Abstract
Objective
To describe the clinical characteristics of pregnant women with influenza-like illness (ILI) during the 2009 H1N1 pandemic with the use of a standardized management algorithm.
Study Design
Between June, 2009 and March, 2010 we assembled a prospective cohort of pregnant women with ILI at a single tertiary care center using a standardized algorithm. Clinical outcomes were compared between women with 2009 H1N1 virus and those without.
Results
45 women were included. 17 had 2009 H1N1 infection and 28 did not. Demographic characteristics were similar between groups. The median temperature upon presentation (99.7 versus 98.8°F, p=0.004) was slightly higher among those with 2009 H1N1. All those with 2009 H1N1 influenza and 89% of those without were treated with oseltamivir. A total of 12 women (27%) were hospitalized. There were no endotracheal intubations or deaths.
Conclusion
Among this cohort of pregnant women, most were managed as outpatients, and had favorable maternal outcomes.
Keywords: Influenza, pandemic, pregnancy, 2009 H1N1
Introduction
The 2009 H1N1 influenza virus contains a unique combination of gene segments that have not been reported previously among swine or human influenza cases in the United States.6, 7 The 2009 H1N1 novel swine-origin influenza virus is influenza A: H1N1 that is a triple recombinant including gene segments of human, swine, and avian origin. The first reports of 2009 H1N1 in pregnancy suggested that pregnancy may be a significant risk factor for mortality in the most recent pandemic. The CDC published a report in July, 2009 detailing 34 cases of 2009 H1N1 across the United States. During a two-month time period, 45 deaths were reported to the CDC. Six of these were among pregnant women.10
Since that time, there have been several reports examining the impact of 2009 H1N1 among hospitalized pregnant women. These have confirmed the original report suggesting that disease is more likely to be severe during pregnancy..11 Among women admitted to intensive care units in Australia and New Zealand, the mortality rate was 11%.12 Subsequent reports have not found mortality rates to be as high as the ICU-specific rate, but suggested that mortality rates were increased in pregnancy and that time from clinical presentation to antiviral therapy was an important determinant of outcome.13, 14
Because many facilities were capable of performing laboratory testing for 2009 H1N1 only in the hospital setting, there have been limited data available from outpatient settings regarding 2009 H1N1 in pregnancy. It is unknown whether 2009 H1N1 is more mortal than other causes of influenza-like illness. In this report, we sought to describe the clinical characteristics of pregnant women with influenza-like illness (ILI) during the 2009 H1N1 pandemic with the use of a standardized management algorithm. Secondarily, we compared the clinical presentation of pregnant women with confirmed 2009 H1N1 to those with other causes of influenza-like illness in both inpatient and outpatient settings.
Materials and Methods
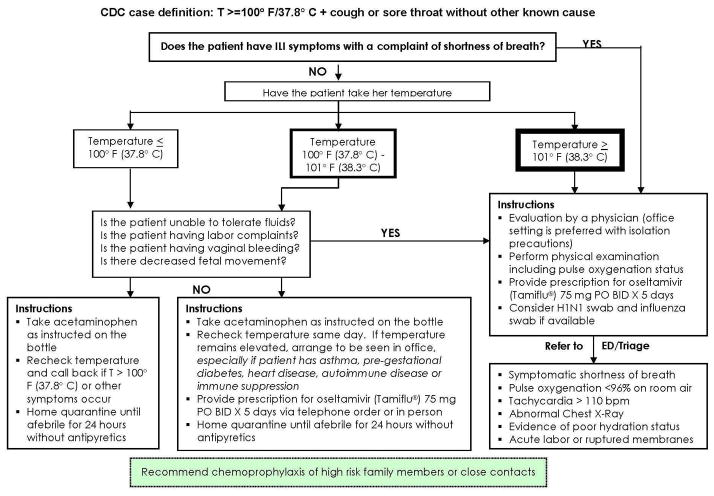
We performed a prospective cohort study of pregnant women who presented with symptoms of influenza-like illness to the triage unit of Women & Infants Hospital of Rhode Island, a large, tertiary care obstetric hospital affiliated with Brown University’ During the recent pandemic, our hospital, like many others across the country, developed a standard patient flow and management algorithm that was disseminated to the community (Figure 1). This algorithm provided guidance to providers regarding when to send patients with ILI to the obstetric triage unit. Potentially eligible subjects were recruited from this unit. Between June, 2009 and March, 2010, pregnant women with symptoms of