Abstract
Background
Several studies have identified a specific fracture in the proximal diaphysis of the femur in patients treated with bisphosphonates. The fractures typically are sustained after a low-energy mechanism with the presence of an existing characteristic stress fracture. However, it is unclear whether these patients are best treated nonoperatively or operatively.
Questions/purposes
What is the likelihood of nonoperatively treated bisphosphonate-associated femoral stress fractures progressing to completion and during what time period? If prophylactic fixation is performed, do patients have a shorter hospital length-of-stay compared with patients having surgical fixation after fracture completion?
Patients and Methods
We retrospectively searched for patients older than 50 years receiving bisphosphonate therapy, with either incomplete, nondisplaced stress fractures or completed, displaced fractures in the proximal diaphysis of the femur between July 2002 and April 2009. After applying exclusion criteria, we identified 34 patients with a total of 40 bisphosphonate-associated fractures. The average duration of bisphosphonate use was 77 months. Twenty-eight of 40 (70%) fractures were completed, displaced fractures. Six of the 12 nondisplaced stress fractures initially were treated nonoperatively. The remaining six stress fractures were treated with prophylactic cephalomedullary nail fixation. The minimum followup was 12 months (mean, 36.5 months; range, 12–72 months).
Results
Five of the six stress fractures treated nonoperatively progressed to fracture completion and displacement at an average of 10 months (range, 3–18 months). The average hospital stay was 3.7 days for patients treated prophylactically and 6.0 days for patients treated after fracture completion.
Conclusions
Our data suggest nonoperative treatment of bisphosphonate-related femoral stress fractures is not a reliable way to treat these fractures as the majority progress to fracture completion. Prophylactic fixation of femoral stress fractures also reduces total hospital admission time.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bisphosphonate medications are indicated for patients with postmenopausal osteoporosis and for patients with metastatic disease to the skeleton [16, 19, 20]. By decreasing the rate of bone resorption, bisphosphonate use causes increases in bone mineral density and stabilization of osteolytic lesions [11]. As alendronate has been approved by the US Food and Drug Administration (FDA) since 1995, long-term use of bisphosphonates is becoming common in these patient populations.
Long-term use of bisphosphonates is not without its complications. Bisphosphonate-associated stress fractures in the femur have been identified and characterized and are considered a risk for patients receiving long-term bisphosphonate therapy [7, 10, 11, 14, 20]. The true fracture risk associated with bisphosphonates is unclear as recent epidemiologic studies suggest modest to no increased risk with relative hazard ratios 1.03–1.50 compared with placebo [2, 15, 22]. However confidence intervals were wide (95% CI, 0.06–16.46) making the results difficult to interpret [2, 15, 22]. These stress fractures can be seen on plain radiographs as simple transverse patterns, with unicortical beaking and hypertrophy of the diaphyseal cortex [14] (Fig. 1). MRI or three-phase skeletal scintigraphy with 99mTc-methylene diphosphonate also can identify these fractures. Completion or displacement of these fractures may result from low-energy mechanisms, and there often is a preceding history of thigh pain in the ipsilateral extremity [7] (Fig. 2). Several studies have identified long-term use (> 5 years) of alendronate is a risk factor for these particular fractures [7, 10, 14, 20]; however, it is unknown whether other types of bisphosphonates also will be risk factors.
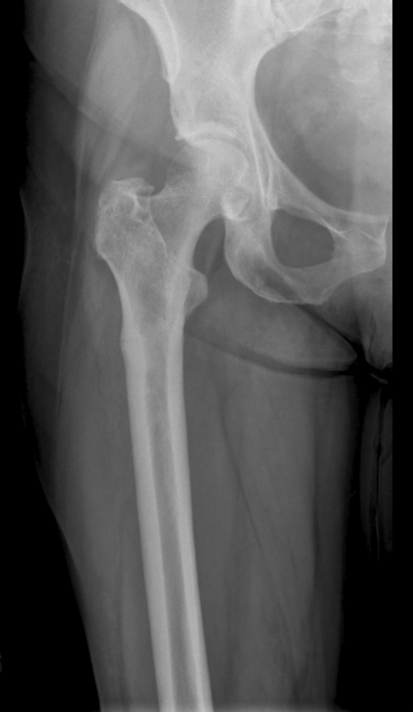
Fig. 1.
An AP radiograph of the hip and proximal femur shows a nondisplaced, bisphosphonate-related femoral stress fracture. Lateral cortical hypertrophy can be seen.
