Abstract
Objective
Transsphenoidal pituitary surgery (TS) is the primary treatment of choice for patients with acromegaly. Macroadenomas (>1 cm) are more difficult to resect than microadenomas (remission rate ± 50% compared to ± 90%). Besides the conventional microscopic TS, the more recently introduced endoscopic technique is nowadays frequently used. However, no large series reporting on its results have yet been published. We evaluated the outcome of endoscopic TS in 40 patients with a growth hormone (GH)-secreting macroadenoma treated in our hospital between 1998 and 2007.
Methods
Medical records were retrospectively reviewed. Remission was defined as disappearance of clinical symptoms of acromegaly, normal serum insulin-like growth factor-1 levels (≤2 SD) and serum GH levels suppressed to <2 mU/l after an oral glucose tolerance test within the first 4 months after TS.
Results
In four patients TS aimed at debulking of the tumour. In the remaining 36 patients, remission was achieved in 20 patients. In the first 5 years remission was achieved in 6 out of 18 patients (33%) compared to 14 out of 22 patients (63%) in the following 5 years (p = 0.06). Thirteen patients had a mild perioperative complication. Before TS 15 patients received hormonal substitution therapy compared to 12 patients (33%) after TS.
Conclusion
Endoscopic TS is a good primary therapeutic option for patients with a GH-secreting macroadenoma, resulting in a remission rate of up to 63% in experienced hands. This technique can potentially improve the outcome of TS in these patients.
Keywords: Pituitary, Acromegaly, Endoscopy, Macroadenoma, Transsphenoidal surgery
Introduction
Untreated acromegaly causes significant morbidity, and is associated with a two- to threefold increase in mortality. When acromegaly is treated successfully and “safe” growth hormone (GH) and insulin growth factor-1 (IGF-1) values are achieved, the mortality rate normalises [32]. Therefore, appropriate treatment of acromegaly is crucial. However, symptoms and signs of acromegaly develop insidiously, and there is often a delay in diagnosis for up to 10 years. Therefore, approximately 70% of GH-secreting adenomas are ≥1 cm (macroadenomas) at the time acromegaly is diagnosed [32].
According to experts, transsphenoidal pituitary surgery (TS) is the treatment of choice for acromegaly [28, 31], potentially rapidly restoring normal physiology by a single intervention. Macroadenomas, however, are difficult to remove by TS, especially when invasive. This may explain the relatively low remission rate of about 50% reported after TS in macroadenomas, whereas remission rates up to 90% are achieved by TS in microadenomas (<1 cm) [34]. Since more recently developed medical therapies achieve good results in controlling acromegaly, some authors have recommended medical therapy as a primary treatment option instead of TS for patients with a GH-secreting macroadenoma that does not cause mass effects [13, 25].
Nowadays, the endoscopic technique of TS is increasingly used by many neurosurgeons instead of the conventional microscopic technique. This technique, offering a panoramic wide angle view with increased illumination, was first developed in the 1990s. Different angles can be used, making it possible to effectively reach supra- and parasellar portions of the lesion and work around the corner [10, 14]. Due to these advantages, it has been suggested that the endoscopic technique may be preferable to the conventional technique, especially in patients with invasive macroadenomas [14, 28, 39]. However, due to the recent introduction of this technique, no large series reporting on the results of endoscopic TS in acromegaly have yet been published.
To gain insight in the role of endoscopic TS as a primary treatment option for patients with GH-secreting macroadenomas, we evaluated the results of endoscopic TS in 40 consecutive patients with a GH-secreting macroadenoma treated in our hospital between 1998 and 2007.
Patients and methods
Patients
Between 1998 and 2007, 40 patients with acromegaly and a macroadenoma on a preoperative magnetic resonance imaging (MRI) scan underwent endoscopic TS in our centre. The medical records of these patients (19 males and 21 females, Table 1) were retrospectively reviewed. Age at time of TS was 47.4 ± 11.4 (mean ± SD) years and BMI was 29.0 ± 4.9 kg/m2. We collected data on preoperative as well as early postoperative evaluation, complications that occurred during TS or in the early postoperative period, and data on the follow-up of these patients.
Table 1.
