Abstract
Epigenetic mechanisms are involved in programming gene expression throughout development. In addition, they are key contributors to the processes by which early-life experience fine-tunes the expression levels of key neuronal genes, governing learning and memory throughout life. Here we describe the long-lasting, bi-directional effects of early-life experience on learning and memory. We discuss how enriched postnatal experience enduringly augments spatial learning, and how chronic early-life stress results in persistent and progressive deficits in the structure and function of hippocampal neurons. The existing and emerging roles of epigenetic mechanisms in these fundamental neuroplasticity phenomena are illustrated.
Keywords: CRH, epigenetic, hippocampus, neonatal, early-life experience, maternal care, learning and memory, stress, corticotropin releasing hormone, glucocorticoids, GR, neuroplasticity, programming, resilience
1. The clinical problem: association of early-life experience with learning and memory
Numerous clinical reports demonstrate a strong association between early-life experience and subsequent cognitive functions. Chronic childhood stress (such as extreme poverty, loss of parent, social deprivation or abuse) correlates with learning and memory impairments later in life (Kaplan et al., 2001; Nelson et al., 2007; Wilson et al., 2007). As examples, lower socioeconomic level early in life correlates with cognitive function in adulthood, and post-institutionalized orphans have abnormal neuronal function in limbic areas including the hippocampus, as shown by functional MRI studies (Chugani et al., 2001), and by worse cognitive performance when compared to never-institutionalized children (Nelson et al., 2007). Improving the experience of these institutionalized infants by placing them in families significantly improves learning and memory long-term. Remarkably, the timing of the placement into foster care is crucial, and placement before the age of two years is associated with improved outcome (Bos, Zeanah, Smyke, Fox, & Nelson, 2010). These data suggest that critical developmental periods exist for the processes by which early-life experience shapes cognitive function throughout life.
The impact of early-life experience, and especially of chronic stress, on the integrity of the hippocampus, a region subserving certain learning and memory processes (Andersen, Moser, Moser & Trommald, 1996; Eichenbaum, Yonelinas, & Ranganath, 2007; Morris et al., 2003; Squire, Wixted, & Clark, 2007), is supported also by clinical studies suggesting that hippocampal volumes in adults that have experienced early-life abuse are smaller (Bremner, et al., 1997). Whereas this view is not universally endorsed (Lenze, Xiong, & Sheline, 2008; Lyons, Yang, Sawyer-Glover, Moseley, & Schatzberg, 2001), this association and similar findings in other human studies (Buss et al., 2007) suggest that chronic early-life stress is associated with impairments in hippocampal structure and function in adulthood. In addition these correlational studies demonstrate the complexity of human research: elucidating the potential causal relationship between early-life stress and later life cognitive outcomes is difficult, because of uncontrollable variables such as genetics and subtle environmental influences that may not be measurable. Such studies lead to the realization that use of animal models benefits our understanding of the causal relationship between early-experiences and life-long learning and memory. These enable prospective longitudinal studies as well as control of genetic background (Nestler & Hyman, 2010). In addition, parameters of interest can be manipulated and subsequent experiences can be controlled throughout the entire period of investigation.
Notably, uncovering the biological mechanisms involved in the long-term consequences of enhanced early-life experience is of paramount importance, because these mechanisms may be employed for therapeutic interventions and improved outcome. This has happened, for example, when infant position was found to govern sudden infant death, and care of infants was changed (Ponsonby, Dwyer, Gibbons, Cochrane, & Wang, 1993). In addition, discovering if chronic early-life stress directly impacts cognitive function is extremely important because over 50% of the world’s children are exposed to chronic stress (UNICEF, 2005), and such stress cannot currently be prevented. Therefore, establishing causality and defining the molecular and cellular mechanisms for potential long-lasting effects of early-life experience and stress on learning and memory are prerequisites to preventive and therapeutic approaches in the future.
2. Animal models enable determination of causality and elucidation of the mechanisms by which early-life experience, including chronic stress, might govern learning and memory throughout life
For the past six decades, scientists have employed models in primates and rodents to manipulate environmental and genetic variables for the study of early-life experience on later-life cognitive functions (for review, see Korosi & Baram, 2009; Levine, 2000). In these animal models, early-life experience was modulated in a bi-directional manner (Figure 1). Acute-intermittent stress, such as daily maternal separation, or chronic stress imposed via alteration of maternal behavior, were designed to mimic human conditions of poverty, illness or neglect/ abuse (Avishai-Eliner, Brunson, Sandman, & Baram, 2002; Brunson, Chen, Avishai-Eliner, & Baram, 2003; Fenoglio, Brunson, & Baram, 2006a; Heim, Plotsky, & Nemeroff, 2004; Levine, 2000). In contrast, enhanced early-life experience was generated via a naturalistic selection of high-caring dams (Hofer, 1994), or via procedures, such as brief daily handling of the pups, that augment maternal care and thus maternal-derived sensory input (Brunson, Avishai-Eliner, Hatalski, & Baram, 2001; Fenoglio, Chen, & Baram, 2006b; Korosi & Baram, 2009; Korosi et al., 2010; Meaney, Aitken, van Berkel, Bhatnagar, & Sapolsky, 1988).
Figure 1. Bidirectional effects of early-life experience on cognitive function throughout life.
Chronic stress in the early postnatal period is associated with impaired cognition during middle-age in people. Animal models demonstrate that this type of stress causes loss of spines and eventual dendritic dying-back (atrophy), attenuated long-term potentiation and progressive deficits in spatial memory. In contrast, enriched early-life experience, and especially augmented sensory input from the mother, results in improved spatial learning compared to controls. In both cases, the mechanisms for the bidirectional plasticity involved persistently altered expression of genes involved in regulation of the ‘stress-system’.