influenza-like illness were approached for enrollment. Women with a fever >100°F and cough and/or sore throat in the absence of other known causes of illness were eligible. Women were also eligible if the provider deemed the patient to be at risk for 2009 H1N1 such that they sent a swab to the Rhode Island State Department of Health for confirmatory testing. This occasionally occurred in the setting of myalgias or other illness with known 2009 H1N1 contact but without fever. When patients meeting these criteria were evaluated in the triage unit, they were approached for enrollment whether they had been referred by a provider or not. No asymptomatic women were included regardless of sick contacts. During this time in the State of Rhode Island, the State Department of Health allowed testing for all pregnant women deemed to be at risk for 2009 H1N1 even if they were not hospitalized. This is a real time RT-PCR test that should theoretically be minimally affected by recent antiviral therapy because it does not rely upon replicating virus for detection. Exclusion criteria were participation in another influenza study, immunocompromise, positive group A streptococcal test for pharyngitis, suspicion of active tuberculosis, or suspicion of acute pyelonephritis.
Figure 1.
Telephone triage instructions were disseminated to the hospital and community based physician offices.
The study was approved by the Women & Infant’s Hospital Institutional Review Board. Eligible women were approached and those that were willing to participate provided written, informed consent. Subjects were interviewed by study personnel and data were collected from their charts until discharge. Basic demographic information, underlying medical conditions, and previous pregnancy data were collected. Complications during the current pregnancy were assessed. The subjects’ presenting symptoms were elicited and data regarding any home therapies prior to presentation were collected. The highest fever at home was recorded and the vital signs upon presentation to the hospital were collected. The subjects’ prior influenza immunization history was elicited. Therapy prescribed including antivirals, antibiotics for presumed pneumonia, and need for oxygen support, was collected. Final results of the state testing for 2009 H1N1 were confirmed. Clinical data during the course of the patient evaluation and, if applicable, hospitalization were collected. Neonatal data will be reported separately.
Because of the observational nature of the study, simple descriptive statistics were used to compare characteristics of those with 2009 H1N1 and those without. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared between groups using two-sided T-test or the Wilcoxon rank-sum test if the variable was not normally distributed.
Results
Fifty-four women with influenza-like illness were enrolled in the cohort, 9 of whom were excluded due to participation in another influenza study. The general characteristics and outcomes of these 9 were not significantly different from those reported here. Forty-five women were thus included in this analysis. Of these 17 tested positive for the 2009 H1N1 pandemic virus, 24 tested negative for any influenza virus, 3 tested positive for seasonal influenza viruses. One woman was not tested. Twelve of the women (27%) were hospitalized24% of those with 2009 H1N1 and 29% of those without. There were no endotracheal intubations or deaths. The demographic characteristics of the women with 2009 H1N1 infection were similar to those without (Table 1). Approximately 59% of the women with 2009 H1N1 reported Hispanic ethnicity compared to 43% of those without, p=0.5. In general, in our hospital, only approximately a third of the deliveries are to women reporting Hispanic ethnicity.
Table 1.