Results of endoscopic transsphenoidal pituitary surgery in patients with acromegaly (1998–2007)
| Patient number, gender, age (years) | Preoperative octreo-tide | MRI (mm) | Invasion on preoperative MRI | Year of TS | Postoperative IGF-1 | Postoperative oGTT | TS result | Additional therapy | Last IGF-1 | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nmol/l | SD | nmol/l | SD | |||||||||
| 1. f, 52 | Y | 20 | Sc r | 1998 | 50.3 | >2 | - | F | RT + cab | 19.0 | 1 | 127 |
| 2. m, 43 | N | 25 | - | 1999 | 24.0 | 1 | 38 | F | Octr | 32.8 | >2 | 38 |
| 3. f, 27 | N | 18 | Sc r | 2000 | 92.7 | >2 | 9 | F | GK | 10.6 | -2 | 109 |
| 4. m, 40 | Y | 10 | - | 2000 | 23.1 | 1 | <2 | R | - | 12.9 | -1 | 106 |
| 5. f, 24 | Y | 17 | - | 2000 | 77.0 | >2 | - | F | Octr + cab | 41.0 | >2 | 78 |
| 6. f, 59 | Y | 20 | Ssphen,sc l | 2001 | 64.5 | >2 | - | F | TS, RT + peg | 15.6 | 0 | 76 |
| 7. f, 54 | N | 11 | Sc l | 2001 | 62.8 | >2 | - | F | RT + octr | 23.5 | 1 | 73 |
| 8. m, 39 | N | 14 | - | 2001 | 73.6 | >2 | - | F | RT + octr, cab | 28.1 | 2 | 68 |
| 9. m, 49 | Y | 43 | Ssphen, sc l + r | 2001 | 60.5 | >2 | - | F | TS + octr, cab | 21.6 | 1 | 91 |
| 10. f, 50 | Y | 12 | - | 2001 | 10.2 | -2 | <2 | R | - | 15.4 | 0 | 74 |
| 11. f, 49 | Y | 13 | - | 2001 | 38.4 | >2 | - | F | Octr | 14.0 | 0 | 91 |
| 12. m, 37 | Y | 20 | - | 2001 | 28.3 | 1 | <2 | R | Octr | 15.6 | 0 | 87a |
| 13. m, 44 | Y | 10 | Sc r | 2001 | 83.6 | >2 | - | F | GK + octr, peg | 27.9 | 2 | 86 |
| 14. f, 34 | Y | 16 | - | 2002 | 10.5 | -2 | <2 | R | - | 11.4 | -2 | 70 |
| 15. f, 55 | Y | 18 | - | 2002 | 30.9 | >2 | 3 | F | Octr | 9.8 | -2 | 86 |
| 16. f, 56 | Y | 11 | Sc r | 2002 | 12.7 | -1 | <2 | R | - | 13.6 | 0 | 79 |
| 17. m, 56 | Y | 14 | - | 2002 | 16.8 | 0 | <2 | R | - | 15.2 | 0 | 77 |
| 18. m, 64 | Y | 12 | - | 2002 | 18.0 | 1 | 2 | F | Octr | 18.3 | 1 | 57 |
| 19. f, 66 | Y | 16 | Ssphen, sc r | 2003 | 27.1 | 2 | <2 | R | - | 25.0 | 2 | 65 |
| 20. f, 45 | Y | 30 | Sc l | 2003 | 24.7 | 1 | <2 | R | - | 29.0 | 2 | 48 |
| 21. m, 35 | Y | 39 | Ssphen | 2003 | 66.3 | >2 | - | F | Octr | 14.1 | -1 | 69 |
| 22. f, 56 | Y | 15 | Sc r | 2003 | 15.3 | 0 | <2 | R | - | 16.5 | 0 | 47 |
| 23. f, 41 | Y | 15 | - | 2003 | 28.0 | 1 | <2 | R | - | 22.7 | 1 | 66 |
| 24. m, 54 | Y | 13 | - | 2004 | 19.8 | 1 | <2 | R | - | 16.6 | 0 | 44 |
| 25. f, 43 | Y | 11 | - | 2004 | 29.5 | 2 | <2 | R | - | 22.8 | 1 | 43 |
| 26. m, 48 | N | 12 | - | 2005 | 35.8 | >2 | - | F | Octr | 23.6 | 1 | 31 |
| 27. f, 29 | Y | 10 | Sc l | 2005 | 25.6 | 1 | <2 | R | - | 23.5 | 1 | 37 |
| 28. f, 51 | Y | 13 | - | 2005 | 11.7 | -1 | <2 | R | - | 15.6 | 0 | 49 |
| 29. m, 64 | Y | 14 | Sc r | 2005 | 30.0 | >2 | - | F | Cab | 21.4 | 1 | 36 |
| 30. m, 68 | Y | 30 | Sc l + r, ssphen | 2005 | 31.5 | >2 | - | F | Octr | 16.8 | 1 | 30 |
| 31. m, 46 | Y | 21 | - | 2005 | 43.1 | >2 | <2 | F | - | 36.2 | >2 | 32 |
| 32. m, 45 | N | 15 | - | 2005 | 46.0 | >2 | <2 | F | - | 31.3 | >2 | 38 |
| 33. m, 35 | Y | 42 | Sc l + r | 2005 | 163.8 | >2 | 595 | F | RT + octr, cab | 56.9 | >2 | 33 |
| 34. f, 28 | Y | 45 | Sc l + r | 2006 | 98.7 | >2 | - | F | RT + octr | 33.3 | >2 | 15 |
| 35. f, 67 | Y | 11 | - | 2006 | 21.5 | 1 | <2 | R | - | 27.4 | >2 | 31a |
| 36. m, 40 | Y | 21 | Sc r | 2007 | 22.9 | 0 | <2 | R | - | 21.3 | 1 | 14 |
| 37. m, 62 | Y | 20 | - | 2007 | 18.4 | 1 | <2 | R | - | 18.4 | 1 | 6 |
| 38. f, 41 | Y | 24 | Sc re | 2007 | 31.7 | 2 | <2 | R | - | 12.0 | -1 | 15 |
| 39. f, 46 | Y | 18 | - | 2007 | 16.2 | 0 | <2 | R | - | 21.3 | 1 | 8 |
| 40. m, 54 | Y | 27 | Sc r | 2007 | 26.6 | 2 | <2 | R | - | 26.6 | 2 | 6 |
f: female; m: male; preoperative octreotide Y: treated with octreotide before surgery; preoperative octreotide N: not treated with octreotide before surgery; MRI: magnetic resonance imaging results given as maximal diameter of the visualised tumour in mm; sc r: cavernous sinus right; sc l: cavernous sinus left; ssphen: shenoid sinus; ssphen: sphenoid sinus; TS: transsphenoidal surgery; IGF-1 nmol/l: value of insulin-like growth factor-1; IGF-1 SD: standard deviation of insulin-like growth factor-1 compared to normal values in people of the same age and sex; oGTT: minimal value of growth hormone achieved during the postoperative oral glucose tolerance test; GTT -: no oral glucose tolerance test performed after surgery; TS result R: remission; TS result F: failure; RT: conventional radiotherapy; octr: octreotide; cab: cabergoline; GK: gamma knife radiosurgery; peg: pegvisomant; a: relapse at last follow-up
Preoperative evaluation and perioperative treatment
The initial diagnosis of acromegaly was based on clinical grounds and biochemical tests, including assessment of serum GH levels (basal and after oral administration of glucose) and serum IGF-1 levels. Furthermore, the thyrotropic, gonadotropic and pituitary-adrenal axes were assessed, as well as the prolactin blood level. Preoperative pituitary imaging by MRI was performed in all patients.