A majority of studies employing these structured alterations of early-life experience have focused on ‘emotional’ outcomes- the vulnerability or resilience of the adult ‘graduates’ of these early-life manipulations to depressive-like or anxiety-like behaviors. However, existing and emerging evidence indicates that early-life experience and stress also contribute significantly to learning and memory throughout life, as suspected from the clinical correlational studies of infants and children. In this paper, we review data that support a causal relationship of enhanced early-life experience (derived from augmented quality of maternal care) and learning and memory function during adulthood. We also discuss the causal relationship of chronic stress during the neonatal / infancy period and cognitive decline commencing in middle age. In both cases we describe the molecular and cellular processes that are involved, and discuss the role of epigenetics in the persistently altered expression of key genes that contribute to these phenotypes.
3. Cellular and molecular changes resulting from enhanced early-life experience and from chronic early-life stress: a common theme
A key determinant of early-postnatal existence involves the interaction of the immature individual with his / her parent(s). Both clinical and experimental studies have confirmed the fundamental role of the presence and sensory input from a mother on the essence of the neonatal and infancy experience. Considered along a continuum, frequent and consistent nurturing care suppresses stress in the immature rat, monkey and human (Dent, Smith, & Levine, 1999; Gunnar, Larson, Hertsgaard, Harris, & Brodersen, 1992; Harlow & Suomi, 1971), whereas absence of the mother or abnormal quality or quantity of maternal care is a major provoker of the newborn / infant “stress system” (Figure 1). Based on this body of information, many manipulations of early-life experience have utilized modulation of mother-infant interactions. In addition, the principal ‘read-outs’ of the effects of these modulations on the infant brain have included acute and persistent alterations in the expression and function of genes that are involved in regulation of the stress response (Avishai-Eliner, Eghbal-Ahmadi, Tabachnik, Brunson, & Baram, 2001a; Fenoglio et al., 2005; Korosi et al., 2010; Plotsky & Meaney, 1993; Weaver et al., 2004).
As shown in Figure 2, the response to stress is governed by a number of neurotransmitters, neuromodulators and steroids (Joels & Baram, 2009; McEwen, 1999; Ulrich-Lai & Herman, 2009). In essence, external (or internal) signals that are interpreted as indicating potential or existing threat result in release of the neuropeptide corticotropin releasing hormone (CRH) from cells within the hypothalamus (Vale, Spiess, Rivier, & Rivier, 1981), as well as within the hippocampus (Chen, Bender, Frotscher, & Baram, 2001a; Chen et al., 2004b; Chen, Fenoglio, Dube, Grigoriadis, & Baram, 2006; Chen et al., 2010), and the amygdala (Roozendaal, Brunson, Holloway, McGaugh, & Baram, 2002). Hypothalamic CRH reaches the pituitary gland releasing corticotropin, and the latter promotes secretion of glucocorticoids. Glucocorticoids prepare the body for stress but, importantly, cross the blood brain barrier and interact with cognate receptors throughout the brain including in principal cells of the hippocampal formation (McEwen, 1999; Reul & de Kloet, 1985). Neurons within the hippocampal formation are activated by salient stresses, as measured by immediate early-gene expression and a number of electrophysiological parameters (Alfarez, Joels, & Krugers, 2003; Kim & Diamond, 2002; Pavlides, Watanabe & McEwen, 1993; Chen, Fenoglio, Dube, Grigoriadis, & Baram, 2006) reviewed by (Joels & Baram, 2009). In contrast, pleasurable or non-stressful experiences often cause minimal and rapidly-decaying release of stress hormones with transient activation of distinct cellular and molecular cascades (Feder, Nestler, & Charney, 2009; Fenoglio et al., 2006b; Sweatt, 2009). Whereas these processes were first uncovered in the adult organism, they function also in the neonatal period (Brunson et al., 2003; Dallman, 2000; Gunnar & Quevedo, 2007). Specifically, stress and other experiences activate hypothalamic (Chen, Hatalski, Brunson & Baram, 2001b) and hippocampal (Hatalski, Brunson, Tantayanubutr, Chen, & Baram, 2000) neurons. CRH is released from the hypothalamus to induce release of peripheral hormones (Yi & Baram, 1994), and CRH and glucocorticoid receptor (GR) signaling are functional in the periphery and within the hippocampus (Avishai-Eliner, Brunson, Sandman, & Baram, 2002; Lupien, McEwen, Gunnar, & Heim, 2009; Rosenfeld, van Eekelen, Levine, & de Kloet, 1993; Yi, Masters & Baram, 1993).
Figure 2. A schematic of the sequence of cells and molecules activated during stress, and their effects on the brain.
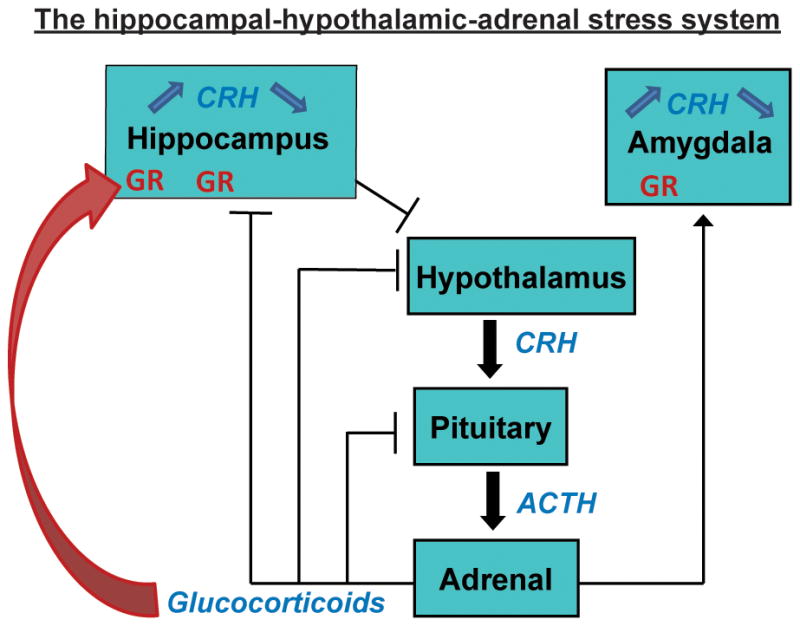
Stress signals release corticotropin releasing hormone (CRH) in amygdala and hypothalamus (see text). Activation of CRH receptors in the pituitary gland as a result of hypothalamic CRH release initiates the peripheral response to stress, including ACTH and glucocorticoid secretion. Glucocorticoids penetrate the blood brain barrier and act on wide-spread brain receptors, including in hippocampus. Activation of CRH receptors in hippocampus by physiological levels of CRH primes LTP. However, activation of the receptors by CRH levels found during severe stress provokes rapid loss of dendritic spines and contributes to defects in spatial memory.
