
| Aortic tissue | |||||
| Carbachol | 19.03 ± 0.97 | 22.63 ± 2.10 | 20.93 ± 3.40 | 24.47 ± 2.14 | 22.20 ± 0.92 |
| GTN | 20.41 ± 2.64 | 20.00 ± 1.88 | 20.80 ± 2.28 | 27.02 ± 3.21 | 27.01 ± 1.78 |
| Cavernosal tissue | |||||
| Carbachol | 7.69 ± 0.55 | 9.68 ± 0.78 | 10.41 ± 0.89 | 10.23 ± 1.68 | 8.78 ± 1.32 |
| GTN | 9.44 ± 0.81 | 11.22 ± 0.89 | 11.14 ± 1.57 | 10.58 ± 1.57 | 9.83 ± 1.50 |
Contractile responses (% of baseline tension) of aortic and cavernosal tissue to phenylephrine before relaxation with carbachol or GTN. No significant difference within all treatment groups (n.s.). Five to ten animals per group. Mean ± SEM.
ApoE−/−, apolipoprotein-E-deficient; GTN, glyceryl trinitrate.
Figure 2.
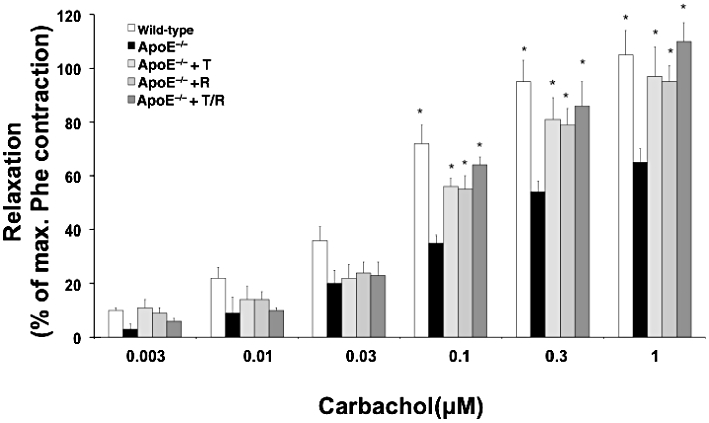
Corpus cavernosum isometric tension recording. Relaxation to carbachol, as % of phenylephrine (Phe)-induced contraction, was assessed in corpora cavernosal strips. Apolipoprotein-E-deficient (ApoE−/−) mice were treated with ramipril (R), or telmisartan (T) or in combination (T/R). Data shown are means ± SEM from six to nine animals per group. *P < 0.05, different from values for ApoE−/− mice.
Table 3.
Parameters (pD2, maximum effect) of endothelium-dependent or -independent relaxations in aortic rings and corpora cavernosal strips
| Wild-type | ApoE−/− | ApoE−/−+ ramipril | ApoE−/−+ telmisartan | ApoE−/−+telmisartan/ramipril | |
|---|---|---|---|---|---|
| Aortic tissue, response to carbachol | |||||
| pD2 (-log) | −5.85 ± 0.22* | −4.71 ± 0.20 | −6.27 ± 0.34* | −5.47 ± 0.18* | −5.70 ± 0.31* |
| Maximum relaxation (%) | 76.4 ± 4.9* | 49.3 ± 5.4 | 81.2 ± 7.7* | 75.7 ± 7.7* | 69.5 ± 4.1* |
| Cavernosal tissue, response to carbachol | |||||
| pD2 (-log) | −7.45 ± 0.07* | −7.16 ± 0.18 | −7.54 ± 0.11* | −7.58 ± 0.08* | −7.66 ± 0.08* |
| Maximum relaxation (%) | 105.3 ± 9.5* | 64.5 ± 4.5 | 95.3 ± 5.6* | 97.2 ± 11.6* | 109.2 ± 13.3* |
| Aortic tissue, response to glyceryl trinitrate | |||||
| pD2 (-log) | −6.22 ± 0.08 | −6.36 ± 0.12 | −6.40 ± 0.20 | −6.69 ± 0.09 | −6.72 ± 0.15 |
| Maximum relaxation (%) | 124.3 ± 11.8 | 141.9 ± 9.1 | 142.9 ± 11.4 | 108.2 ± 6.1 | 113.5 ± 7.1 |
| Cavernosal tissue, response to glyceryl trinitrate | |||||
| pD2 (-log) | −5.56 ± 0.14 | −5.69 ± 0.13 | −5.54 ± 0.20 | −5.57 ± 0.13 | −5.73 ± 0.19 |
| Maximum relaxation (%) | 46.4 ± 6.5 | 57.3 ± 6.9 | 43.5 ± 3.3 | 46.6 ± 5.9 | 53.6 ± 7.4 |
Endothelium-dependent relaxations were induced by carbachol and endothelium-independent relaxations by glyceryl trinitrate. Data shown are means ± SEM from six to ten mice per group.