Long-acting somatostatin analogues (SA) were given preoperatively in 34 patients for a median period of 7 months (range 1–28), 1 patient received 10 mg/4 weeks, 20 patients received 20 mg/4 weeks and 13 patients received 30 mg/4 weeks.
One hour before surgery, administration of glucocorticoids (prednisolone, 25 mg i.v. every 8 h) was started. Two days after surgery glucocorticoid administration was changed from i.v. to oral, and the dose was tapered rapidly.
Surgical technique
The endoscopic technique of TS was introduced in our hospital in 1994 and first used for acromegaly in 1998. From 1998 onward practically all TSs (n = 365) were performed endoscopically. The surgeries were exclusively performed by two neurosurgeons. The technique is very similar to the technique that Jho et al. and Cappabianca et al. have described previously [7, 8, 21, 22]. However, a binostril transsphenoidal approach to the sella turcica was used, during which the endoscope was handheld.
For the endoscopic transnasal TS, 0º and 30° rigid endoscopes with a lens diameter of 4 mm with a separate shaft were used, which allow easy and comfortable holding, while offering a suction-irrigation-system for cleaning the lens (Karl Storz GmbH, Tuttlingen, Germany). The instruments used are principally the same as used with the microsurgical technique. Because an adenoma was visible on preoperative MRI, a selective adenomectomy was performed in all patients.
Postoperative evaluation
A complication of TS was defined as any event occurring during or after TS that required treatment. As intraoperative cerebrospinal fluid (CSF) leakage is inherent to the surgical procedure and is closed during TS with a fat graft, it was not regarded as a complication, whereas postoperative CSF leakage was considered a complication.
On the 7th day postoperatively, at least 48 h after the last dose of glucocorticoids, early biochemical evaluation was carried out by measuring the serum concentrations of IGF-1, fasting cortisol, adrenocorticotropic hormone (ACTH), thyrotropin (TSH), free thyroxine (FT4), gonadotropines (LH and FSH), testosterone, estradiol and prolactin.
Patients were re-evaluated every 2 to 4 weeks during the first 3 months after surgery. Serum GH and IGF-1 levels were measured at each visit. Four months after surgery a new MRI of the pituitary was performed to check for tumour remnants. An oral glucose tolerance test (OGTT; 100 g of glucose [36]) was performed if the IGF-1 level had normalised or was marginally elevated. Thereafter patients who were in remission were evaluated at least once a year or earlier in case of clinical suspicion of a relapse.
Criteria for remission and relapse
Remission was defined as disappearance of clinical symptoms of active GH hypersecretion with in addition normal serum IGF-1 levels (≤ mean + 2 standard deviations for age) and suppression of serum GH levels to <2 mU/l during OGTT within the first 4 months after surgery [19, 20].
Relapse was defined as development of clinical signs of active GH hypersecretion with elevated serum IGF-1 levels (> mean + 2 standard deviations for age) and serum GH levels ≥2 mU/l during OGTT [19, 20].
Imaging
All preoperative and postoperative MRI scans were evaluated by the same neurosurgeon to prevent bias. Maximal diameter of the adenoma was defined as the largest distance that could be measured in any direction of the adenoma. Invasion was defined as suspected growth of the adenoma beyond the sella into the cavernous sinus or the sphenoid sinus.
Analysis of factors influencing outcome and statistics
Data were analysed using SPSS 16.0. Characteristics of patients operated on in the first and second 5 years were compared using unpaired T-test and Pearson’s chi-square test. The influence of various factors on the chance to achieve remission by TS was analysed by binary logistic regression. The factors analysed were: date of operation (as a surrogate measure for experience of the neurosurgeons), age, gender, the level of preoperative IGF-1 and GH, the diameter of the adenoma on preoperative MRI, evidence of invasion on the preoperative MRI, occurrence of perioperative complications and the need for hormonal substitution therapy after TS. The influence of dichotomous variables (gender, substitution therapy before TS, evidence of invasion on the preoperative MRI, TS in the first or second 5 years, occurrence of perioperative complications and the need for hormonal substitution therapy after TS) on the chance of remission were also analysed using Pearson’s chi-square test. Statistical significance was defined as p = <0.05 (two-sided).