The ‘tone’ of the system, i.e., the magnitude of the neuronal and hormonal response to a given stressor, is governed, at least in part, by levels of expression of key ligands and receptors (Heim et al., 2009; Holsboer & Ising, 2008). CRH levels in hypothalamus and in the hippocampus are typically correlated with magnitude of release during stress (Chen et al., 2004b; Kovacs & Sawchenko, 1996). GR levels in hippocampus are generally correlated with efficient shut-off of the stress response, via negative feedback, as well as with resilience to depression and other stress-related disorders (Holsboer, 2001; McEwen, 1999; Schmidt et al., 2005). Thus, augmented early-life experience has been found to be associated with enduring attenuation of stress response, as well as augmented learning and memory (Fenoglio et al., 2005; Meaney et al., 1991). At the molecular level, reduction of the expression of CRH in the hypothalamus and increased expression of GR in hippocampus have been documented (Plotsky & Meaney, 1993). In contrast, as will be described below, chronic early stress has recently been found to upregulate expression of CRH in hippocampus (Ivy et al., 2010). Taken together, these data suggest that, among the many enduring changes in the expression of key neuronal genes (Roth, Lubin, Funk, & Sweatt, 2009), persistent alteration in the expression levels of genes governing the stress-response, specifically GR and CRH, might contribute crucially to enduring phenotypic alteration of learning and memory that are induced by early-life experience and stress. The mechanism by which these changes are initiated and the bases of their persistence involve epigenetic processes (Bale et al., 2010; Borrelli, Nestler, Allis, & Sassone-Corsi, 2008).
4. Early-life experience regulates CRH expression in the hypothalamus via epigenetic mechanisms
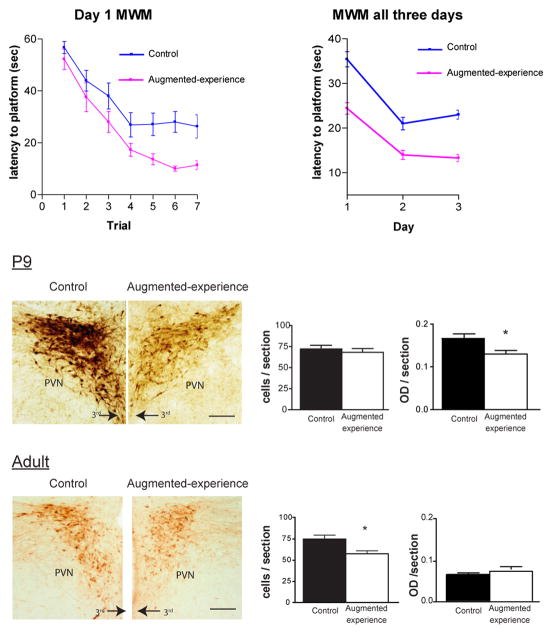
Enriched early-life experience during the first week(s) of life leads to a phenotype of improved hippocampus-dependent learning and memory (assessed using the Morris water maze and the novel object recognition tests) that last throughout adulthood (Fenoglio et al., 2005; Korosi & Baram, 2009; Meaney, 2001; Figure 3 left panel). What might the cellular and molecular basis of this enduring effect be? When the early postnatal experience is enriched by augmented sensory input from the mother, hypothalamic CRH expression is repressed and glucocorticoid receptor expression in hippocampal area CA1 is augmented, and both of these changes appear to be life-long. Time course analyses demonstrated that CRH repression (Figure 3 right panel), and the resulting chronic reduction in stress-induced release of hypothalamic CRH and of plasma glucocorticoid levels preceded changes in GR expression (Avishai-Eliner et al., 2001a). In addition, blocking the binding of CRH to its receptor in non-enriched pups led to improved spatial learning in these non-enriched animals as adults (Fenoglio, et al., 2005). Low plasma glucocorticoid levels are the major inducer of GR expression in hippocampus (Herman & Spencer, 1998), so the above data together suggest that the enduring changes in gene expression set in motion by enriched early-life experience might commence at the level of regulation of the Crh gene within the hypothalamus (Korosi et al., 2010; Weaver et al., 2004; Figure 3). If so, then how might the Crh gene be regulated persistently, and by what putative epigenetic processes?
Figure 3. Augmented spatial learning after enriched early-life experience correlates with early and enduring repression of CRH expression in hypothalamus.
Top panel: Mean latency to reaching the hidden platform in the 3-day Morris water maze (MWM) test variant. Two month old male rats that had experienced enriched maternal care on postnatal days 2–9 learned to find a hidden platform faster than littermate controls during the first day of the MWM, and this advantage persisted throughout the test. This finding suggests that their spatial learning and memory were augmented (modified from Fenoglio et al., 2005, with permission). Bottom panels: Neurons expressing CRH in the hypothalamic paraventricular nucleus were visualized using immunocytochemistry. P9: already at the end of the one week-period of enriched experience, the intensity of the CRH signal was lower in ‘enriched’ rat hypothalamus. Adult: This reduction of CRH expression (verified also at the mRNA level) persisted to adult life (modified from Korosi et al., 2010, with permission).