P < 0.05 versus ApoE−/− mice.
ApoE−/−, apolipoprotein-E-deficient.
Vascular endothelial function
Endothelium-dependent relaxation of aortic tissue to carbachol was impaired in untreated ApoE−/− animals in comparison to WT mice at all concentrations. All three treatments improved this endothelial function equally, that is, there were no differences between the treatment groups (Figure 3). The pD2 of carbachol also improved significantly in all treatment groups, whereas endothelium-independent relaxations to glyceryl trinitrate did not differ significantly at any concentration between groups (Table 3).
Figure 3.
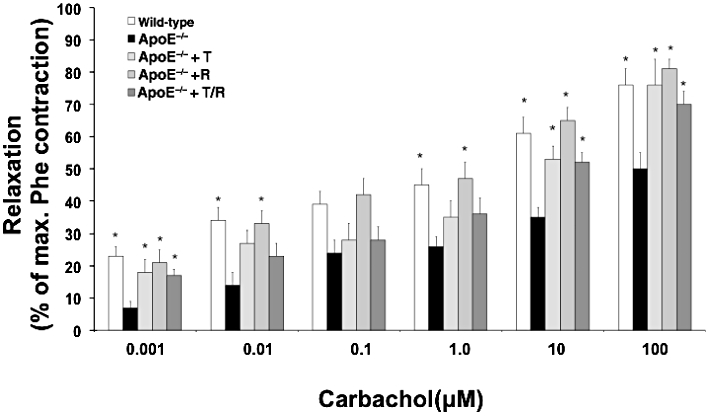
Aortic ring isometric tension recording. Relaxation to carbachol in % of phenylephrine (Phe)-induced contraction was assessed in aortic rings. Apolipoprotein-E-deficient (ApoE−/−) mice were treated with ramipril (R), or telmisartan (T) or in combination (T/R). Data shown are means ± SEM from seven to ten animals per group. *P < 0.05, different from values for ApoE−/− mice.
Dihydroethidium fluorescence
Intensity of the fluorescence signals measured subsequent to staining with DHE was higher in ascending aorta and CCS of untreated ApoE−/− mice in contrast to that in tissues from WT animals. ApoE−/− animals treated with telmisartan, ramipril, or both showed a significant reduction in DHE fluorescence signals in comparison to untreated ApoE−/− mice while there were no differences in this variable between treatment groups (Figure 4).
Figure 4.
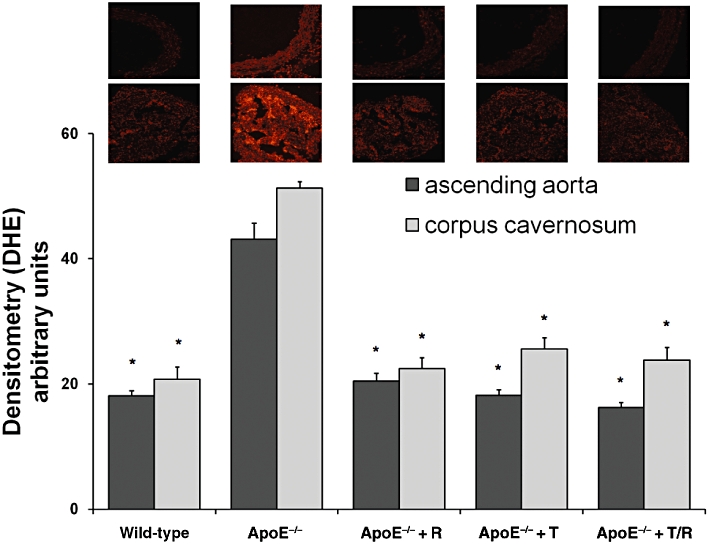
Oxidative stress [dihydroethidium (DHE) fluorescence]. Ascending aortae (representative images above) and corpora cavernosa (representative images below) were stained with DHE (five per group). Apolipoprotein-E-deficient (ApoE−/−) mice were treated with ramipril (R), or telmisartan (T) or in combination (T/R).Data shown are means ± SEM. *P < 0.05, different from values for ApoE−/− mice.