Results
Remission rates after TS
The results of endoscopic TS in the 40 patients with a GH-secreting macroadenoma are shown in Fig. 1. The individual data per patient are presented in Table 1. Histological investigation of the removed tissue showed evidence of a GH-producing adenoma in all cases. The overall remission rate in our series is 50%. However, four patients (patients 21, 30, 33 and 34, Table 1) had an invasive adenoma of more than 30 mm in diameter and suffered from local mass effects. The intention of the TS in these patients was to debulk the adenoma, as it was appreciated that cure could not be achieved by TS. In the remaining 36 patients, in whom the intent was cure, remission was achieved in 20 patients. In this group the remission percentage thus was 56%. Median follow-up was 56 months (range 6–126). Recently, two patients (patients 12 and 35) developed a mild relapse. Patient 12 is now being treated with octreotide, while the relapse of patient 35 was very mild and no treatment had yet been initiated.
Fig. 1.
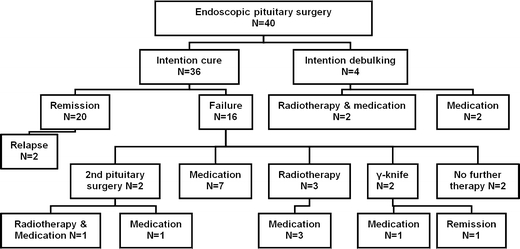
Results of endoscopic pituitary surgery in patients with a growth hormone secreting macroadenoma (1998–2007)
The date of TS significantly influenced the chance of remission after TS. If a patient was operated on at a later date, the chance of achieving remission after TS was higher (p = 0.04). If the results of TS during the first 5-year interval after the introduction of the endoscopic technique are compared with the second 5-year interval, remission was achieved in 6 out of 18 patients (33%) during the first 5 years, whereas in the next 5 years, 14 of 22 patients (63%) were in remission after TS (p = 0.06). However, the four patients who only underwent debulking were all operated on in the last 5 years. If these patients are excluded, the remission rate achieved in the last 5 years is 77%, which is significantly better than the remission rate over the first 5 years (p = 0.01). Table 2 shows that baseline characteristics of patients operated on during the first 5 years do not significantly differ with baseline characteristics of patients operated on during the last 5 years, except for preoperative IGF-1 levels, which were significantly higher in the second group. This does not change if the four patients who underwent debulking are excluded.
Table 2.
Comparison of baseline characteristics of the patients operated on during the first 5 years and the patients operated on during the second 5 years
| First 5 years (n = 18) | Second 5 years (n = 22) | Significance | |
|---|---|---|---|
| Gender (% male) | 8 (44%) | 11 (50%) | p = 0.76 |
| Age (years) | 46.2 (±11.0) | 48.4 (±11.9) | p = 0.56 |
| BMI (kg/m2) | 29.8 (±4.4) | 26.6 (±5.3) | p = 0.32 |
| Preoperative medication | 13 (72%) | 20 (91%) | p = 0.12 |
| Adenoma diameter (cm) | 16.9 (±7.8) | 20.9 (10.4) | p = 0.18 |
| Invasion on preoperative MRI | 7 (39%) | 12 (54%) | p = 0.32 |
| Preoperative IGF-1 value (nmol/l) | 93.3 (±23.5) | 116.6 (±36.9) | p = 0.03 |
Data are expressed as means and standard deviations in case of continuous variables and as exact numbers and percentages in case of nominal or ordinal variables. BMI: Body mass index; IGF-1: insulin-like growth factor-1; MRI: magnetic resonance imaging
There were no statistically significant differences between the patients who underwent successful or unsuccessful TS with respect to age, gender, occurrence of perioperative complications, preoperative IGF-1 levels or need for hormonal substitution therapy after TS. There was a trend that if the diameter of the adenoma was larger, the chance to achieve remission was smaller (p = 0.06 in all patients); however if the four patients who underwent only debulking were excluded, this trend was no longer present (p = 0.56). Eight of 19 patients (42%) with evidence of invasion on preoperative MRI, and 12 of 21 patients (57%) without invasion achieved remission after TS (p = 0.34). If the four patients in whom the intention of the TS was to debulk the adenoma were not taken into account, the remission rate in patients with suspected invasion was 53%, indicating that in this study invasion did not significantly influence the chance to achieve remission (p = 0.82).
Additional treatment and benefits of TS in patients with persistent acromegaly after TS
Although remission was not achieved via TS in 20 patients, the maximal diameter of the adenoma was reduced from a median of 18 mm (range 10–45) on the preoperative MRI to a median of 7 mm (range 0–35) on the MRI performed 4 months after surgery. The adenoma was reduced in size in all patients, and in six patients no residual adenoma was visible on the postoperative MRI. In three patients (patients 11, 15 and 18), normal IGF-1 levels could be achieved with a dose of octreotide that was the same or even lower than the dose prescribed before the operation and that had been insufficient to suppress IGF-1 to normal levels before TS.
Figure 1 shows how the 20 patients with persisting acromegaly after TS were treated. A second TS was attempted in two patients but failed to result in cure. Of the eight patients receiving additional conventional radiotherapy, none is presently in remission and all are still receiving medical treatment to control the acromegaly. Radiosurgery (γ-knife) was performed in two patients and resulted in remission in one of them. Of the remaining patients not cured by the TS, all patients except patient 32 and 33 were exclusively treated with medication. Patient 32 and 33 did not receive any further treatment. In patient 32 the IGF-1 level was only slightly elevated with no symptoms of active acromegaly, GH was suppressed to <2 mU/l after OGTT, and the mean GH values are below 6.5 mU/l. Patient 33 refused to be tested or treated further after TS because the symptoms of acromegaly had disappeared.