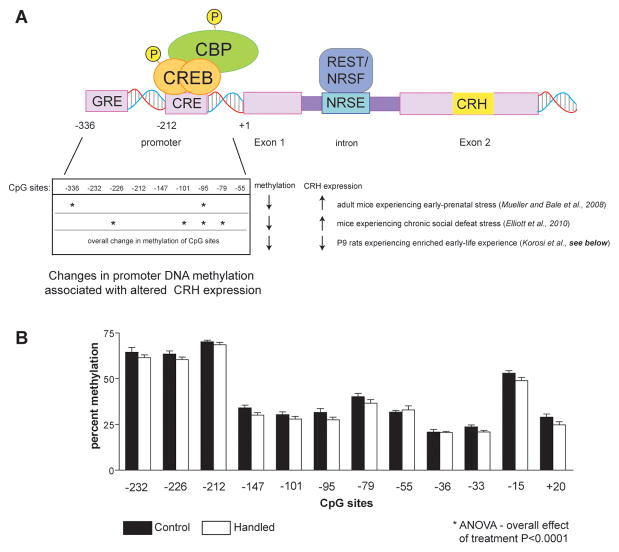
The Crh gene is composed of two exons and an 800bp intron (Figure 4). The entire protein-coding region is contained in the second exon. The promoter region of the Crh gene contains several putative cis-regulatory elements (Figure 4 top panel) including a cAMP response element (CRE), AP-1 sequence and glucocorticoid response element (GRE), and these contribute to tissue-specific (Seasholtz, Thompson & Douglass, 1988; Vamvakopoulos, et al., 1990), circadian (Watts, Tanimura, & Sanchez-Watts, 2004) and stress-provoked (Hatalski & Baram, 1997; Shepard, Liu, Sassone-Corsi, & Aguilera, 2005) expression of this gene. There are a number of CpG sequences in both the promoter region and in the first intron (Elliott, Ezra-Nevo, Regev, Neufeld-Cohen, & Chen, 2010; McGill et al., 2006; Mueller & Bale, 2008; Figure 4 bottom panel). Indeed, the Crh gene is regulated also by methyl CpG-binding protein 2 (MeCP2; Chahrour et al., 2008; McGill et al., 2006) a transcriptional repressor that binds methylated CpG dinucleotides and recruits corepressors and chromatin remodeling proteins (Nan et al., 1998).
Figure 4. Architecture and epigenetic changes in the Crh gene.
A. Top panel: The Crh gene consists of two exons and a single intron. The promoter region has a number of regulatory sites including a cyclic AMP response element (CRE) that interacts with the CRE binding protein (CREB) and related co-factors such as CREB binding protein (CBP). A second site, functional in mature but not neonatal hypothalamus, consists of a glucocorticoid receptor (GR) response element (GRE). The intron includes a sequence (NRSE) that functionally interacts with the repressor REST1/NRSF. Bottom panel: Methylation of DNA dinucleotides (CpG) within the promoter region of the Crh gene is modulated by experience, including pre-and postnatal stress. CpG sites with significant effect of the early-life manipulation are denoted by asterisks. B. Changes in the methylation of CpG sites within the Crh gene promoter in hypothalami of postnatal day 9 rats that have received enriched experience for the preceding week. A modest yet significant reduction of methylation of the promoter DNA was found (two-way ANOVA; effect of group F(1,96) = 16.58, p < .0001)Notably, it was associated with reduced expression of the gene.
In addition, the Crh gene intron contains a functional restrictive silencing element-1/neuron restrictive silencing element (RE-1/NRSE) sequence, that binds restrictive element silencing transcription factor/neuron restrictive silencing factor (REST/NRSF; Seth & Majzoub, 2001). Whereas NRSF was originally described in non-neuronal cells, where it restricts neuronal gene expression, the repressor is now known to be expressed in neurons (Calderone, et al., 2003; Palm, Belluardo, Metsis, & Timmusk, 1998) and to regulate neuronal gene expression (Andres et al., 1999; Gillies, Haddley, Vasiliou, Bubb, & Quinn, 2009; Korosi et al., 2010; Seth & Majzoub, 2001; McClelland et al., 2011, in revision). NRSF functions by recruiting chromatin-modifying cofactors (Naruse, Aoki, Kojima, & Mori, 1999; Roopra, Huang, & Dingledine, 2001; Roopra et al., 2000; Zheng, Zhao, & Mehler, 2009).
The specific epigenetic mechanisms by which early-life experience attenuates CRH expression acutely and long-term are not fully elucidated. At the end of the enriched-experience epoch (postnatal day 9), NRSF levels have been found to be augmented in hypothalamic tissue, and the repressor was bound to the Crh gene chromatin, as found using chromatin immunoprecipitation (Korosi et al., 2010). Both of these findings support a role for NRSF in an epigenetic down-regulation of CRH expression that initiates augmented spatial learning for life (Fenoglio et al., 2005; Korosi et al., 2010; Figure 3). In this study, methylation of CpG dinucleotides of the Crh gene promoter was modestly yet significantly lower in rats immediately following enriched early-life experience (P9) than in controls(Figure 4). Notably, reduced methylation is generally associated with enhanced transcription, whereas Crh gene expression was reduced in this group that performed better in learning and memory tests later in life. Thus, whereas altered methylation of the Crh gene after early-life (Mueller & Bale, 2008) or adult manipulations is clearly operant, its role in the effects of early-life enriched experience on lifelong memory function remains to be fully studied. Additional remaining questions include the nature of the ‘pathways’ from the maternal-derived sensory input to the hypothalamus (Fenoglio et al., 2006b; Korosi & Baram, 2009), and the nature of the signals that converge on the CRH-expressing hypothalamic neuron and provoke it to initiate epigenetic mechanisms that suppress the Crh gene. It has already been established that enriched early-life experience results in reduced numbers of excitatory synapses and excitatory input to CRH-expressing hypothalamic neurons, without altered GABAergic neurotransmission (Korosi, et al., 2010), and this phenomenon might be the signal that sets in motion the epigenetic machinery within the cell (Korosi et al., 2010).
5. Enduring alterations of learning and memory after chronic early-life stress
As mentioned above, epidemiological and experimental evidence suggests that adverse early-life experience, and particularly chronic psychological stress, may predispose to cognitive dysfunction (Ammerman, Kolko, Kirisci, Blackson, & Dawes, 1999; Huot, Plotsky, Lenox, & McNamara, 2002; Kaplan et al., 2001; Meaney et al., 1988; Poeggel et al., 2003; van Oers, de Kloet, & Levine, 1999) that appears much later in life. This implies that early-life stress can have effects that impact neuronal function significantly and enduringly throughout adulthood and aging.
