Abstract
Background
Tranexamic acid (TEA) reportedly reduces perioperative blood loss in TKA. However, whether it does so in minimally invasive TKA is not clear.
Questions/purposes
We asked whether TEA would reduce blood loss and blood transfusion requirements after minimally invasive TKA.
Patients and Methods
We prospectively enrolled 100 patients who underwent minimally invasive TKAs: 50 received one intravenous injection of TEA before deflation of the tourniquet and a control group of 50 patients received an equivalent volume of placebo. We compared changes in hemoglobin, postoperative drainage, total blood loss, and transfusion rates between the two groups.
Results
The total blood loss was less for patients in the TEA group than for the control group: 833 mL (374–1014 mL) versus 1453 mL (733–2537 mL), respectively. The rate of blood transfusion also was less for patients in the TEA group than in the control group (4% versus 20%). The hemoglobin levels on the second and fourth postoperative days were greater for patients in the TEA group than in the control group.
Conclusions
Our data suggest one intraoperative injection of TEA decreased the total blood loss and need for transfusion after minimally invasive TKA.
Level of Evidence
Level II, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA is associated with considerable blood loss, ranging from 1000 to 1500 mL after conventional TKA, with an average transfusion rate of 1 to 2 U of allogeneic blood [8, 17, 21]. The fibrinolytic system is activated transiently after any surgery [30], and the use of a pneumatic tourniquet to create a bloodless field is associated with increased fibrinolytic activity in the surgically treated limb in animal models [23] and for patients having elective orthopaedic surgery [10, 29]. The increased fibrinolytic activity may increase blood loss after knee arthroplasty during first few postoperative hours [5, 13]. TEA, an inhibitor of fibrinolysis [25], decreases postoperative blood loss after conventional TKA according to some reports [5, 13, 26, 32].
Minimally invasive surgery has become used more commonly for TKAs in recent years [35]. Previous studies suggest minimally invasive TKA (MIS-TKA) reduces hospital stay [6], decreases postoperative pain [6, 12], decreases rehabilitation needs [6, 12], and enables return to normal function earlier than a standard TKA [33]. Theoretically, owing to the shorter operative wound length and less muscle splitting involved in MIS-TKA, compared with conventional TKA, less intraoperative and postoperative blood loss is anticipated. However, the literature contains contradictory results regarding perioperative blood loss after MIS-TKA. Laskin et al. [19] reported greater postoperative drainage in the MIS-TKA group than in the standard TKA group. However, Tenholder et al. [34] reported that patients with MIS-TKA required fewer transfusions than the patients with the standard approach. Furthermore, the effect of TEA on reducing postoperative bleeding after MIS-TKA has not been reported.
We therefore asked whether a one-injection protocol of TEA would reduce blood loss and blood transfusion requirements after MIS-TKA.
Patients and Methods
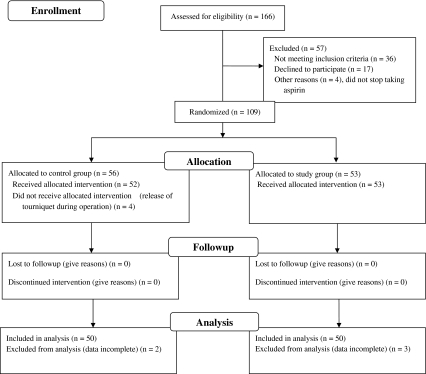
We conducted a prospective comparative study to answer the above question. Between October 2008 and June 2009 we treated 166 patients with late-stage arthritis of the knee who underwent a unilateral MIS-TKA. We excluded 57 patients: 36 patients with thrombocytopenia or hemophilia, prior surgery of the affected knee, hemoglobin (Hb) less than 10 g/dL on the day of admission, a history of thromboembolic disease or lifelong warfarin therapy for thromboembolism prophylaxis; 17 declined to participate in the study; and four who did not withhold use of aspirin for 1 week before admission. This left 109 patients who were assigned to either the study (TEA) or the control group (Fig. 1). Patients who underwent surgery on days ending in an odd number were assigned to the TEA group, and patients who underwent surgery on days ending in an even number were assigned to the control group. In the TEA group, 10 mg/kg of TEA (Transamin 50 mg/mL; China Chemical and Pharmaceutical Co, Taiwan, ROC) was administered through an intravenous drip before deflation of the tourniquet, usually at the end of wound closure. In the control group, an equivalent volume of normal saline was used. Four patients subsequently were excluded from the study owing to release of the tourniquet during surgery. Five patients (two in the control group and three in the study group) were excluded owing to incomplete data collection. Therefore, 100 patients were included in the statistical analysis (Fig. 1). The minimum followup was 12 weeks (mean, 36 weeks; range, 12–84 weeks). No patients were lost to followup. This study was approved by the Institutional Review Board and all patients signed informed consent.
Fig. 1.
A flow diagram of the patients through the trial phases is shown.