Complications of TS and influence of TS on deficiencies of pituitary hormones
Only mild complications occurred in our series. Fourteen patients developed a very mild transient diabetes insipidus (DI) for a maximum of 2 to 3 days. This was not regarded as a complication. Only one patient (patient 33) had a more severe transient DI. Five patients, of whom two had had mild transient DI early after the operation, were treated with fluid restriction when they developed a mild hyponatremia because of inappropriate ADH secretion. Four patients had mild epistaxis, controlled with nasal tampons. Three patients had cerebrospinal fluid (CSF) leakage postoperatively. They were treated successfully with an external lumbar drain.
Fifteen patients (38%) already received substitution therapy for deficiency of one or more hormones before TS. After TS 33% of all patients receive long-term hormonal substitution. Twenty percent of the patients receive substitution with levothyroxine, 12.5% receive androgens, 12.5% receive glucocorticoids, 2.5% receive GH therapy and 2.5% receive desmopressin (Table 3).
Table 3.
Influence of transsphenoidal surgery on substitution therapy in 40 patients with acromegaly caused by a macroadenoma
| Levothyroxine substitution | Androgen substitution | Glucocorticoid substitution | GH substitution | Desmopressin substitution | Total | |
|---|---|---|---|---|---|---|
| Discontinued after TS | 5 | 5 | 1 | 11 | ||
| Started after TS | 4 | 2 | 2 | 1 | 1 | 10 |
| Continued after TS | 4 | 3 | 3 | 10 |
TS: transsphenoidal surgery, GH: growth hormone.
Discussion
In this study we report on the results of endoscopic TS in 40 consecutive patients with acromegaly and a pituitary macroadenoma on preoperative MRI, operated on in our hospital between 1998 and 2007. Although some results of endoscopic TS in small numbers of patients with acromegaly have been mentioned in large series of patients with different pituitary tumours [6, 11, 26, 42], no series focussing on the results of endoscopic TS in patients with acromegaly have yet been published. All previous published series on results of TS in patients with GH-secreting macroadenomas used the conventional technique of TS.
Remission rates after conventional TS reported in these larger series of patients with GH-secreting macroadenomas (including giant macroadenoma) vary widely, from 15 to 71% (Table 4) [1, 2, 4, 15, 18, 23, 29, 34, 40, 41, 43, 45]. The overall remission rate of 50% in this study is in concordance with these results. However, not all series have used the same criteria to describe remission. The studies that used the criteria for remission formulated by Giustina et al. in 2000 [19, 20], as we did in our series, reported remission rates in patients with macroadenomas of maximally 50%. Therefore, the remission rate of 50% reported in our series is comparable to the best previously published remission rates achieved in patients operated on by the conventional microscopic method of TS.
Table 4.
Review of the criteria to define remission of acromegaly and remission percentages in macroadenomas reported in the most recently published series (1997–2005)
| Author | N | Criteria of remission | Remission % |
|---|---|---|---|
| van Lindert et al. [45] | 40 | GH <2 ng/ml after OGTT, IGF1 N | 55 |
| Abosch et al. [2] | 254 | Basal GH <5 ng/ml | 71 |
| Swearingen et al. [41] | 129 | GH <2 ng/ml after OGTT or IGF1 N or basal GH <2.5 ng/ml | 48 |
| Gittoes et al. [18] | 45 | GH <2 mU/l after OGTT or basal GH <5 mU/l | 51 |
| Laws et al. [29] | 51 | GH ≤1 ng/ml after OGTT or IGF1 N or basal GH ≤2.5 ng/ml | 51 |
| Kaltsas et al. [23] | 50 | Basal GH <2.5 ng/ml, IGF1 N | 26 |
| Abe and Ludecke [1] | 126 | Basal GH <2.5 ng/ml, IGF1 N | 68 |
| Shimon et al. [40] | 44 | GH <2 ng/ml basal or after OGTT, IGF1 N | 64 |
| Beauregard et al. [4] | 77 | GH ≤1 ng/ml after OGTT or IGF1 N or basal GH ≤2.5 ng/ml | 49 |
| Trepp et al. [43] | 64 | GH <1 ng/ml after OGTT or IGF1 N or basal GH <2.5 ng/ml | 39 |
| Erturk et al. [15] | 19 | GH ≤2 ng/ml basal or after OGTT | 15 |
| Nomikos et al. [34] | 364 | GH <1 ng/ml after OGTT or IGF1 N or GH <2.5 ng/ml | 50 |
N: number of patients included; GH: growth hormone; OGTT: oral glucose tolerance test; IGF1: insulin-like growth factor type 1
However, the remission rate of 63% (or 77% if the patients who underwent debulking are excluded) we achieved in the last 5 years, compared to a remission rate of 33% in the first 5 years, is very promising for the future. The characteristics of patients operated on in the first 5 years and second 5 years were comparable (Table 2). The only significant difference was that the patients operated upon in the second 5 years had a significantly higher IGF-1 level. Therefore, we believe that the higher remission rate achieved in the last 5 years is not biased by patients on whom it was easier to operate. So, it is more likely that the large difference between the remission rate achieved in the first 5 years and the second 5 years after introduction of the endoscopic technique of TS can be explained by the increasing experience of the two neurosurgeons who performed all endoscopic TSs in our hospital. Strong evidence exists that success rates of microscopic TS critically depend on the skills and experience of the neurosurgeon [3, 15, 18]. Our data indicate that this is no different for endoscopic TS. This argues in favour of concentrating endoscopic TS for acromegaly in a limited number of experienced centres.