The hippocampus is critical for a variety of memory processes (Hollup, Kjelstrup, Hoff, Moser & Moser, 2001) and is unusually vulnerable to stress (Bremner et al., 1997; Kim & Diamond, 2002; McEwen, 1999; Sapolsky, 2002). The bases for this vulnerability include high expression of glucocorticoid receptors, for example in CA1 pyramidal cells (Herman, Patel, Akil, & Watson, 1989; de Kloet, Karst, & Joels, 2008), direct and powerful afferent input from stress-activated brain regions (see Segal, Richter-Levin, & Maggio, 2010; Ulrich-Lai & Herman, 2009; Joels & Baram, 2009 for review), and stress-induced release of endogenous neuromodulators within the hippocampus including CRH (Chen et al., 2004b; Chen et al., 2006; Chen et al., 2010). This susceptibility of hippocampal function to stress likely plays an adaptive role, by enhancing synaptic plasticity (Blank, Nijholt, Eckart, & Spiess, 2002) and learning and memory during acute stress that lasts for seconds to minutes (Joels & Baram, 2009). However, it also renders the hippocampus vulnerable to potentially deleterious effects of severe or chronic stress (McEwen, 1999; Brunson et al., 2003). Chronic stress (or long-term elevation of plasma stress hormones) in adult and aging rats, affects hippocampus-dependent cognitive function (Alfarez et al., 2003; Bodnoff et al., 1995; Dachir, Kadar, Robinzon, & Levy, 1993; Kerr, Campbell, Applegate, Brodish, & Landfield, 1991; Kim & Diamond, 2002; Landfield, McGaugh, & Lynch, 1978; Luine, Villegas, Martinez, & McEwen, 1994), but these disturbances are generally transient (Pavlides, Nivon, & McEwen, 2002). In contrast, as mentioned above, clinical studies have suggested that when occurring early in life, chronic stress might impact learning and memory in hippocampus in an enduring and potentially progressive manner (Wilson et al., 2007).
To examine if a causal relationship exists between chronic early-life stress and enduring and progressive deficits in learning and memory, and to better understand the mechanisms by which such stress might impact cognitive function long-term, a wide variety of experimental manipulations during development have been designed. These stresses have included acute / intermittent separation of dams from the pups (de Kloet, Oitzl, & Joels, 1999; Huot et al., 2002; Oomen et al., 2009; Oomen et al., 2010; Schmidt, Oitzl, Levine, & de Kloet, 2002; van Oers et al., 1999), a single prolonged separation of dams from the pups (Avishai-Eliner, Yi, Newth, & Baram, 1995; Dent, Smith, & Levine, 2000; Levine, 2000), and a chronic alteration of maternal behavior, resulting in stress to the pups (Brunson et al., 2005; Gilles, Schultz, & Baram, 1996; Ivy et al., 2010; Roth & Sullivan, 2005). The timing of the stress has also been an important variable, with some groups aiming to recreate prenatal stress, and others focusing on the immediate neonatal period, or slightly later. In view of this diversity of approaches and of endpoints, it is remarkable that the results have generally been in accord: early-life stress has adverse effects on spatial learning and these results endure, or emerge later in life (Brunson et al., 2005; Huot et al., 2002). Aiming for a naturalistic rodent model of chronic early-life stress, a paradigm involving maintaining the dam with the pups within an impoverished cage environment was designed, and this manipulation led to chronic stress in both dams and pups (Avishai-Eliner, Gilles, Eghbal-Ahmadi, Bar-El, & Baram, 2001b; Brunson et al., 2005; Ivy, Brunson, Sandman, & Baram, 2008; Rice, Sandman, Lenjavi, & Baram, 2008). In essence, chronic stress was generated during postnatal days 2-9, a period when hippocampal development is comparable to that found late in human gestation and the early postnatal period (Avishai-Eliner et al., 2002). For the stress group, cage environment and maternal behavior were altered by placing pups and dams in cages with limited nesting / bedding material on postnatal day 2. Cages were fitted with soft plastic mesh bottom, bedding was essentially removed, and nesting material consisted of one paper towel that was used by the dam to construct a rudimentary nest area. The abnormal cage environment prevented the dam from constructing satisfactory nests. This resulted in maternal stress, promoting fragmented and erratic care to the pups (Ivy et al., 2008). The disrupted maternal care led to chronic stress in the pups, as measured both by plasma glucocorticoid levels and by the presence of hypertrophied adrenal glands (Avishai-Eliner et al., 2001b; Gilles et al., 1996). This model, where stress was constant and persistent, has been adapted to the mouse (Rice et al., 2008; Schmidt et al., 2002) and has been adopted and modified by several groups around the world (Moriceau, Roth, & Sullivan, 2010; Moriceau, Shionoya, Jakubs, & Sullivan, 2009; Roth & Sullivan, 2005; Schmidt, Wang,& Meijer, 2010).
At the end of the stress period, dams and pups were moved to normal-bedded cages. Maternal behavior normalized within hours, and by the time they reached adulthood, the neuroendocrine parameters of the stress system returned to baseline in the early-life stress graduates, and were indistinguishable from those of conventionally raised rats (Brunson et al., 2005). However, despite apparent dissipation of the physiological correlates of stress, the early-life chronic stress led to enduring and profound changes of the structure and function of hippocampal neurons that are likely generated via epigenetic modulation of key stress hormone genes.