Demographic characteristics of Women with Influenza-like illness
| Characteristic | 2009 H1N1 positive (n=17) | Other ILI (n=28) | P-value* |
|---|---|---|---|
| Age (y), mean (SD) | 24.8 (4.2) | 25.4 (5.9) | 0.7 |
| Range | 19–33 | 16–39 | |
| Gestational age (wks), median (range) | 26 (4.6–32) | 18 (6–38) | 0.8 |
| Race/ethnicity n(%) | |||
| Hispanic | 10 (58.8) | 12 (42.9) | 0.5 |
| White, non-Hispanic | 31 (17.7) | 11 (39.3) | |
| Black, non-Hispanic | 1 (5.9) | 1 (3.6) | |
| Other/multi-racial | 3 (17.7) | 4 (14.3) | |
| Marital status n(%) | |||
| Single | 10 (58.8) | 15 (53.6) | 0.9 |
| Married/Partnered | 7 (42.2) | 12 (42.9) | |
| Employment status n(%) | |||
| Unemployed | 11 (64.7) | 10 (35.7) | 0.06 |
| Insurance n(%) | |||
| Medicaid/Uninsured | 13 (76.5) | 18 (64.3) | 0.8 |
| Private | 3 (17.7) | 7 (25.0) | |
| BMI, mean (SD) | 29.0 (4.9) | 29.8 (6.5) | 1.0 |
by Fisher’s exact test, Student’s t-test, or Wilcoxon rank sum as appropriate ILI: influenza-like illness, y: years, SD: standard deviation, wks: weeks, BMI: body mass index
Relatively few women reported underlying medical illnesses. Women with 2009 H1N1 infection were not significantly more likely to report underlying illnesses than those without, although there were slightly more women reporting a history of asthma in the 2009 H1N1 group. Those with 2009 H1N1 were less likely to report tobacco use, 5.9% versus 25%, p=0.1. Women with 2009 H1N1 were not more likely to have experienced early pregnancy complications prior to enrollment. Approximately a quarter in each group reported a history of hyperemesis with weight loss earlier in the pregnancy. Forty-one and 37% of women in each group reported having been vaccinated for seasonal influenza during the 2008–2009 influenza season (Table 2).
Table 2.
Underlying characteristics of women with influenza-like illness
| Characteristic | 2009 H1N1 positive (n=17) | Other ILI (n=28) | P-value* |
|---|---|---|---|
| Symptoms n(%) | |||
| Fever | 15 (88.2) | 20 (71.4) | 0.3 |
| If yes: | |||
| Temp, median (range) | 101.3 (99.1–103.0) | 100.7 (99.5–103.2) | 0.4 |
| Cough | 16 (94.1) | 23 (82.1) | 0.4 |
| Stuffiness | 12 (70.6) | 18 (64.3) | 0.8 |
| Sore throat | 13 (76.5) | 21/27 (77.8) | 1.0 |
| Body aches | 16 (94.1) | 23/27 (85.2) | 0.6 |
| Nausea | 9 (52.9) | 17 (60.7) | 0.8 |
| Vomiting | 7 (41.2) | 12/27 (44.4) | 1.0 |
| Diarrhea | 3 (17.7) | 10/27 (37.0) | 0.2 |
| Difficult breathing | 11 (64.7) | 17 (60.7) | 1.0 |
| Contractions | 2 (11.8) | 3 (11.1) | 1.0 |
| Pain relievers prior to presentation n(%) | |||
| Acetaminophen | 10 (58.8) | 14 (50.0) | 0.8 |
| NSAID | 2 (11.8) | 1 (3.6) | 0.5 |
| Cold medicine | 1 (5.9) | 3 (10.7) | 1.0 |
| Received 2008/2009 flu vaccine, n(%) | 7 (41.2) | 10/27 (37.0) | 1.0 |
| Received 2009/2010 flu vaccine, n(%) | |||
| No | 4 (23.5) | 10 (35.7) | 0.3 |
| Yes | 3 (17.7) | 9 (32.1) | |
| N/A | 10 (58.8) | 9 (32.1) | |
| Vital signs, median (range) | |||
| Temperature (F) | 99.7 (97.8–102.7) | 98.8 (97.7–100.6) | 0.004 |
| Heart rate | 100 (76–150) | 98 (70–150) | 0.6 |