Previously published series on conventional microscopic TS in patients with acromegaly and a macroadenoma found that the chance of remission after TS could be predicted by the suspected invasiveness of the macroadenoma on the preoperative MRI scan [2, 4, 18]. However, in this study, although we observed a non-significant trend towards a lower chance of successful TS if tumour invasion was suspected, remission was still achieved in 42% of patients with suspected invasion. This may be explained by the fact that the endoscopic technique enables the use of different angles to operate, making it possible to reach suprasellar and parasellar portions of the lesion effectively [10, 14]. If this is the case, the endoscopic technique might be preferable in case of invasive macroadenomas.
Due to the good results that have been achieved by medical therapy in patients with acromegaly and the relatively low remission rates after TS for patients with a GH-secreting macroadenoma, some authors have recommended medical therapy as a primary treatment option instead of TS for patients with a GH-secreting macroadenoma not causing mass effects [13, 25, 39]. Nowadays long-acting somastatin analogues (SA) have the potential to normalise IGF-1 levels in two thirds of patients, additionally controlling tumour size [17]. The more recently developed GH receptor antagonist pegvisomant can normalise IGF-1 in up to 97% of patients [44]. Furthermore, studies on combination therapies with SA and pegvisomant or SA and dopamine agonists have shown that combination therapy may be successful when monotherapy has failed [16, 33, 38]. Although medical treatment can result in long-term remission, it cannot cure acromegaly. Moreover, pegvisomant, which is effective by preventing GH action in the target tissues (organs), lacks a direct effect on the tumour to control long-term tumour growth. This might limit its use as primary therapy for patients with macroadenomas until more long-term data on safety are available. Last but not least, lifelong use of expensive medication is required with the risk of serious side effects.
Studies have shown that surgical debulking can improve control of acromegaly by SA [12, 24, 35]. So even if a patient cannot be cured by TS, TS should still be considered, especially if acromegaly cannot be controlled by SA before TS. In this study TS reduced the size of the adenoma in all patients who were not cured by TS and improved the response to SA treatment in at least five of these patients. Unfortunately, a preoperative IGF-1 value during SA therapy was not available in all patients, so possibly more patients benefited from the TS to control their acromegaly.
Preoperative treatment with SA has been associated with improved results of TS, especially in macroadenomas [1, 5, 9, 30]. This could possibly be explained by adenoma shrinkage or a change in the consistency of the adenoma [9]. However, most published studies have limitations. They are retrospective, have poor remission rates or small numbers of patients. Furthermore, other studies have not confirmed this positive effect [27, 37]. A negative effect of preoperative treatment with SA on the outcome of TS results, however, has never been found. Therefore, and because pretreatment with SA improves metabolic control, we prescribed preoperative therapy with octreotide in all but six patients. Of these six patients, none achieved remission after TS. However, because of the small number of patients who did not receive preoperative treatment and the retrospective character of this study, it was not possible to evaluate whether preoperative treatment had an effect on the results of TS.
Thirteen patients in our study had a perioperative complication. All complications were mild, and no serious complications occurred. This is in concordance with the incidence of complications associated with TS via the microscopic technique [1, 2, 4, 15, 18, 23, 29, 34, 40, 41, 43, 45]. However, the endoscopic technique is probably more comfortable for the patients as the nose septum is almost left intact and usually no nasal packing is required after surgery. Besides the four patients with mild epistaxis, no rhinologic/local complications occurred, which seems to be less than those reported with the conventional technique. However, most patients that are operated upon via the microscopic technique do not need nasal packaging, but receive it because of a longstanding surgical habit.
In this series the number of hormonal deficiencies caused by TS was equal to the number of deficiencies cured by TS. All patients had a macroadenoma, which frequently causes a hormonal deficiency by itself before surgery. If the adenoma is selectively removed, normal pituitary function can potentially be restored [45]. Therefore, in macroadenomas, the fear of creating new hormonal deficiencies should probably not be a reason to restrain from TS.
Conclusion
Endoscopic TS is a treatment that should be considered as a primary therapeutic option for patients with a GH-secreting macroadenoma. In this series of patients operated on by experienced surgeons, it resulted in a remission rate of at least 50%, with only mild complications. The relatively high remission rate of 63% (or 77% excluding the patients who underwent primary debulking) we achieved in the last 5 years indicates that operation results can improve further if experience is gained. Because the endoscopic technique enables the surgeon to use different angles, this technique can potentially improve the outcome of TS in macroadenomas, especially in patients with invasive macroadenomas. However, a randomised clinical trial comparing endoscopic and conventional TS in patients with a GH-secreting macroadenoma is needed to determine the exact pros and cons of both techniques.
Acknowledgments
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Funding
No specific grants from any funding agency in the public, commercial, or not-for-profit sector were received for this research.