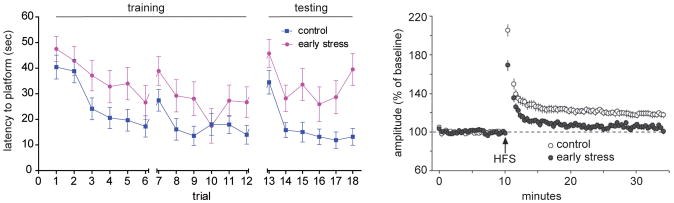
Functionally, young-adult graduates of chronic early-life stress performed reasonably well in the Morris water maze (MWM) test of spatial learning and memory. Long-term potentiation (LTP) in response to high frequency stimulation was normal in both areas CA1 and CA3, though subtle changes in the properties of CA3 pyramidal cells were apparent (Brunson et al., 2005). However, by 7–10 months, performance in the MWM as well as in the novel object recognition test declined in comparison to age-matched controls (Figure 5 left panel). Performance in the MWM might be confounded in rats that had experienced stress early-in life, because the MWM includes adverse elements (forced swim), and it could be argued that the ability of the early-stressed group to ‘cope’ with this additional stress was modulated by the early-life experience. Therefore, an independent memory test that interrogates the hippocampal-limbic circuit and is relatively devoid of intrinsic ‘stress’ was also employed. Performance in the novel object recognition test was also impaired in early-stress rats and this was accompanied by profound attenuation of LTP in both CA3 and CA1 (Figure 5 right panel). The structural correlates of these spatial and recognition memory defects included impoverishment (atrophy) of apical dendritic trees of CA1 and CA3 pyramidal neurons, with commensurate loss of dendritic spines and synapses (Brunson et al., 2005; Ivy et al., 2010; Figure 6 left panel) in the stratum radiatum, the site of commissural/ associational synapses. Thus, a single week of chronic stress early in the postnatal period led to enduring and potentially progressive disturbances in synaptic plasticity and in memory processes, at least in part via loss of dendrites, dendritic spines and excitatory synapses (Chen et al., 2010; Ivy et al., 2010).
Figure 5. Reduced spatial learning and memory skills and attenuated long term potentiation in middle aged rats that had experienced chronic early-life stress.
Left panel: comparison of training and reversal performance of control middle-aged male rats to a cohort experiencing chronic stress for a week early in life. Latency of the stressed rats to finding a hidden platform using spatial cues is impaired. Right panel: Long-term potentiation in response to high-frequency stimulation is attenuated in early-stressed (CES) rats compared with controls. (Modified from Ivy et al., 2010, with permission)
Figure 6. Dendritic atrophy and enduring upregulation of CRH expression in hippocampus of middle-aged rats experiencing chronic early-life stress.
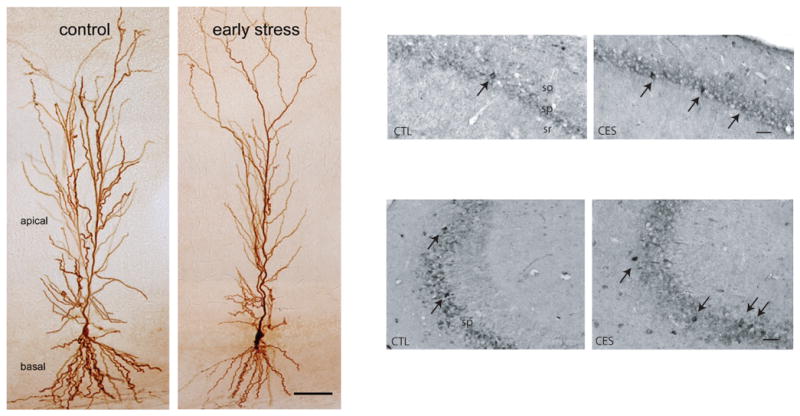
Left Panel: Atrophy of the apical dendritic trees of CA1 pyramidal cells accompanied loss of spatial memory and attenuated LTP in 12 month old rats that experienced one week of chronic stress during postnatal days 2–9. The image depicts representative neurons filled with biocytin (From Brunson et al., 2005, with permission). Right panel: Increased number of immunocytochemistry-detectable CRH-expressing interneurons in hippocampi of middle-aged rats that experienced early-life stress (highlighted by arrows). All sections were run together, and cells were quantified in the pyramidal cells layers of areas CA3 and CA1 (from Ivy et al., 2010, with permission).
6. Putative mechanisms of the enduring and progressive consequences of chronic early-life stress on hippocampus- mediated learning and memory
The chain of events that bridges a single neonatal week of chronic stress with enduring and progressive disturbances of hippocampal structure and function are not yet fully understood. Because, as described above, stress involves the release and subsequent expression of specific mediators, including neurotransmitters, neuropeptide and steroid hormones (Joels & Baram, 2009; McEwen, 1999; Ulrich-Lai & Herman, 2009), it has been a general assumption that these stress mediators were involved in the processes by which stress impacts learning and memory. Indeed, much work has identified the actions of glucocorticoid receptor activation in the consequences of early stress on cognitive function (Alfarez et al., 2009). However, a key enigma remains: how does a transient elevation of stress hormones result in enduring or progressive disturbance in learning and memory? In the paragraphs below, we describe recent studies that support the idea that chronic early-life stress results in epigenetic ‘programming’ of stress-related genes, including hippocampal CRH expression, at higher levels. The enduring elevation of CRH expression and release impairs dendritic spines, with eventual dying back of dendrites. Loss of dendritic spines and the synapses that they carry might thus underlie the loss of synaptic plasticity and memory function in ‘graduates’ of early-life stress (Figure 5, Figure 6 left panel).
Ivy (2010) found a major increase in the protein levels of CRH in hippocampal pyramidal cells of middle-aged rats stressed early in life (Figure 6 right panel), and similar augmentation was apparent at the mRNA levels (Fenoglio et al., 2006a). In addition, the group was able to block the consequences of chronic early-life stress by interfering with the interaction of CRH with its receptor, CRHR1 within the brain (Ivy et al., 2010). Hippocampal CRH is released during stress throughout life (Chen et al., 2001a; Chen et al., 2004b), and interacts with CRHR1 to cause rapid retraction of dendritic spines (Chen, Dube, Rice, & Baram, 2008; Chen et al., 2010). Exposure of hippocampal organotypic cultures to stress levels of CRH for a week not only reduced spine number, but also led to dying back of dendrites (Chen et al., 2004a). Thus, exposure of the developing hippocampus to stress-levels of CRH during the week of early-life stress, and / or release of pathological levels of the peptide throughout the months that follow might contribute to the loss of structural integrity of hippocampal neurons that characterizes adult rats after chronic early-life stress. This loss of dendrites, spines and synapsesseems to underlie the disturbances of learning and memory in the same rats (Chen et al., 2008; Chen et al., 2010; Ivy et al., 2010).