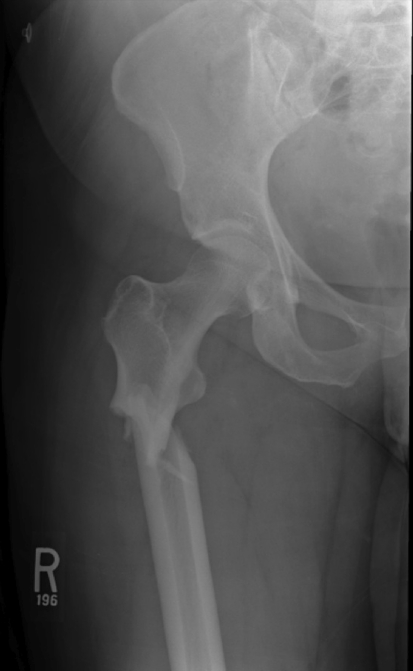
Fig. 2.
An AP radiograph of the hip and proximal femur shows a completed, displaced fracture. Lateral cortical hypertrophy, unicortical beaking, and a transverse fracture pattern can be seen.
Nonoperative treatment of femoral neck fractures generally is limited to stress fractures in the compressive trabeculae, while operative fixation has been recommended for fractures on the tension side of the femoral neck [5]. Whether bisphosphonate-related stress fractures in the femoral subtrochanteric or high diaphyseal region should be treated nonoperatively or operatively is unclear. Nonoperative treatment includes modification of activities with partial weightbearing on the affected extremity using assistive devices and discontinuation of bisphosphonate use. Although this avoids surgical risks, the impact on quality of life and the costs associated with temporary disability need to be considered. Some surgeons at our institution anecdotally recommend prophylactic fixation as they believe this will prevent patient suffering, completion of the fracture, and related morbidities. However, prophylactic fixation requires a surgical procedure with associated risks.
Some studies of bisphosphonate-associated femoral stress fractures describe patients presenting with acute fractures after low-energy mechanisms [7, 10, 11, 14, 20]. These studies have been case reports or retrospective reviews of two to 25 patients that correlate the use of bisphosphonates, particularly alendronate, with the presence of the characteristic fracture. Taken together, these studies do not provide adequate information to suggest the best management practices for patients with bisphosphonate-associated stress fractures that have not yet completely fractured.
We therefore addressed the following questions: (1) What is the likelihood of nonoperatively treated bisphosphonate-associated femoral stress fractures progressing to fracture completion and during what time period? (2) If prophylactic fixation is performed, do patients have a shorter hospital length-of-stay compared with patients having surgical fixation after fracture completion?
Patients and Methods
We conducted a search of fracture databases from two Level One trauma centers for patients older than 50 years with nondisplaced, proximal diaphyseal stress fractures and completed, displaced proximal diaphyseal femoral fractures from July 2002 to April 2009. A total of 613 patients were identified. We searched for patients older than 50 years because of the greater likelihood of bisphosphonate use in this age group. We excluded patients with fractures sustained by high-energy mechanisms, fractures associated with malignancy, intertrochanteric fractures, distal femur fractures, and periprosthetic fractures [17]. Of the 613 patients, we identified 34 patients (40 fractures) with fractures radiographically characteristic of a bisphosphonate-associated completed femur fracture including unicortical beaking, a thin transverse fracture line, cortical hypertrophy, or they had a bisphosphonate-associated stress fracture in the same corresponding region (Fig. 3). All included patients had a documented history of bisphosphonate use at the time of presentation. All patients were female (Table 1). Twenty-nine of the 34 patients (85%) had been treated with alendronate (70 mg every week), three patients were treated with zoledronic acid (4 mg every 6 months), and two patients were treated with pamidronate (90 mg every 4 weeks). The average length of treatment receiving bisphosphonates was 77 months (range, 36–120 months). The minimum followup was 12 months (mean, 36.5 months; range, 12–72 months). No patients were lost to followup. All patients were recalled specifically for this study to assess current physical status; data also were obtained from medical records and radiographs. The design and protocol of this study were approved by the Institutional Review Board.
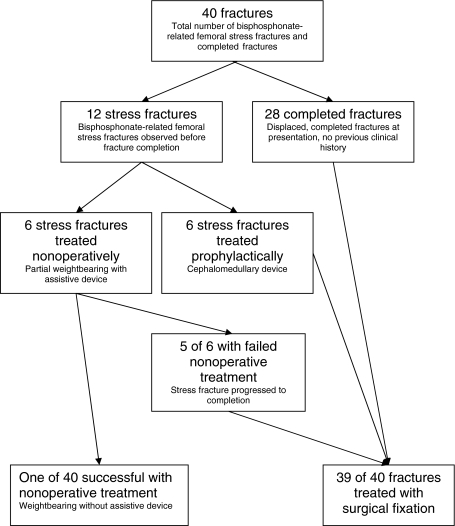
Fig. 3.