Lipid peroxidation
The content of hydroperoxides in aortic and cavernosal tissue of ApoE−/− mice which had not received any treatment exceeded that measured in WT animals. Intervention with telmisartan, ramipril or their combination reduced the formation of lipid hydroperoxides in both tissues, without differences between the treatment groups (Figure 5).
Figure 5.
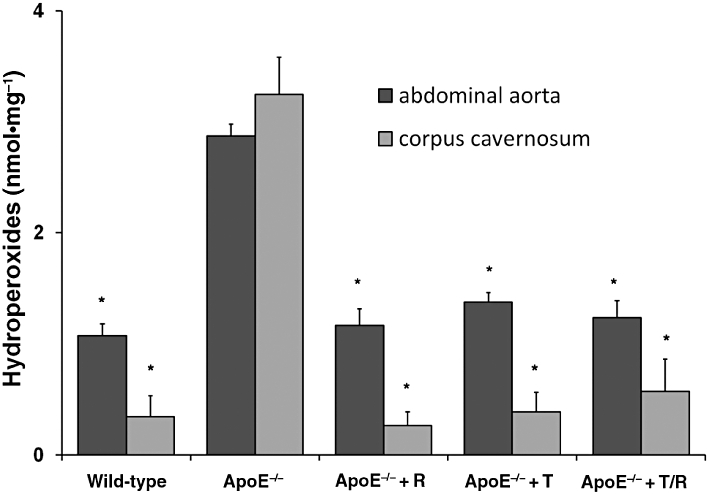
Oxidative stress (lipid peroxidation). Levels of hydroperoxides were assessed in abdominal aortic segments and corpora cavernosal tissue and expressed in nmol (mg protein)−1. Apolipoprotein-E-deficient (ApoE−/−) mice were treated with ramipril (R), or telmisartan (T) or in combination (T/R). Data shown are means ± SEM from five animals per group. *P < 0.05, different from values for ApoE−/− mice.
Endothelial nitric oxide synthase
The eNOS was quantified by immunohistochemistry-staining of corpora cavernosa sections. In ApoE−/− mice, eNOS was significantly decreased and could partly restored by treatment with telmisartan, ramipril, or the combination (Figure 6).
Figure 6.
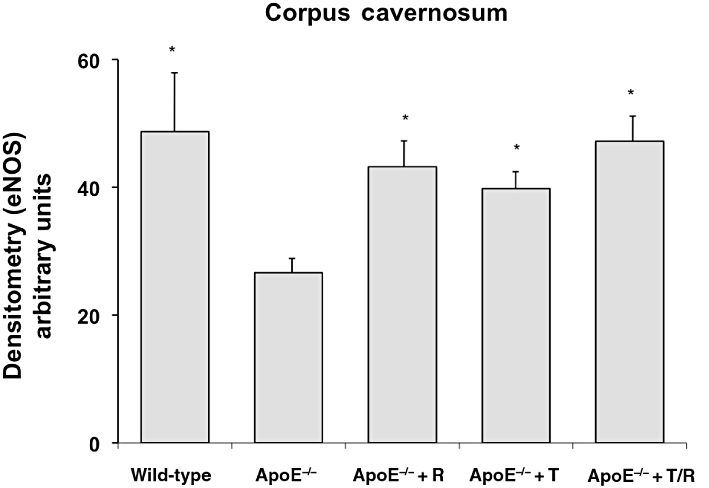
Immunohistochemical staining for eNOS. The amounts of eNOS were quantified by immunohistochemical staining of corpora cavernosa sections following fluorescence microscopy. Apolipoprotein-E-deficient (ApoE−/−) mice were treated with ramipril (R), or telmisartan (T) or in combination (T/R). Data shown are means ± SEM from five mice per group. *P < 0.05, different from values for ApoE−/− mice.