| BP-systolic | 116 (95–126) | 116 (11–138) | 0.8 |
| BP-diastolic | 68 (54–80) | 69.5 (55–84) | 0.8 |
| Respiratory rate | 20 (12–26) | 19 (12–28) | 0.9 |
| Oxygen saturation | 98 (86–100) | 98 (88–100) | 0.7 |
| Fetal heart rate | 150 (125–180) | 156 (120–194) | 0.2 |
| Therapy, n(%) | |||
| Oseltamivir: twice daily | 17 (100) | 25 (89.3) | 0.3 |
| Antibiotics: oral | 1 (5.9) | 3 (10.7) | 1.0 |
| Antibiotics: IV | 0 | 1 (3.6) | 1.0 |
| Nasal cannula | 1 (5.9) | 1 (3.6) | 1.0 |
| Face mask | 1 (5.9) | 2 (7.1) | 1.0 |
| Non-rebreather mask | 0 | 1 (3.6) | 1.0 |
| Nebulizer | 2 (11.8) | 4 (14.3) | 1.0 |
by Fisher’s exact test, Student’s t-test, or Wilcoxon rank sum as appropriate ILI: influenza-like illness, NSAID: non-steroidal anti-inflammatory drug, N/A: not applicable because vaccine not yet available, F: Fahrenheit, BP: blood pressure, IV: intra-venous
At the time of presentation, there were no statistically significant differences in the frequency of contact with ill individuals or in the presence of clinical symptoms. Nausea and vomiting were reported frequently in both groups (Table 2). The median self-reported temperature prior to presentation at triage was higher among those with 2009 H1N1 but did not reach statistical significance, 101.3°F versus 100.7°F, p=0.4. The median temperature on presentation was higher among those with 2009 H1N1, 99.7°F versus 98.8°F, p=0.004. Approximately half the women in each group took acetaminophen at home prior to presentation. Overall 69% of the women used over-the-counter medications prior to presentation at the triage area. The median heart rate among those with 2009 H1N1 was 100 beats per minute beats versus 98 in those without 2009 H1N1, p=0.6 and the range in both groups included heart rates as high as 150 beats per minute. One hundred percent of those with 2009 H1N1 and 89% of those without were prescribed twice daily oseltamivir at or prior to enrollment; 5.9% of those with and 10.7% of those without 2009 H1N1 were also prescribed oral antibiotics. 23.5% of those with and 28.6% of those without 2009 H1N1 required oxygen or nebulized beta agonist therapy.
Among the 12 hospitalized patients, 3 of 4 of those with 2009 H1N1 and 6 of 8 of those without had an obstetric concern listed as one of the reasons for admission. All admitted patients had an ILI symptom as the reason for hospitalization. These included persistent tachycardia, dyspnea, hypoxia, persistent fever, or chest radiograph abnormality. Of the obstetric concerns listed as reasons for hospitalization, one patient without 2009 H1N1 had fetal tachycardia. One had elevated blood pressure and was evaluated for pre-eclampsia. The remainder had a concern for dehydration that might lead to preterm labor, but none actually developed preterm labor. Among those that were hospitalized, the median maternal heart rate was significantly tachycardic, 130 (100-150) and 123 (95-150) beats per minute respectively. There were no clinically significant laboratory abnormalities among the hospitalized women that required intervention (Table 3).
Table 3.