This scenario raises the obvious question regarding the nature of the mechanism that increase the ‘set-point’ of hippocampal CRH expression throughout the life of early-stressed rats. Based on a large body of work (Bale et al., 2010; Borrelli et al., 2008; Franklin et al., 2010; Haggarty et al., 2010; McGill et al., 2006; Mueller & Bale, 2008; Sweatt, 2009), epigenetic mechanisms are excellent candidates for this enduring, augmented expression of the Crh gene in hippocampus. For example, regulation of DNA methylation (Levenson & Sweatt, 2006) and of histone acetylation and methylation (Gupta et al., 2010) has been found to play key roles in fundamental learning and memory processes subserved by the hippocampal network. Obviously, disruption of such processes by stress would impair cognitive performance. However, whereas there is clear evidence for the involvement of epigenetic regulation in processes of learning and memory in the mature hippocampus (Abel & Zukin, 2008; Fischer, Sananbenesi, Mungenast, & Tsai, 2010; Miller, Campbell, & Sweatt, 2008; Na & Monteggia, 2010; Stefanko, Barrett, Ly, Reolon, & Wood, 2009; Sweatt, 2009), the involvement of such mechanisms in the upregulation of the Crh gene is under active study (Figure 4).
7. Why CRH?
This review focused on the role of transcriptional, and likely epigenetic regulation of the CRH gene in hypothalamus and hippocampus, and the consequences of these changes in gene expression on learning and memory. Two questions that arise are (1) are these changes selective to CRH? and (2) why regulation of CRH rather than other stress-related genes?
The answers to both these questions are speculative at this point. As mentioned above, early-environment-induced resilience to stress is associated not only with reduced CRH expression in hypothalamus, but also in augmented glucocorticoid receptor expression in hippocampus (Plotsky & Meaney, 1993; Avishai-Eliner et al., 2001a). Temporally, the reduction in CRH expression precedes, and might initiate (Fenoglio et al., 2005), the changes in GR. Unlike the resilience to drug abuse, where large-scale changes in gene expression have been delineated (Feder, Nestler, & Charney, 2009), the extent of epigenetic changes of families of genes that might govern the observed enhancement of hippocampal function after enriched early-life environment remain to be fully studied. Weaver et al. identified over 900 genes with altered expression in hippocampus of adult rats that have experienced augmented maternal care early in life (Weaver, Meaney, & Szyf, 2006). Interestingly, the group found reversal of these alterations of gene expression upon treatment with trichostatin or with a source of methyl group (Weaver et al., 2005; Weaver et al., 2006). Whereas the transcriptome arrays were carried out only in adulthood, these data support the idea that epigenetic regulation of the expression of numerous genes takes place after enriched experience early in life that promotes learning and memory long-term.
As mentioned above, levels of stress hormone (and receptor) gene expression are both influenced by stress, and govern the release and / or signaling of stress mediators in hippocampus and throughout the brain. Interestingly, enduring changes in the expression of receptors to systemic stress hormones (GR and MR) have not been observed after early-life stress that promotes deterioration in learning and memory later in life. This observation is intuitively logical, because this type of adaptation would be expected to result in major changes in the body’s response to stress in general, including disruption of the negative feedback by which the hippocampus restrains the hormonal response to stress (Ulrich-Lai & Herman, 2009). In contrast, CRH belongs to a class of modulators, neuropeptides, that play less prominent roles in the peripheral hormonal response (Joels & Baram, 2009). Similar to other peptides, CRH has a peripheral role in mediating the body’s response to stress and, in parallel is released in hippocampus to modulate hippocampal function during stress. Peptides with similar dual roles include ghrelin (Diano et al., 2006) and somatostatin (Bloom, 1986). These peptides, including CRH, are released in specific brain regions and modulate the function of a limited number of neurons within selective populations and networks. In the temporal domain, peptides bridge neurotransmitter function (seconds), and hormonal effects (up to hours). Thus, selective epigenetic regulation of hippocampal CRH expression enriches the repertoire of hippocampal plasticity (Joels & Baram, 2009), enabling augmented fine-tuning of hippocampal function in the context of graded stress situations.
8. Summary
Early-life experience influences learning and memory processes throughout life in a bidirectional manner. The mechanisms for such enduring neuroplasticity include stable alteration in the expression of key neuronal genes, including those that regulate the salience and stressfulness of experience. Enriched postnatal experience enduringly augments learning and memory, at least in part via persistent suppression of CRH expression. In contrast, chronic early-life stress results in long-lasting and progressive deficits in the structure and function of hippocampal neurons, potentially via epigenetic programming of the expression ‘set-point’ of the same gene in hippocampus at a higher level. Many questions remain about the initiation and nature of the epigenetic mechanisms that are involved in programming the enduring effects of early-life experience on learning and memory throughout life.