A flowchart shows the distribution of fractures and their treatment in this study.
Table 1.
Patient demographics
| Parameter | Value |
|---|---|
| Number of patients | 34 |
| Gender | |
| Male | 0 (0%) |
| Female | 34 (100%) |
| Age (years)* | 68.5 (53–87) |
| Bisphosphonate | |
| Alendonate | 29 (85.3%) |
| Zoledronic acid | 3 (8.8%) |
| Pamidonate | 2 (5.9%) |
| Duration of bisphosphonate use† | |
| < 5 years | 3 (15.8%) |
| > 5 years | 16 (84.2%) |
* Values are expressed as mean, with range in parentheses; †only 19 of 34 patients had documentation of duration of bisphosphonate use.
Twenty-four patients (28 of the 40 fractures [70%]) presented with completed fractures and had no prior documentation of a stress fracture. The mechanism of injury in all of these patients was a fall from standing. Two of the 28 patients reported a “snap” in the affected extremity while ambulating just before the fall. None of the patients presented with bilateral completed bisphosphonate-associated femur fractures. Nine patients (12 of the 40 fractures [30%]) presented with bisphosphonate-associated femoral stress fractures. Six of these 12 fractures (50%) initially were treated nonoperatively, and six were treated with prophylactic cephalomedullary nailing.
Nonoperative management consisted of partial weightbearing with assistive devices (crutches) on the affected extremity until resolution of pain and radiographic documentation of fracture line disappearance. Patients were considered to have nonoperative treatment only if they were actively being followed for the stress fracture. Leg pain alone in the ipsilateral leg before presentation with a fracture was not considered nonoperative treatment.
The indications for surgery were: completed femur fracture or femoral stress fracture in the femoral subtrochanteric or high femoral diaphyseal region. Fractures were treated with a cephalomedullary nail device (Synthes™ Femoral Nail or Trochanteric Fixation Nail, Synthes Inc, Paoli, PA, USA), which uses proximal fixation with a blade/screw in the femoral neck and distal locking screws (Fig. 4). Patients were positioned on a fracture table or laterally on a standard table.
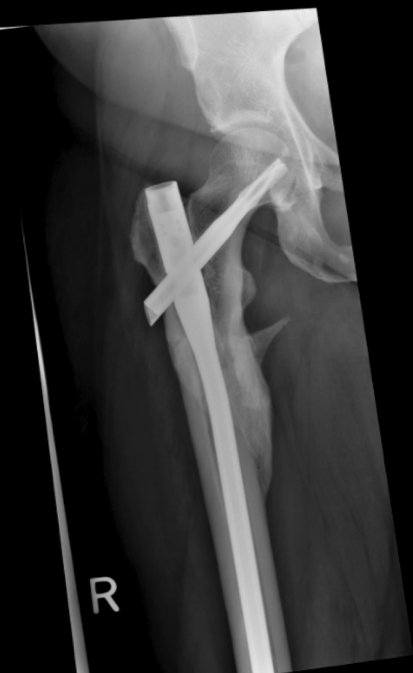
Fig. 4.