References
- 1.Abe T, Ludecke DK. Effects of preoperative octreotide treatment on different subtypes of 90 GH-secreting pituitary adenomas and outcome in one surgical centre. Eur J Endocrinol. 2001;145:137–145. doi: 10.1530/eje.0.1450137. [DOI] [PubMed] [Google Scholar]
- 2.Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998;83:3411–3418. doi: 10.1210/jc.83.10.3411. [DOI] [PubMed] [Google Scholar]
- 3.Bates PR, Carson MN, Trainer PJ, Wass JA. Wide variation in surgical outcomes for acromegaly in the UK. Clin Endocrinol (Oxf) 2008;68:136–142. doi: 10.1111/j.1365-2265.2007.03012.x. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard C, Truong U, Hardy J, Serri O. Long-term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol (Oxf) 2003;58:86–91. doi: 10.1046/j.1365-2265.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- 5.Beckers A. Does preoperative somatostatin analog treatment improve surgical cure rates in acromegaly? A new look at an old question. J Clin Endocrinol Metab. 2008;93:2975–2977. doi: 10.1210/jc.2008-1351. [DOI] [PubMed] [Google Scholar]
- 6.Cappabianca P, Alfieri A, Colao A, Ferone D, Lombardi G, de Divitiis E. Endoscopic endonasal transsphenoidal approach: an additional reason in support of surgery in the management of pituitary lesions. Skull Base Surg. 1999;9:109–117. doi: 10.1055/s-2008-1058157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS) Minim Invasive Neurosurg. 1998;41:66–73. doi: 10.1055/s-2008-1052019. [DOI] [PubMed] [Google Scholar]
- 8.Cappabianca P, Alfieri A, Thermes S, Buonamassa S, de Divitiis E. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery. 1999;45:392–395. doi: 10.1097/00006123-199908000-00041. [DOI] [PubMed] [Google Scholar]
- 9.Carlsen SM, Lund-Johansen M, Schreiner T, Aanderud S, Johannesen O, Cooper SJ, JG HJK, Fougner SL, Bollerslev J. Preoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trial. J Clin Endocrinol Metab. 2008;93:2984–2990. doi: 10.1210/jc.2008-0315. [DOI] [PubMed] [Google Scholar]
- 10.Ceylan S, Koc K, Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112:99–107. doi: 10.3171/2009.4.JNS09182. [DOI] [PubMed] [Google Scholar]
- 11.Charalampaki P, Reisch R, Ayad A, Conrad J, Welschehold S, Perneczky WC. Endoscopic endonasal pituitary surgery: surgical and outcome analysis of 50 cases. J Clin Neurosci. 2007;14:410–415. doi: 10.1016/j.jocn.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Colao A, Attanasio R, Pivonello R, Cappabianca P, Cavallo LM, Lasio Lodrini A, Lombardi G, Cozzi R. Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2006;91:85–92. doi: 10.1210/jc.2005-1208. [DOI] [PubMed] [Google Scholar]
- 13.Danoff A, Kleinberg D. Somatostatin analogs as primary medical therapy for acromegaly. Endocr. 2003;20:291–297. doi: 10.1385/ENDO:20:3:291. [DOI] [PubMed] [Google Scholar]
- 14.de Divitiis E, Cappabianca P, Cavallo LM. Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery. 2002;51:699–705. doi: 10.1097/00006123-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Erturk E, Tuncel E, Kiyici S, Ersoy C, Duran C, Imamoglu S. Outcome of surgery for acromegaly performed by different surgeons: importance of surgical experience. Pituitary. 2005;8:93–97. doi: 10.1007/s11102-005-3280-9. [DOI] [PubMed] [Google Scholar]
- 16.Feenstra J, de Herder WW, ten Have SM, van den Beld AW, Feelders RA, Janssen JA, van der Lely AJ. Combined therapy with somatostatin analogues and weekly pegvisomant in active acromegaly. Lancet. 2005;365:1644–1646. doi: 10.1016/S0140-6736(05)63011-5. [DOI] [PubMed] [Google Scholar]
- 17.Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2005;90:4465–4473. doi: 10.1210/jc.2005-0260. [DOI] [PubMed] [Google Scholar]
- 18.Gittoes NJ, Sheppard MC, Johnson AP, Stewart PM. Outcome of surgery for acromegaly—the experience of a dedicated pituitary surgeon. QJM. 1999;92:741–745. doi: 10.1093/qjmed/92.12.741. [DOI] [PubMed] [Google Scholar]
- 19.Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526–529. doi: 10.1210/jc.85.2.526. [DOI] [PubMed] [Google Scholar]
- 20.Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141–148. doi: 10.1210/jc.2009-2670. [DOI] [PubMed] [Google Scholar]
- 21.Jho HD, Alfieri A. Endoscopic transsphenoidal pituitary surgery: various surgical techniques and recommended steps for procedural transition. Br J Neurosurg. 2000;14:432–440. doi: 10.1080/02688690050175229. [DOI] [PubMed] [Google Scholar]
- 22.Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44–51. doi: 10.3171/jns.1997.87.1.0044. [DOI] [PubMed] [Google Scholar]
- 23.Kaltsas GA, Isidori AM, Florakis D, Trainer PJ, Camacho-Hubner C, Afshar F, Sabin I, Jenkins JP, Chew SL, Monson JP, Besser GM, Grossman AB. Predictors of the outcome of surgical treatment in acromegaly and the value of the mean growth hormone day curve in assessing postoperative disease activity. J Clin Endocrinol Metab. 2001;86:1645–1652. doi: 10.1210/jc.86.4.1645. [DOI] [PubMed] [Google Scholar]