Characteristics of women hospitalized with influenza-like illness
| Characteristic | 2009 H1N1 positive (n=4) | Other ILI (n=8) | P- value* |
|---|---|---|---|
| Gestational age (weeks), median (range) | 20.5 (17–26) | 23.5 (6–36) | 0.9 |
| Recorded vital signs, median (range) | |||
| Highest temp (F) | 100.3 (98.6–102.4) | 99.7 (98.1–102.7) | 0.8 |
| Highest respiratory rate | 24 (22–35) | 23 (20–30) | 0.6 |
| Highest heart rate | 130 (100–150) | 123 (95–150) | 0.7 |
| Lowest BP systolic | 84 (74–110) | 89 (77–108) | 0.7 |
| Lowest BP diastolic | 46 (28–60) | 45.5 (38–62) | 0.9 |
| Highest BP systolic | 121.5 (120–122) | 122 (110–160) | 0.7 |
| Highest BP diastolic | 77.5 (68–84) | 75 (62–93) | 0.9 |
| Therapy prescribed | |||
| Oseltamivir: once daily | 0 | 1 (12.5) | 1.0 |
| Oseltamivir: twice daily | 4 (100) | 7 (87.5) | 1.0 |
| Antibiotics: IV | 1 (25.0) | 4 (50.0) | 1.0 |
| Steroids: oral prednisone | 1 (25.0) | 2 (25.0) | 1.0 |
| Oxygen supplementation | |||
| Nasal cannula | 1 (25.0) | 1 (12.5) | 1.0 |
| Face mask | 1 (25.0) | 2 (25.0) | 1.0 |
| Non-rebreather mask | 1 (25.0) | 1 (12.5) | 1.0 |
| Nebulizer | 3 (75.0) | 4 (50.0) | 0.6 |
| Labs at admission, median (range) | |||
| WBC (×103) | 10.2 (3.7–12.4) | 10.4 (8.0–14.7) | 0.7 |
| HGB | 11.9 (11.4–12.0) | 12.0 (11.3–13.0) | 0.6 |
| HCT | 36 (31–37) | 36.6 (35.1–40.3) | 0.4 |
| Platelets (×103) | 177 (151–204) | 215 (161–379) | 0.1 |
| AST | 36 (22–50) (n=2) | 22.5 (19–26) (n=2) | 0.7 |
| ALT | 31 (17–45) (n=2) | 14.5 (10–19) (n=2) | 0.7 |
| BUN | 5 (5–8) | 5 (5–6) | 1.0 |
| Creatinine (mg/dL) | 0.8 (0.7–0.8) | 0.6 (0.5–0.7) | 0.06 |
| Most abnormal labs, median (range) | |||
| Highest WBC (×103) | 10.2 (6.8–12.4) | 10.4 (8.0–15.9) | 0.7 |
| Lowest HGB | 9.0 (8.6–11.9) | 11.0 (9.3–12.2) | 0.2 |
| Lowest HCT | 27.6 (27.0–37.0) | 34.0 (28.7–36.8) | 0.2 |
| Lowest Platelets (×103) | 148.5 (121–204) | 184 (139–379) | 0.2 |
| Highest AST | 36 (22–50) (n=2) | 20.5 (16–26) (n=4) | 0.4 |
| Highest ALT | 33.5 (17–50) (n=2) | 16.5 (10–19) (n=4) | 0.5 |
| Highest BUN | 5 (5–45) | 5.5 (5–7) | 0.8 |
| Highest Creatinine (mg/dL) | 0.8 (0.7–0.8) | 0.6 (0.6–0.7) | 0.06 |
by Fisher’s exact test, Student’s t-test, or Wilcoxon rank sum as appropriate ILI: influenza-like illness, NSAID: non-steroidal anti-inflammatory drug, N/A: not applicable because vaccine not yet available, F: Fahrenheit, BP: blood pressure, IV: intra-venous, WBC: leukocyte count, HGB: hemoglobin (g/dL), HCT: hematocrit (%), AST: aspartate aminotransferase (U/L), ALT: alanine aminotransferase (U/L), Blood urea nitrogen (mg/dL)
Comment
We found that the majority of women with 2009 H1N1 infection and other causes of influenza-like illness were able to be managed as outpatients during pregnancy. Maternal tachycardia was almost universally present. Nausea and vomiting were commonly reported among both those with and without 2009 H1N1. There are a number of reports that have emerged since the 2009 H1N1 pandemic began suggesting a disproportionately high rate of hospitalization and death among pregnant women compared to non-pregnant people11–14. Our study was not designed to make this comparison, but rather to examine whether 2009 H1N1 is more virulent and might require different therapies and management schema than other causes of ILI. Within the limitations of our sample size, we did not find worse outcomes but did find that 2009 H1N1 experienced higher fevers than those with other causes of ILI. The neonatal data are being reported in a separate analysis.
