Abstract
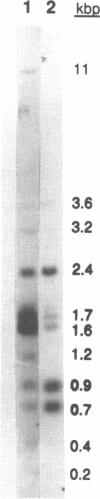
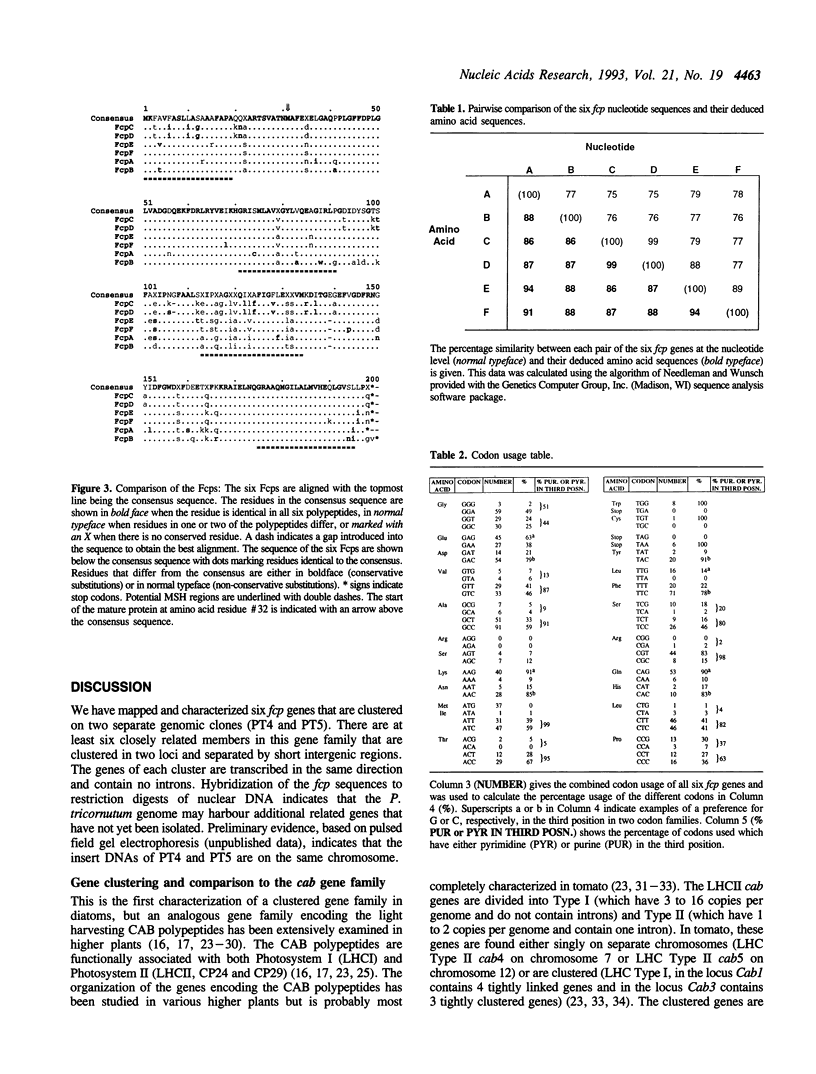
We are studying the multigene family encoding the fucoxanthin-chlorophyll binding proteins (fcp genes) that constitute the major component of the photosystem II-associated light harvesting complex in diatoms and brown algae. The characteristics of clusters of fcp genes on the genome of the diatom Phaeodactylum tricornutum are described. Sequence analysis of two genomic clones, PT5 and PT4, has demonstrated the presence of four fcp genes (fcpA, fcpB, fcpC, fcpD) on the former and two fcp genes (fcpE, fcpF) on the latter. The proteins encoded by the six characterized fcp genes range in similarity from 86% to 99%. The genes within each cluster are separated by short intergenic sequences (between 0.5 to 1.1 kb). None of these genes contain introns and all appear to be transcribed with short 5' transcribed, untranslated leader sequences; the transcription initiation sites were mapped 26 to 48 bases upstream of the ATG translation start site. Small conserved motifs are found among all of the genes just upstream of both the translation and the transcription start sites. The codon bias is similar in all of the fcp genes, with a predominance of pyrimidines in the third positions of codons of the four codon families. The two fcp genes that are most similar are fcpC and fcpD, and might represent a recent gene duplication. Southern analyses using fcp cDNAs as hybridization probes suggest that there may be additional sequences on the P. tricornutum genome that resemble the characterized fcp sequences.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Friedman A. L., Gustafson D. L., Rudnick M. S., Lyman H. Light-harvesting systems of brown algae and diatoms. Isolation and characterization of chlorophyll a/c and chlorophyll a/fucoxanthin pigment-protein complexes. Biochim Biophys Acta. 1981 Apr 13;635(2):304–316. doi: 10.1016/0005-2728(81)90029-3. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bhaya D., Grossman A. Targeting proteins to diatom plastids involves transport through an endoplasmic reticulum. Mol Gen Genet. 1991 Oct;229(3):400–404. doi: 10.1007/BF00267462. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Demmin D. S., Stockinger E. J., Chang Y. C., Walling L. L. Phylogenetic relationships between the chlorophyll a/b binding protein (CAB) multigene family: an intra- and interspecies study. J Mol Evol. 1989 Sep;29(3):266–279. doi: 10.1007/BF02100210. [DOI] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawley M. W., Grossman A. R. Polypeptides of a Light-Harvesting Complex of the Diatom Phaeodactylum tricornutum Are Synthesized in the Cytoplasm of the Cell as Precursors. Plant Physiol. 1986 May;81(1):149–155. doi: 10.1104/pp.81.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. R., Pichersky E., Kloppstech K. Chlorophyll a/b-binding proteins: an extended family. Trends Biochem Sci. 1991 May;16(5):181–186. doi: 10.1016/0968-0004(91)90072-4. [DOI] [PubMed] [Google Scholar]
- Green B. R., Shen D., Aebersold R., Pichersky E. Identification of the polypeptides of the major light-harvesting complex of photosystem II (LHCII) with their genes in tomato. FEBS Lett. 1992 Jun 22;305(1):18–22. doi: 10.1016/0014-5793(92)80646-x. [DOI] [PubMed] [Google Scholar]