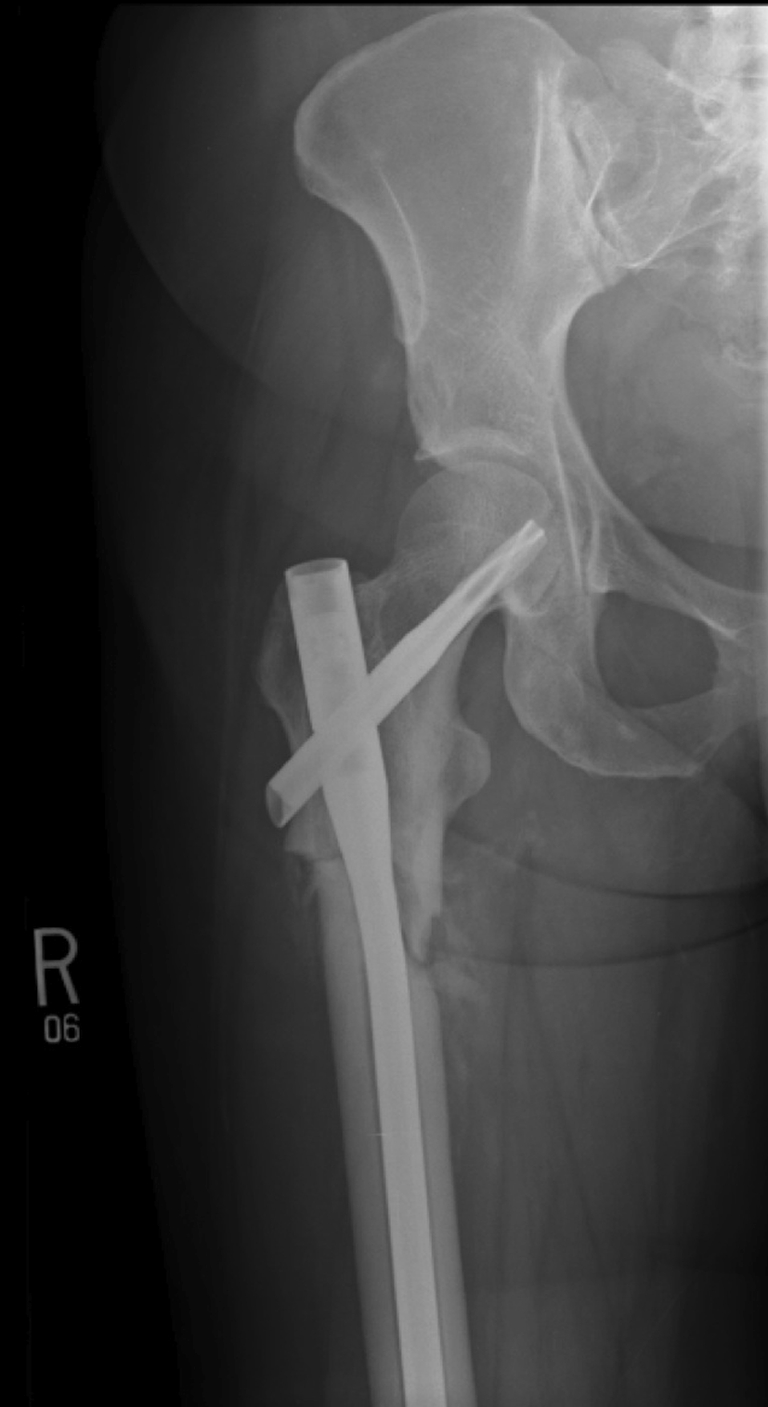
An AP radiograph shows the hip and proximal femur after operative fixation.
All patients were allowed weightbearing as tolerated after the procedure and began mobilization with physical therapy on postoperative Day 1. Patients received physical therapy twice daily for approximately 30 minutes during their hospital admission. Assistive devices were curtailed based on patients’ needs, typically beginning with a walker and progressing to crutches. Bisphosphonate therapy was not continued.
Patients returned to clinic 2 weeks postoperatively for radiographic evaluation and to evaluate wound healing and progression with weightbearing. Outpatient physical therapy was started at this 2-week point. AP and lateral view radiographs of the affected hip and femur were obtained. Patients then returned 6 weeks later (2 months postoperatively) to ensure full weightbearing, gait analysis, and repeat radiographic evaluation. After the 2-month period, patient followup was based on clinical symptoms. Fracture union was determined by radiographs and clinical resolution of pain (Fig. 5). Two of us (MBB, JAA) evaluated all radiographs to ascertain the state of healing at last followup. The criteria for healing were bridging of the fracture by bone at three of four cortices and obliteration of the fracture line with cortical continuity [6].
Fig. 5.
An AP radiograph of the hip and proximal femur obtained 12 months postoperative shows healing.
Data collected included fracture type, mechanism of injury, presence of contralateral stress fracture, incidence/dose/duration of bisphosphonate use, medical history, course of nonoperative treatment if a known stress fracture was present, type of fixation, and length of hospital stay. Statistical analysis was performed with Student’s t test to compare the length of hospital stay between the two groups. Normality of data was assessed with skewness and kurtosis values (SPSS 19.0, Chicago, IL, USA).
Results
Of the six bisphosphonate-associated femoral stress fractures treated nonoperatively, five progressed to fracture completion. The time to fracture completion in this group occurred at an average of 10 months (range, 3–18 months). Only one stress fracture did not have fracture completion after 1 year of nonoperative treatment.