There were no maternal deaths in our cohort and endotracheal intubation was not necessary. This may be due to several factors. Consistent with CDC recommendations and because it took 24–72 hours to get the result from the State Department of Health testing, patients with ILI were treated as though they had 2009 H1N1. Our hospital developed a cautious, directive, patient triage algorithm which may have allowed providers to perform earlier intervention and care than some of the earlier reports were able to have11–14. A recent reported supported the probable safety of antivirals in pregnancy15 and our algorithm complied with ACOG and CDC guidelines that directed early, liberal use of these medications16, 17.
Another possibility is that we could have missed more severely ill patients because they went directly to the intensive care unit. Our research team regularly screened the intensive care units, labor and delivery, and the triage unit so we do not believe that there were any severely ill women who were not approached for inclusion. The State of Rhode Island reported all 2009 H1N1 deaths weekly and weekly conference calls with the Department of Health reviewed the cases of severe illness in the state. Some women declined participation in the study but to our knowledge, there were no intubations or deaths of any pregnant women with 2009 H1N1 in the State of Rhode Island during this timeframe.
A strength of our study is the prospective nature of the data collection and the fact that we did not know whether patients were actually infected with 2009 H1N1 at the time of enrollment because of the time necessary to obtain results from the Department of Health. Use of rapid influenza testing was discouraged at our institution during this timeframe because of its previously reported low sensitivity for detecting 2009 H1N1. Patients who met the criteria for suspicion of ILI were treated aggressively without waiting for the test results. Previous reports also suggested that patients with 2009 H1N1 were likely to experience nausea and vomiting, a symptom not typical of seasonal influenza11. This was reported in approximately 20–40% of patients with 2009 H1N1 in other reports10, 11, 18, 19. We found that pregnant women with ILI were likely to report nausea and vomiting even if they were not found to be infected with 2009 H1N1. It is unclear why so many women who did not have 2009 H1N1 infection in this study reported this symptom. Because so many patients in the study did not have any form of influenza, it may be that those women were suffering from a primary gastrointestinal virus rather than a respiratory illness despite the high frequency of upper respiratory tract symptoms reported. There were not enough subjects in the study with non-pandemic influenza to make comparisons between seasonal influenza and the pandemic virus in terms of outcomes.
There are a number of studies suggesting that both seasonal and pandemic influenza are more serious in pregnancy than in the non-pregnant state. Hospitalization rates are higher among pregnant and puerperal women during influenza season.20 Influenza-attributable acute cardiopulmonary events are more than five times higher in pregnancy as in the non-pregnant state, 10.5 (95% CI 6.7–14.3) versus 1.91 (95% CI 1.51–2.31) per 10,000 women-months.21 The risk of morbidity during pregnancy appears to be even higher during influenza pandemics. Reports published from the 1918 pandemic revealed that in over 1,300 cases of influenza in pregnancy, approximately 50% were complicated by pneumonia. Of those that developed pneumonia, mortality was 54%. The mortality rates appeared to be highest in the third trimester. Among women who did not develop pneumonia, 25% experienced pregnancy “interruption.” The rate of pregnancy loss was even higher, 50%, for those that experienced pneumonia.4 In the study from the 1957 pandemic, influenza was the leading cause of maternal mortality during that timeframe. The study was a population based cohort and compared hospitalization rates for respiratory illness during influenza season with rates in non-influenza seasons. They found that the hospitalization rate ratio in pregnancy was 5.1 (95%CI 3.6–7.3) among women without significant co-morbidities and even higher among those with co-morbidities.5 There are a number of reasons why we did not find severe outcomes such as these. Vaccination for 2009 H1N1 became available during the course of our study. The outcomes may have been much more severe if many more women had become infected. Because of the intense attention to early therapy and media education of patients during this pandemic, patients may have sought care and begun antiviral therapy much earlier than in previous pandemics.