Discussion
The present data show that the AT1 receptor antagonist telmisartan and the ACE inhibitor ramipril were equally effective in restoring aortic and cavernosal endothelial function and in reducing atherosclerosis in a murine model of cardiovascular disease, supposedly via reduction of excessive amounts of reactive oxygen species (ROS) in both tissues with restoration of eNOS as the pathophysiological link. Combination of both drugs did, however, not provide any further amelioration of vascular damage.
The predictive value of end-organ damage for cardiovascular events and, vice versa, their regression by treatment for cardiovascular protection is well accepted. The effects of differentially blocking the RAAS on end-organ damage in individuals bearing cardiovascular risk factors such as hypertension and dyslipidaemia have been widely discussed in terms of left ventricular function, renal function, cerebrovascular function and congestive heart failure (Friedrich et al., 2006; Werner et al., 2008). Little attention has been paid to functional changes in erectile tissue as a very sensitive target-organ in cardiovascular disease. The corpora cavernosa of the penis represent the most abundantly endothelialized tissue in the human body as physiology of erection is strongly dependent on the release of nitric oxide from the cavernous endothelium, making ED an indicator of endothelial dysfunction appearing 3 to 12 years prior to other cardiovascular manifestations of end-organ damage (Bookstein et al., 1990; Harrison, 1997; Speel et al., 2003; Baumhakel and Bohm, 2007).
Despite evidence for the improvement of ED via monotherapy with inhibitors of the RAAS, the ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and the Telmisartan Randomized AssessmeNT Study in ACE-INtolerant Subjects with Cardiovascular Disease (TRANSCEND), were the first long-term prospective trials on cardioprotective actions in high-risk patients to address the effects of dual RAAS blockade on erectile function (Bohm et al., 2007; Yusuf et al., 2008a,b). Effects due to reduction in tissue Ang II effects through telmisartan on the one hand and reduced formation of Ang II and degradation of bradykinin via ramipril, on the other, could have exerted synergistic effects on erectile tissue, given its sensitivity to changes in erectile function.
The present study evaluated functional and molecular effects of dual RAAS blockade on endothelial tissues in ApoE−/− mice, an established murine model of atherosclerosis and ED (Behr-Roussel et al., 2006; Kolovou et al., 2008). All ApoE−/− mice developed equal hypercholesterolaemia, as a pre-condition of the severity of onset in metabolic changes initiating endothelial dysfunction and haemodynamic changes (Kolovou et al., 2008). However, there was a not significant reduction in serum levels of total cholesterol as well as LDL- and high density lipoprotein-cholesterol in the combination treatment group. This trend might be explained by complete inhibition of detrimental effects of Ang II on inflammation parameters and insulin signalling with consequent increase of cholesterol levels (Munoz et al., 2009). Nevertheless, these effects are not likely to influence results on endothelial function and atherosclerosis.
Blood pressure was reduced by either means of RAAS blockade, in comparison to untreated animals. Lowering of systolic blood pressure in the telmisartan and combination therapy groups was slightly and not significantly greater than that achieved by ramipril treatment alone. RAAS blockade by any intervention significantly improved endothelium-dependent relaxation, equally for all treatments. Similarly, RAAS blockade attenuated atheromatous plaque formation in ApoE−/− mice, with no differences between treatments.
Reduction in blood pressure below a certain level in patients treated with telmisartan, ramipril or both has recently been shown not to any increased benefit in myocardial infarction, while cardiovascular mortality was unchanged or increased in a large population of cardiovascular high-risk patients from the ONTARGET-trial (Sleight et al., 2009). Furthermore, it has been suggested that cardioprotective effects attributed to blockade of the RAAS are only in part due to a reduction in blood pressure (Baumhakel et al., 2008; Jankowski et al., 2009). The AT1 receptor promotes pro-inflammatory, pro-fibrotic, pro-thrombotic and apoptotic effects, which lead to endothelial dysfunction and are largely independent of pressure-mediated endothelial damage in experimental settings (Jankowski et al., 2009). Activation of interleukins, cytokines, adhesion molecules and growth factors is dependent on AT1 receptor stimulation by Ang II (Skultetyova et al., 2007). Activation of AT1 receptors also leads to imbalance of the oxidative homeostasis of endothelial cells through excessive production of ROS, mainly via AT1 receptor-responsive enzymes such as nicotinamide adenine dinucleotide phosphate-oxidase (Taniyama and Griendling, 2003). Excessive production of ROS leads to dysfunction of the endothelium by scavenging nitric oxide and subsequently reducing its protective effects on the endothelium (Li and Forstermann, 2000; Landmesser and Harrison, 2001). Consistent with these earlier reports, oxidative stress was increased in aortic and penile tissues in this study.