For the six patients with six stress fractures prophylactically nailed before fracture completion, the average postoperative hospital stay was 3.7 days (range, 3–5 days; SD, 0.81 days; skewness, 0.9; kurtosis, −0.3). The 30 patients with 34 acute completed bisphosphonate-associated femur fractures had an average hospital stay of 6.0 days (range, 3–17 days; SD, 3.1 days; skewness, 1.9; kurtosis, 4.6), which was longer (p < 0.001) than that of patients who had prophylactic nailing of their stress fractures. Looking at only the four patients (five stress fractures) treated nonoperatively with fractures that progressed to fracture completion, the average length of hospital stay was 6.0 days (range, 5–7 days; SD, 0.83 days; skewness, .512; kurtosis, −0.612), which was longer (p < 0.003) than that of the patients who had prophylactic nailing of their stress fractures.
Of the 39 fractures (33 patients) treated with surgical fixation, radiographic union was evident in six of six (100%) prophylactically nailed stress fractures, five of five (100%) failed nonoperative displaced stress fractures, and 27 of 28 (96%) completed stress fractures. No patients who received prophylactic nailing experienced complications. Among patients presenting after fracture completion, one had a nonunion develop after surgery requiring a subsequent surgical procedure whereas another had a delayed union, which healed via nonoperative measures by 26 months followup. Two patients had hardware removed for symptomatic reasons after completion of fracture healing. There were no infections documented in any of the study patients.
Discussion
Bisphosphonates have become the first-line therapy for patients with osteoporosis, with use of alendronate in women reducing the risk of clinical hip fractures by 36% and vertebral fractures by 44% [4]. It is estimated approximately 190 million prescriptions for bisphosphonates have been made worldwide [1]. Studies have described a specific fracture pattern that occurs in patients receiving long-term bisphosphonate therapy [7, 10, 11, 14, 20]. Neviaser et al. [14] performed a retrospective review of patients presenting with low-energy femoral shaft fractures. Of patients taking alendronate, 76% had a characteristic fracture in the femoral diaphysis. They describe this characteristic fracture as a “simple transverse fracture with a unicortical beak in an area of cortical hypertrophy.” The true mechanism of this bisphosphonate-related stress fracture is unknown, but it is believed bisphosphonate-related decreased bone turnover can lead to an accumulation of microtrauma, which may precipitate the generation of an insufficiency fracture that does not heal [13]. Recognition of these femoral stress fractures after long-term bisphosphonate use is critical for orthopaedic surgeons given the widespread use of bisphosphonates. There are no current recommendations for the duration of treatment with bisphosphonates [21]. Furthermore, the natural history and optimal management of bisphosphonate-associated fractures have not been well described. In this retrospective review, we addressed two questions: (1) What is the likelihood of nonoperatively treated bisphosphonate-associated femoral stress fractures progressing to completion and during what time period? (2) If prophylactic fixation is performed, do patients have a shorter hospital length-of-stay compared with patients having surgical fixation after fracture completion?
There are limitations to this study. First, with the retrospective study design some information was missing. In 19 of the 34 patients, there was clear evidence providing the precise duration of bisphosphonate use; the remaining patients had documented current use of bisphosphonates without known duration. In addition, not all patients had bilateral radiographs. It is our current practice to obtain bilateral radiographs for all patients presenting with possible bisphosphonate-related fractures. Second, the sample size of our study is small, particularly for patients presenting with nondisplaced femoral stress fractures. One explanation for the low number in this subgroup could be sampling bias as our patients were selected retrospectively from a fracture database with potential for underreporting of nondisplaced stress fractures. Third, our study did not aim to address what percentage of patients receiving bisphosphonate therapy had femoral stress fractures; this question would be better suited for large-scale epidemiologic studies. Likewise, there was no risk/benefit assessment of how many fractures may have been prevented by bisphosphonates compared with how many fractures might have been caused. Finally, our study was not randomized and potential confounders could have affected length of stay (age, comorbidities). A prospective randomized trial ultimately is needed to compare nonoperative versus prophylactic management.
Our observations suggest nonoperative management (partial weightbearing) of bisphosphonate-associated stress fractures has a high likelihood of failure with the majority of fractures progressing to fracture completion. Unlike femoral neck stress fractures, the clinical course and treatment algorithm for stress fractures in the subtrochanteric or high diaphyseal region are not well described. Ha et al. [8] recently reviewed the clinical course of 11 patients (14 fractures) with bisphosphonate-associated femoral stress fractures and found that five of the 14 fractures progressed to completion at a mean of 10 months. Five additional fractures were treated surgically owing to intractable pain even though secondary displacement did not develop. Although patients in our study had a higher rate of fracture completion, our study results are consistent with those of Ha et al. [8], in that the majority of the stress fractures required surgical intervention within 1 year.