This study leaves unanswered several questions that deserve further study. The impact of seasonal influenza in pregnancy in modern times is still unknown. The effect of antiviral therapy and antibiotics on the need for ventilatory support or death has not been well studied. The efficacy and optimal dosage of vaccination in pregnancy is also unknown. While multiple reports have shown severe outcomes among pregnant women with 2009 H1N1, our study shows that in some instances, pregnant women with ILI can be managed in the outpatient setting without severe outcomes or mortality.
Acknowledgments
The authors thank Linda Nelson, Pharm.D. and Raymond Powrie, M.D. for their collaborative efforts in the development of the standardized patient algorithm.
Funding: Time spent on this study was partially supported by 1K23HD062340-01 (Anderson-PI) and departmental funds
Footnotes
Reprints will not be available for this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in poster format at the 37th Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Santa Fe, NM, August 5-7, 2010
References
- 1.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerging Infectious Diseases. 2008;14(1):95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerging Infectious Diseases. 2006;12(11):1638–43. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saleeby E, Chapman J, Morse J, Bryant A. H1N1 influenza in pregnancy: cause for concern. Obstetrics & Gynecology. 2009;114(4):885–91. doi: 10.1097/AOG.0b013e3181bb44bb. [DOI] [PubMed] [Google Scholar]
- 4.Harris JW. Influenza in pregnant women. JAMA. 1919;72(14):978–80. [Google Scholar]
- 5.Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–5. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Swine Influenza A (H1N1) infection in two children-Southern California, March-April 2009. MMWR. 2009;58:400–2. [PubMed] [Google Scholar]
- 7.Garten RJ, Davis CT, Russell CA, et al. Antigenic and Genetic Characteristics of Swine-Origin 2009 A(H1N1) Influenza Viruses Circulating in Humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Update: Swine Influenza A (H1N1) Infections-California and Texas, April, 2009. MMWR. 2009;58(16):435–7. [PubMed] [Google Scholar]
- 9.Novel Swine-Origin Influenza AVIT. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med. 2009;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. The Lancet. 2009;374(9688):451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 11.Louie JK, Acosta M, Jamieson DJ, Honein MA the California Pandemic Working G. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 12.The Anzic Influenza Investigators and Australasian Maternity Outcomes Surveillance SystemCritical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340(mar18_3):c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 Influenza A(H1N1) Virus Illness Among Pregnant Women in the United States. JAMA. 2010;303(15):1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 Pandemic Influenza A (H1N1) Virus Infection in Pregnant Women. Obstetrics & Gynecology. 2010;115(4):717–26. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 15.Greer LG, Sheffield JS, Rogers VL, Roberts SW, McIntire DD, Wendel GD., Jr Maternal and neonatal outcomes after antepartum treatment of influenza with antiviral medications. Obstetrics & Gynecology. 115(4):711–6. doi: 10.1097/AOG.0b013e3181d44752. [DOI] [PubMed] [Google Scholar]
- 16.ACOG Committee on Obstetric Practice, ACOG committee opinion number 305. Influenza vaccination and treatment during pregnancy. Obstetrics & Gynecology. 2004;104:1125–6. [PubMed] [Google Scholar]
- 17.National Center for Immunization and Respiratory Diseases CDC. Use of Influenza A (H1N1) 2009 Monovalent Vaccine, Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR. 2009;58(Early Release):1–8. [PubMed] [Google Scholar]
- 18.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 19.Miller AC, Safi F, Hussain S, Subramanian RA, Elamin EM, Sinert R. Novel Influenza A(H1N1) Virus Among Gravid Admissions. Arch Intern Med. 170(10):868–73. doi: 10.1001/archinternmed.2010.126. [DOI] [PubMed] [Google Scholar]
- 20.Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ: Canadian Medical Association Journal. 2007;176(4):463–8. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of Influenza on Acute Cardiopulmonary Hospitalizations in Pregnant Women. Am J Epidemiol. 1998;148(11):1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]