- Green L. S., Laudenbach D. E., Grossman A. R. A region of a cyanobacterial genome required for sulfate transport. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1949–1953. doi: 10.1073/pnas.86.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A., Manodori A., Snyder D. Light-harvesting proteins of diatoms: their relationship to the chlorophyll a/b binding proteins of higher plants and their mode of transport into plastids. Mol Gen Genet. 1990 Oct;224(1):91–100. doi: 10.1007/BF00259455. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Pichersky E., Malik V. S., Castresana C., Ko K., Darr S. C., Cashmore A. R. A cDNA clone encoding a photosystem I protein with homology to photosystem II chlorophyll a/b-binding polypeptides. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8844–8848. doi: 10.1073/pnas.84.24.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson S., Gustafsson P. Evolutionary conservation of the chlorophyll a/b-binding proteins: cDNAs encoding type I, II and III LHC I polypeptides from the gymnosperm Scots pine. Mol Gen Genet. 1991 Sep;229(1):67–76. doi: 10.1007/BF00264214. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Harris S. Processes of gene duplication. Nature. 1982 Mar 4;296(5852):9–10. doi: 10.1038/296009a0. [DOI] [PubMed] [Google Scholar]
- Kellmann J. W., Pichersky E., Piechulla B. Analysis of the diurnal expression patterns of the tomato chlorophyll a/b binding protein genes. Influence of light and characterization of the gene family. Photochem Photobiol. 1990 Jul;52(1):35–41. doi: 10.1111/j.1751-1097.1990.tb01752.x. [DOI] [PubMed] [Google Scholar]
- McGrath J. M., Terzaghi W. B., Sridhar P., Cashmore A. R., Pichersky E. Sequence of the fourth and fifth Photosystem II type I chlorophyll a/b-binding protein genes of Arabidopsis thaliana and evidence for the presence of a full complement of the extended CAB gene family. Plant Mol Biol. 1992 Aug;19(5):725–733. doi: 10.1007/BF00027069. [DOI] [PubMed] [Google Scholar]
- Owens T. G. Light-Harvesting Function in the Diatom Phaeodactylum tricornutum: II. Distribution of Excitation Energy between the Photosystems. Plant Physiol. 1986 Mar;80(3):739–746. doi: 10.1104/pp.80.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Wold E. R. Light-Harvesting Function in the Diatom Phaeodactylum tricornutum: I. Isolation and Characterization of Pigment-Protein Complexes. Plant Physiol. 1986 Mar;80(3):732–738. doi: 10.1104/pp.80.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancic P. G., Strotmann H. Structure of the nuclear encoded gamma subunit of CF0CF1 of the diatom Odontella sinensis including its presequence. FEBS Lett. 1993 Mar 29;320(1):61–66. doi: 10.1016/0014-5793(93)81658-m. [DOI] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Breidenbach R. B., Kausch A. P., Cashmore A. R. Molecular characterization and genetic mapping of two clusters of genes encoding chlorophyll a/b-binding proteins in Lycopersicon esculentum (tomato). Gene. 1985;40(2-3):247–258. doi: 10.1016/0378-1119(85)90047-2. [DOI] [PubMed] [Google Scholar]
- Pichersky E. Nomad DNA--a model for movement and duplication of DNA sequences in plant genomes. Plant Mol Biol. 1990 Sep;15(3):437–448. doi: 10.1007/BF00019160. [DOI] [PubMed] [Google Scholar]
- Piechulla B., Kellmann J. W., Pichersky E., Schwartz E., Förster H. H. Determination of steady-state mRNA levels of individual chlorophyll a/b binding protein genes of the tomato cab gene family. Mol Gen Genet. 1991 Dec;230(3):413–422. doi: 10.1007/BF00280298. [DOI] [PubMed] [Google Scholar]
- Polans N. O., Weeden N. F., Thompson W. F. Inheritance, organization, and mapping of rbcS and cab multigene families in pea. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5083–5087. doi: 10.1073/pnas.82.15.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J. M., Bhaya D., Block M. A., Grossman A. R. Isolation, transcription, and inactivation of the gene for an atypical alkaline phosphatase of Synechococcus sp. strain PCC 7942. J Bacteriol. 1991 Jul;173(14):4297–4309. doi: 10.1128/jb.173.14.4297-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling L. L., Chang Y. C., Demmin D. S., Holzer F. M. Isolation, characterization and evolutionary relatedness of three members from the soybean multigene family encoding chlorophyll a/b binding proteins. Nucleic Acids Res. 1988 Nov 25;16(22):10477–10492. doi: 10.1093/nar/16.22.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]