We also observed that patients with stress fractures treated prophylactically with a cephalomedullary nail before fracture completion had a shorter postoperative hospital stay than patients who had surgical stabilization after fracture completion. One study suggests orthopaedic hospitalizations after hip fractures cost, on average, $391.93 per day [9]. This number does not include the increased cost of intensive care unit stays, which one may speculate may be needed more frequently for patients who were not optimized for surgery in the elective setting.
Our review also suggests bisphosphonates other than alendronate are associated with bisphosphonate-associated proximal diaphyseal fractures. Current studies in the literature documenting bisphosphonate-associated femur fractures cite only alendronate as the bisphosphonate that patients were taking chronically [7, 10, 14]. Alendronate has been the most available bisphosphonate as it was the first approved by the FDA. The effects of alendronate continue for 5 years even after its discontinuation [3]. The half-life of alendronate is reported as 126 months, whereas that of zoledronic acid is 146 hours and that of pamidronate is 28 hours [18]. Despite the varying half-lives, the mechanism of action of these bisphosphonates is similar in that they all affect the osteoclast, leading to the accumulation of microtrauma and ultimately an insufficiency fracture [12, 13].
Our study adds to the literature of bisphosphonate-associated stress fractures and addresses a potential method of treatment. Given our observations, we recommend prophylactic fixation of bisphosphonate-associated stress fractures when identified. The number of patients who underwent prophylactic fixation in this study was small, but the high risk of stress fracture displacement and apparently more economic postoperative course for these patients suggest prophylactic fixation is a reasonable option.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Massachusetts General Hospital and Brigham and Women’s Hospital.
References
- 1.Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;3:369–376. [DOI] [PubMed]
- 2.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, Papp A, Bauer DC. Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 5.Devas MB. Stress fractures of the femoral neck. J Bone Joint Surg Br. 1965;47:728–738. [PubMed] [Google Scholar]
- 6.Dijkman BG, Sprague S, Schemitsch EH, Bhandari M. When is a fracture healed? Radiographic and clinical criteria revisited. J Orthop Trauma. 2010;24(suppl 1):S76–S80. doi: 10.1097/BOT.0b013e3181ca3f97. [DOI] [PubMed] [Google Scholar]
- 7.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 8.Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphoante therapy? Clin Orthop Relat Res. 2010;468:3393–3398. doi: 10.1007/s11999-010-1583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haentjens P, Autier P, Barette M, Boonen S, Belgian Hip Fracture Study Group The economic cost of hip fractures among elderly women: a one-year, prospective, observational cohort study with matched-pair analysis. Belgian Hip Fracture Study Group. J Bone Joint Surg Am. 2001;83:493–500. [PubMed] [Google Scholar]
- 10.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 12.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 14.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 15.Nieves JW, Bilezikian JP, Lane JM, Einhorn TA, Wang Y, Steinbuch M, Cosman F. Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int. 2010;21:399–408. doi: 10.1007/s00198-009-0962-6. [DOI] [PubMed] [Google Scholar]
- 16.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1897–1899. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 17.Orthopaedic Trauma Association Committee for Coding and Classification. Fracture and dislocation compendium. J Orthop Trauma. 1996;10(suppl 1):v–ix, 1–154. [PubMed]
- 18.Physicians’ Desk Reference. 63rd Ed. Montvale, NJ: Thomson Reuters; 2009: 2005, 2341.
- 19.Ross JR, Saunders Y, Edmonds PM, Patel S, Broadley KE, Johnston SR. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ. 2003;327:469. doi: 10.1136/bmj.327.7413.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayed-Noor AS, Sjoden GO. Case reports: two femoral insufficiency fractures after long-term alendronate therapy. Clin Orthop Relat Res. 2009;467:1921–1926. doi: 10.1007/s11999-009-0725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider JP. Bisphosphonates and low-impact femoral fractures: current evidence on alendronate-fracture risk. Geriatrics. 2009;64:18–23. [PubMed] [Google Scholar]
- 22.Shane E. Evolving data about subtrochanteric fractures and bisphosphonates. N Engl J Med. 2010;362:1825–1827. doi: 10.1056/NEJMe1003064. [DOI] [PubMed] [Google Scholar]