- 24.Karavitaki N, Turner HE, Adams CB, Cudlip S, Byrne JV, Fazal-Sanderson V, Rowlers S, Trainer PJ, Wass JA. Surgical debulking of pituitary macroadenomas causing acromegaly improves control by lanreotide. Clin Endocrinol (Oxf) 2008;68:970–975. doi: 10.1111/j.1365-2265.2007.03139.x. [DOI] [PubMed] [Google Scholar]
- 25.Katznelson L. Drug insight: primary medical therapy of acromegaly. Nat Clin Pract Endocrinol Metab. 2006;2:109–117. doi: 10.1038/ncpendmet0096. [DOI] [PubMed] [Google Scholar]
- 26.Koc K, Anik I, Ozdamar D, Cabuk B, Keskin G, Ceylan S. The learning curve in endoscopic pituitary surgery and our experience. Neurosurg Rev. 2006;29:298–305. doi: 10.1007/s10143-006-0033-9. [DOI] [PubMed] [Google Scholar]
- 27.Kristof RA, Stoffel-Wagner B, Klingmüller D, Schramm J. Does octreotide treatment improve the surgical results of macro-adenomas in acromegaly? A randomized study. Acta Neurochir (Wien) 1999;141:399–405. doi: 10.1007/s007010050316. [DOI] [PubMed] [Google Scholar]
- 28.Laws ER. Surgery for acromegaly: evolution of the techniques and outcomes. Rev Endocr Metab Disord. 2008;9:67–70. doi: 10.1007/s11154-007-9064-y. [DOI] [PubMed] [Google Scholar]
- 29.Laws ER, Vance ML, Thapar K. Pituitary surgery for the management of acromegaly. Horm Res. 2000;53(Suppl 3):71–75. doi: 10.1159/000023538. [DOI] [PubMed] [Google Scholar]
- 30.Mao Z, Zhu Y, Tang H, Wang D, Zhou J, He D, Lan H, Luo B, Wang H. Preoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trial. Eur J Endocrinol. 2010;162:661–666. doi: 10.1530/EJE-09-0908. [DOI] [PubMed] [Google Scholar]
- 31.Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(2558–2573):14–12. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 32.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neggers SJ, van Aken MO, Janssen JA, Feelders RA, de Herder WW, van der Lely AJ. Long-term efficacy and safety of combined treatment of somatostatin analogs and pegvisomant in acromegaly. J Clin Endocrinol Metab. 2007;92:4598–4601. doi: 10.1210/jc.2007-1234. [DOI] [PubMed] [Google Scholar]
- 34.Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical 'cure'. Eur J Endocrinol. 2005;152:379–387. doi: 10.1530/eje.1.01863. [DOI] [PubMed] [Google Scholar]
- 35.Petrossians P, Borges-Martins L, Espinoza C, Daly A, Betea D, Valdes-Socin H, Stevenaert A, Chanson P, Beckers A. Gross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogs. Eur J Endocrinol. 2005;152:61–66. doi: 10.1530/eje.1.01824. [DOI] [PubMed] [Google Scholar]
- 36.Pieters GF, Smals AG, Kloppenborg PW. Defective suppression of growth hormone after oral glucose loading in adolescence. J Clin Endocrinol Metab. 1980;51:265–270. doi: 10.1210/jcem-51-2-265. [DOI] [PubMed] [Google Scholar]
- 37.Plöckinger U, Quabbe HJ (2005) Presurgical octreotide treatment in acromegaly: no improvement of final growth hormone (GH) concentration and pituitary function [DOI] [PubMed]
- 38.Selvarajah D, Webster J, Ross R, Newell-Price J. Effectiveness of adding dopamine agonist therapy to long-acting somatostatin analogues in the management of acromegaly. Eur J Endocrinol. 2005;152:569–574. doi: 10.1530/eje.1.01888. [DOI] [PubMed] [Google Scholar]
- 39.Sheppard MC. Primary medical therapy for acromegaly. Clin Endocrinol (Oxf) 2003;58:387–399. doi: 10.1046/j.1365-2265.2003.01734.x. [DOI] [PubMed] [Google Scholar]
- 40.Shimon I, Cohen ZR, Ram Z, Hadani M. Transsphenoidal surgery for acromegaly: endocrinological follow-up of 98 patients. Neurosurgery. 2001;48:1239–1243. doi: 10.1097/00006123-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Swearingen B, Barker FG, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–3426. doi: 10.1210/jc.83.10.3419. [DOI] [PubMed] [Google Scholar]
- 42.Tabaee A, Anand VK, Barron Y, Hiltzik DH, Brown SM, Kacker A, Mazumdar M, Schwartz TH. Endoscopic pituitary surgery: a systematic review and meta-analysis. J Neurosurg. 2009;111:545–554. doi: 10.3171/2007.12.17635. [DOI] [PubMed] [Google Scholar]
- 43.Trepp R, Stettler C, Zwahlen M, Seiler R, Diem P, Christ ER. Treatment outcomes and mortality of 94 patients with acromegaly. Acta Neurochir (Wien) 2005;147:243–251. doi: 10.1007/s00701-004-0466-2. [DOI] [PubMed] [Google Scholar]
- 44.Van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML, Freda PU, Stewart PM, Friend KE, Clemmons DR, Johannsson G, Stavrou S, Cook DM, Phillips LS, Strasburger CJ, Hackett S, Zib KA, Davis RJ, Scarlett JA, Thorner MO. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759. doi: 10.1016/S0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- 45.Van Lindert EJ, Hey O, Boecher-Schwarz H, Perneczky A. Treatment results of acromegaly as analyzed by different criteria. Acta Neurochir (Wien) 1997;139:905–912. doi: 10.1007/BF01411298. [DOI] [PubMed] [Google Scholar]