In the corpus cavernosum, Ang II leads to trabecular contraction, thereby initiating detumescence, by activating cGMP-dependent protein kinases which reduce endothelial nitric oxide production via eNOS, thereby reducing trabecular smooth muscle cell relaxation via guanylyl cyclase while impeding the nitric oxide-mediated inhibition of calcium influx into trabecular smooth muscle cell (Blatter and Wier, 1994; Kifor et al., 1997; Park et al., 1997). This interaction of Ang II and nitric oxide in erectile tissue provides the mechanistic rationale for RAAS blockade to improve erectile function. The more than twofold increase of ROS in ApoE−/− tissues might be involved in the development of endothelial dysfunction in aortic and cavernosal tissues in the present study. Consistent with this possibility, RAAS blockade led to significant reduction in ROS formation in both tissues along with the observed improvement in endothelial function. Moreover, semiquantitative measurement of eNOS by immunohistological methods, revealed a recovery of eNOS in animals with RAAS blockade. The combination of RAAS blockers was no better than each blocker alone in improving endothelial dysfunction and atherogenesis and also in reducing ROS load.
The fact that telmisartan and ramipril monotherapies improved vascular and cavernosal endothelial function and oxidative stress to values, almost equal to those in WT animals, shows that appropriate doses of either an AT1 receptor antagonist or an ACE inhibitor will provide adequate vascular protection, leaving little room for improvement by dual RAAS blockade. In clinical trials on heart failure patients, additional therapy with a AT1 receptor antagonist had favourable effects when ACE inhibitor doses below the median were used (Krum et al., 2004). Furthermore, the theoretical assumption that the reduced bradykinin inactivation under dual RAAS blockade, compared to monotherapy with a AT1 receptor antagonist might yield favourable effects seems to be less applicable to states of advanced vascular injury. Bradykinin effects in rabbits fed atherogenic diet were less pronounced when compared to animals on a standard Western diet (Weckler et al., 2003). However, the role of bradykinin remains unclear in the results presented. One indication of additional effects of bradykinin on endothelial function might be that, in ramipril-treated animals, the improvement of endothelial function was comparable to that in telmisartan treated animals, in spite of a more pronounced blood pressure reduction in the latter animals.
Finally, it is possible that potentially protective AT2 receptor-mediated pathways might be diminished in presence of ACE inhibitors, due to decreased Ang II levels. Regardless of the contribution of these possibilities to the physiologically observed equivalence of dual RAAS blockade in this study, our results show that oxidative stress in aortic and cavernosal tissue and subsequent peroxidation of membrane lipids can be effectively reduced by any type of RAAS blockade at appropriate doses.
Limitations of this study
The ApoE−/− mouse used for the experiments is a transgenic model of hypercholesterol-induced atherosclerosis. Thus, a significant decrease of blood pressure in the treatment groups in these normotensive animals might be an expression of overdosage and translation of the results into humans remains to be demonstrated. Nevertheless, the lack of intergroup difference might be the same in humans.
Functional organ bath chamber experiments assessed endothelial function of the corpus cavernosum, which plays a major role in physiology of penile erection. However, despite evidence for impaired erectile function in ApoE−/− mice, we did not measure improvement of erectile function itself, by treatment with RAAS blockade, but used a suggested surrogate for this function (Behr-Roussel et al., 2006).
In conclusion, telmisartan and ramipril proved to be equally effective in restoring impairment of endothelial function of the aorta and corpus cavernosum, the latter as a possible surrogate of improvement of erectile function, in ApoE−/− mice. Furthermore atheromatous plaque load occurring subsequent to endothelial dysfunction was reduced to a similar extent. These beneficial effects are largely blood pressure-independent and are associated with a reduction of vascular and cavernosal oxidative stress as well as expression of eNOS, as one pathophysiological link. Dual RAAS blockade did not lead to further improvement of these parameters at the respective doses.