Acknowledgments
Supported by NIH grants NS28912; MH 73136. We thank Mrs. B. Cartwright for editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarez DN, De Simoni A, Velzing EH, Bracey E, Joels M, Edwards FA, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–836. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Ammerman RT, Kolko DJ, Kirisci L, Blackson TC, Dawes MA. Child abuse potential in parents with histories of substance use disorder. Child Abuse Negl. 1999;23:1225–1238. doi: 10.1016/s0145-2134(99)00089-7. [DOI] [PubMed] [Google Scholar]
- Andersen P, Moser E, Moser MB, Trommald M. Cellular correlates to spatial learning in the rat hippocampus. J Physiol Paris. 1996;90:349. doi: 10.1016/s0928-4257(97)87917-x. [DOI] [PubMed] [Google Scholar]
- Andres ME, Burger C, Peral-Rubio MJ, Battaglioli E, Anderson ME, Grimes J, et al. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci U S A. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001a;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001b;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci Lett. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE. Whither neuropeptides? Res Publ Assoc Res Nerv Ment Dis. 1986;64:335–349. [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Zeanah CH, Jr, Smyke AT, Fox NA, Nelson CA., 3rd Stereotypies in children with a history of early institutional care. Arch Pediatr Adolesc Med. 2010;164:406–411. doi: 10.1001/archpediatrics.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001a;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hatalski CG, Brunson KL, Baram TZ. Rapid phosphorylation of the CRE binding protein precedes stress-induced activation of the corticotropin releasing hormone gene in medial parvocellular hypothalamic neurons of the immature rat. Brain Res Mol Brain Res. 2001b;96:39–49. doi: 10.1016/s0169-328x(01)00265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004a;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004b;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behav Neural Biol. 1993;60:103–109. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Moments in time--the neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Karst H, Joels M. Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. The ontogeny of the neuroendocrine response to endotoxin. Brain Res Dev Brain Res. 1999;117:21–29. doi: 10.1016/s0165-3806(99)00091-7. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. 2000;141:1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front Neuroendocrinol. 2006a;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006b;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010 doi: 10.1016/j.tips.2010.09.003. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S, Haddley K, Vasiliou S, Bubb VJ, Quinn JP. The human neurokinin B gene, TAC3, and its promoter are regulated by Neuron Restrictive Silencing Factor (NRSF) transcription factor family. Neuropeptides. 2009;43:333–340. doi: 10.1016/j.npep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Larson MC, Hertsgaard L, Harris ML, Brodersen L. The stressfulness of separation among nine-month-old infants: effects of social context variables and infant temperament. Child Dev. 1992;63:290–303. [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, et al. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty P, Hoad G, Harris SE, Starr JM, Fox HC, Deary IJ, Whalley LJ. Human intelligence and polymorphisms in the DNA methyltransferase genes involved in epigenetic marking. PLoS One. 2010;5:e11329. doi: 10.1371/journal.pone.0011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Suomi SJ. Production of depressive behaviors in young monkeys. J Autism Child Schizophr. 1971;1:246–255. doi: 10.1007/BF01557346. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Baram TZ. Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3',5'-monophosphate-regulatory element binding activity. Mol Endocrinol. 1997;11:2016–2024. doi: 10.1210/mend.11.13.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hollup SA, Kjelstrup KG, Hoff J, Moser MB, Moser EI. Impaired recognition of the goal location during spatial navigation in rats with hippocampal lesions. J Neurosci. 2001;21:4505–4513. doi: 10.1523/JNEUROSCI.21-12-04505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemio. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Campbell LW, Applegate MD, Brodish A, Landfield PW. Chronic stress-induced acceleration of electrophysiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991;11:1316–1324. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother's love to baby's future. Front Behav Neurosci. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J Mol Neurosci. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150:85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- Lenze SN, Xiong C, Sheline YI. Childhood adversity predicts earlier onset of major depression but not reduced hippocampal volume. Psychiatry Res. 2008;162:39–49. doi: 10.1016/j.pscychresns.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci. 2006;63:1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- McClelland S, Flynn C, Dubé C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C, Baram TZ. NRSF-mediated HCN channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011 doi: 10.1002/ana.22479. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Mitchell JB, Aitken DH, Bhatnagar S, Bodnoff SR, Iny LJ, et al. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Dev Psychobiol. 2010;52:651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, et al. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Monteggia LM. The role of MeCP2 in CNS development and function. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.05.014. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Naruse Y, Aoki T, Kojima T, Mori N. Neural restrictive silencer factor recruits mSin3 and histone deacetylase complex to repress neuron-specific target genes. Proc Natl Acad Sci U S A. 1999;96:13691–13696. doi: 10.1073/pnas.96.24.13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers H, Joels M, et al. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4:e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc Natl Acad Sci U S A. 2003;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsonby AL, Dwyer T, Gibbons LE, Cochrane JA, Wang YG. Factors potentiating the risk of sudden infant death syndrome associated with the prone position. N Engl J Med. 1993;329:377–382. doi: 10.1056/NEJM199308053290601. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopra A, Huang Y, Dingledine R. Neurological disease: listening to gene silencers. Mol Interv. 2001;1:219–228. [PubMed] [Google Scholar]
- Roopra A, Sharling L, Wood IC, Briggs T, Bachfischer U, Paquette AJ, et al. Transcriptional repression by neuron-restrictive silencer factor is mediated via the Sin3-histone deacetylase complex. Mol Cell Biol. 2000;20:2147–2157. doi: 10.1128/mcb.20.6.2147-2157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P, van Eekelen JA, Levine S, de Kloet ER. Ontogeny of corticosteroid receptors in the brain. Cell Mol Neurobiol. 1993;13:295–319. doi: 10.1007/BF00711575. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Chickens, eggs and hippocampal atrophy. Nat Neurosci. 2002;5:1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Levine S, Oitzl MS, van der Mark M, Muller MB, Holsboer F, et al. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–1464. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Oitzl MS, Levine S, de Kloet ER. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 2010 Nov 18; doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2:1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- Segal M, Richter-Levin G, Maggio N. Stress-induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus. 2010;20:1332–1338. doi: 10.1002/hipo.20751. [DOI] [PubMed] [Google Scholar]
- Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J Biol Chem. 2001;276:13917–13923. doi: 10.1074/jbc.M007745200. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci. 2005;25:4073–4081. doi: 10.1523/JNEUROSCI.0122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF. State of the world's children 2006. New York: 2005. [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Karl M, Mayol V, Gomez T, Stratakis CA, Margioris A, et al. Structural analysis of the regulatory region of the human corticotropin releasing hormone gene. FEBS Lett. 1990;267:1–5. doi: 10.1016/0014-5793(90)80272-k. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Levine S. Persistent effects of maternal deprivation on HPA regulation can be reversed by feeding and stroking, but not by dexamethasone. J Neuroendocrinol. 1999;11:581–588. doi: 10.1046/j.1365-2826.1999.00329.x. [DOI] [PubMed] [Google Scholar]
- Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology. 2004;145:529–540. doi: 10.1210/en.2003-0394. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide's gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Brain Res Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- Zheng D, Zhao K, Mehler MF. Profiling RE1/REST-mediated histone modifications in the human genome. Genome Biol. 2009;10:R9. doi: 10.1186/gb-2009-10-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]