Acknowledgments
Animal studies were supported by grants by Boehringer Ingelheim, Germany. M Böhm is supported by the Deutsche Forschungsgemeinschaft (KFO 196Z).
Glossary
Abbreviations
- ACE
angiotensin converting enzyme
- Ang II
angiotensin II
- CCS
corpora cavernosal strips
- DHE
dihydroethidium
- ED
erectile dysfunction
- eNOS
endothelial nitric oxide synthase
- L-NAME
Nω-nitro-l-arginine methyl ester
- RAAS
renin-angiotensin-aldosterone system
- WT
wild-type
Conflict of interest
M Böhm was a member of the steering committee of the ONTARGET/TRANSCEND trials.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhakel M, Bohm M. Erectile dysfunction correlates with left ventricular function and precedes cardiovascular events in cardiovascular high-risk patients. Int J Clin Pract. 2007;61:361–366. doi: 10.1111/j.1742-1241.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- Baumhakel M, Custodis F, Schlimmer N, Laufs U, Bohm M. Improvement of endothelial function of the corpus cavernosum in apolipoprotein E knockout mice treated with irbesartan. J Pharmacol Exp Ther. 2008;327:692–698. doi: 10.1124/jpet.108.140533. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Uckert S, Stief CG, Truss MC, Machtens S, Scheller F, et al. Possible role of bradykinin and angiotensin II in the regulation of penile erection and detumescence. Urology. 2001;57:193–198. doi: 10.1016/s0090-4295(00)00881-5. [DOI] [PubMed] [Google Scholar]
- Behr-Roussel D, Darblade B, Oudot A, Compagnie S, Bernabe J, Alexandre L, et al. Erectile dysfunction in hypercholesterolemic atherosclerotic apolipoprotein E knockout mice. J Sex Med. 2006;3:596–603. doi: 10.1111/j.1743-6109.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Billups KL, Bank AJ, Padma-Nathan H, Katz SD, Williams RA. Erectile dysfunction as a harbinger for increased cardiometabolic risk. Int J Impot Res. 2008;20:236–242. doi: 10.1038/sj.ijir.3901634. [DOI] [PubMed] [Google Scholar]
- Blatter LA, Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994;15:122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Bohm M, Baumhakel M, Probstfield JL, Schmieder R, Yusuf S, Zhao F, et al. Sexual function, satisfaction, and association of erectile dysfunction with cardiovascular disease and risk factors in cardiovascular high-risk patients: substudy of the ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNT Study in ACE-INtolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) Am Heart J. 2007;154:94–101. doi: 10.1016/j.ahj.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bookstein JJ, Vandeberg J, Machado T. The cavernosal acetylcholine/papaverine response. A practical in vivo method for quantification of endothelium-dependent relaxation. Rationale and experimental validation. Invest Radiol. 1990;25:1168–1174. [PubMed] [Google Scholar]
- Buyukafsar K, Un I. Effects of the Rho-kinase inhibitors, Y-27632 and fasudil, on the corpus cavernosum from diabetic mice. Eur J Pharmacol. 2003;472:235–238. doi: 10.1016/s0014-2999(03)01905-8. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Lewis RW, Mills TM. Captopril treatment reverses erectile dysfunction in male Stroke Prone Spontaneously Hypertensive Rats. Int J Impot Res. 2002;14:494–497. doi: 10.1038/sj.ijir.3900915. [DOI] [PubMed] [Google Scholar]
- Dusing R. Effect of the angiotensin II antagonist valsartan on sexual function in hypertensive men. Blood Press Suppl. 2003;2:29–34. doi: 10.1080/08038020310021967. [DOI] [PubMed] [Google Scholar]
- Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328–338. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- Fogari R, Zoppi A, Poletti L, Marasi G, Mugellini A, Corradi L. Sexual activity in hypertensive men treated with valsartan or carvedilol: a crossover study. Am J Hypertens. 2001;14:27–31. doi: 10.1016/s0895-7061(00)01214-0. [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Teo KK, Bohm M. ACE inhibition in secondary prevention: are the results controversial? Clin Res Cardiol. 2006;95:61–67. doi: 10.1007/s00392-006-0334-6. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, et al. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148:1688–1696. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- Jankowski P, Safar ME, Benetos A. Pleiotropic effects of drugs inhibiting the renin-angiotensin-aldosterone system. Curr Pharm Des. 2009;15:571–584. doi: 10.2174/138161209787315747. [DOI] [PubMed] [Google Scholar]
- Kifor I, Williams GH, Vickers MA, Sullivan MP, Jodbert P, Dluhy RG. Tissue angiotensin II as a modulator of erectile function. I. Angiotensin peptide content, secretion and effects in the corpus cavernosum. The J Urology. 1997;157:1920–1925. [PubMed] [Google Scholar]
- Kirby M, Jackson G, Betteridge J, Friedli K. Is erectile dysfunction a marker for cardiovascular disease? Int J Clin Pract. 2001;55:614–618. [PubMed] [Google Scholar]
- Kolovou G, Anagnostopoulou K, Mikhailidis DP, Cokkinos DV. Apolipoprotein E knockout models. Curr Pharm Des. 2008;14:338–351. doi: 10.2174/138161208783497769. [DOI] [PubMed] [Google Scholar]
- Krum H, Carson P, Farsang C, Maggioni AP, Glazer RD, Aknay N, et al. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. Eur J Heart Fail. 2004;6:937–945. doi: 10.1016/j.ejheart.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: ox marks the spot. Circulation. 2001;104:2638–2640. [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Munoz MC, Giani JF, Dominici FP, Turyn D, Toblli JE. Long-term treatment with an angiotensin II receptor blocker decreases adipocyte size and improves insulin signaling in obese Zucker rats. J Hypertens. 2009;27:2409–2420. doi: 10.1097/HJH.0b013e3283310e1b. [DOI] [PubMed] [Google Scholar]
- NIH. NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- Park JK, Kim SZ, Kim SH, Park YK, Cho KW. Renin angiotensin system in rabbit corpus cavernosum: functional characterization of angiotensin II receptors. J Urol. 1997;158:653–658. [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov. 2007;2:23–27. doi: 10.2174/157489007779606130. [DOI] [PubMed] [Google Scholar]
- Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27:1360–1369. doi: 10.1097/HJH.0b013e32832d7370. [DOI] [PubMed] [Google Scholar]
- Speel TG, van Langen H, Meuleman EJ. The risk of coronary heart disease in men with erectile dysfunction. Eur Urol. 2003;44:366–370. doi: 10.1016/s0302-2838(03)00304-x. discussion 70–71. [DOI] [PubMed] [Google Scholar]
- Speel TG, Kiemeney LA, Thien T, Smits P, Meuleman EJ. Long-term effect of inhibition of the angiotensin-converting enzyme (ACE) on cavernosal perfusion in men with atherosclerotic erectile dysfunction: a pilot study. J Sex Med. 2005;2:207–212. doi: 10.1111/j.1743-6109.2005.20230.x. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- Weckler N, Leitzbach D, Kalinowski L, Malinski T, Busch AE, Linz W. Effect of chronic treatment with the vasopeptidase inhibitor AVE 7688 and ramipril on endothelial function in atherogenic diet rabbits. J Renin Angiotensin Aldosterone Syst. 2003;4:191–196. doi: 10.3317/jraas.2003.031. [DOI] [PubMed] [Google Scholar]
- Werner C, Baumhakel M, Teo KK, Schmieder R, Mann J, Unger T, et al. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol. 2008;97:418–431. doi: 10.1007/s00392-008-0668-3. [DOI] [PubMed] [Google Scholar]
- Xie D, Odronic SI, Wu F, Pippen AM, Donatucci CF, Annex BH. A mouse model of hypercholesterolemia-induced erectile dysfunction. J Sex Med. 2007;4:898–907. doi: 10.1111/j.1743-6109.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Yavuzgil O, Altay B, Zoghi M, Gurgun C, Kayikcioglu M, Kultursay H. Endothelial function in patients with vasculogenic erectile dysfunction. Int J Cardiol. 2005;103:19–26. doi: 10.1016/j.ijcard.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008a;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008b;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]