The sample size was calculated as follows. Camarasa et al. reported the total blood loss after traditional total knee replacement in a control group was 1784 (660) mL [9]. Assuming a mean difference of 400 mL or greater total blood loss between two groups, to obtain a statistical power of 0.85 and alpha error of 0.05, 45 patients were required in each group. Expecting a 10% loss to followup and 10% being excluded from analysis, 110 patients were enrolled.
All patients taking aspirin or nonsteroidal antiinflammatory medicine for prophylaxis of thromboembolism were asked to abstain from taking them for 1 week before surgery. On the day of admission, all patients underwent baseline blood tests including Hb level, hematocrit (Hct), prothrombin time (PT), activated partial thromboplastin time (APTT), and platelet count. The preoperative severity of knee deformities and functional status of the limb were documented by knee ROM and mechanical axis of the knee. The preoperative characteristics, including age, gender, disease of the knee, body mass index (BMI), preoperative Hb level, Hct, PT-international normalized ratio (INR), APTT-INR, American Society of Anesthesiologists (ASA) risk, preoperative mechanical axis, and ROM of the knee, were comparable in both groups (Table 1).
Table 1.
Patient profiles
| Parameter | Control group (n = 50) | Tranexamic acid group (n = 50) | p Value |
|---|---|---|---|
| Age (years) | 68.3 (8.4; 54–87) | 69.2 (6.3; 55–85) | 0.508 |
| BMI (kg/L2) | 28.2 (3.8; 21.2–37.7) | 27.7 (4.7;19.7–37.6) | 0.469 |
| % women | 82.0% (41/50) | 88% (44/50) | 0.278 |
| Osteoarthritis | 100% (50) | 94% (47) | 0.079 |
| ASA risk (%) | |||
| ASA I | 1 (2%) | 0 | 0.409 |
| ASA II | 39 (78%) | 36 (72%) | 0.409 |
| ASA III | 10 (20%) | 14 (28%) | 0.409 |
| Diabetes mellitus | 18% (9) | 18% (9) | 1 |
| Hemoglobin (g/dL) | 13.5 (1.08; 11.1–15.5) | 13.0 (1.4; 10–16.9) | 0.075 |
| Hematocrit | 40.8 (3.0; 34.3–46.8) | 39.9 (3.9; 31.1–48.9) | 0.270 |
| PT-INR | 0.95 (0.033; 0.89–1.04) | 0.96 (0.037; 0.88–1.13) | 0.227 |
| APTT-INR | 0.94 (0.05; 0.79–1.06) | 0.93 (0.08; 0.80–1.18) | 0.475 |
| Preoperative mechanical axis | 11° varus (27° varus to 7° valgus) | 11° varus (28° varus to 11° valgus) | 0.688 |
| Preoperative range of motion | 112° (26.4°, range 80°–135°) | 107° (29.3°, range 40°–140°) | 0.330 |
Numbers in parenthesis are standard deviation and range; ASA = American Society of Anesthesiologists; PT-INR = prothrombin time-international normalized ratio; APTT-INR = activated partial thromboplastin time-international normalized ratio.
All surgery was performed or supervised by the same surgeon (JWW) using the mini-midvastus approach for MIS-TKA, as described by Haas et al. [12]. All patients received general anesthesia. A tourniquet was applied around the thigh after elevation of the limb and exsanguination with an Esmarch band and inflated to a pressure of 300 mm Hg before skin incision. All TKAs were unilateral and cemented using the same prosthesis (Nex-Gen, Legacy Posterior Stabilized Prosthesis; Zimmer, Warsaw, IN, USA). We used an intramedullary alignment rod for femoral cutting and an extramedullary guide system for tibial cutting. The femoral canal for intramedullary guiding was routinely plugged with bone. Meticulous electric cauterization of the soft tissue bleeding points was performed throughout the surgery. The tourniquet was not released until skin closure and application of a compressive dressing. We used two intraarticular drains connected to a vacuum bag (Hemovac, Zimmer) without compression of the bag (ie, without vacuum) during the first 12 hours after surgery. Our protocol of fluid resuscitation after TKA was 100 mL per hour of ¼ saline for 12 hours after surgery. After 12 hours, 60 mL per hour was given until the morning of the second postoperative day as long as the patients’ vital signs were stabilized. The mean lengths of the incisions in full knee extension were similar (p = 0.125) in the two groups: 10.4 cm in the control group and 10.1 cm in the TEA group. Intraoperative blood loss was negligible in all patients because the tourniquet was not deflated until wound closure.
Twelve hours postoperatively, the bag was fully compressed, creating a negative pressure (60 mm Hg). It was our standard protocol that the drains were removed on the second postoperative day no matter what the drain output was. All patients received intravenous prophylactic antibiotic therapy consisting of 1 g cefazolin preoperatively, followed by 1 g every 8 hours for three doses postoperatively. Standard thromboprophylaxis was prescribed postoperatively for all patients, by subcutaneous administration of 20 mg enoxaparin (Clexane, Glaxo-Smith-Kline, Brentford, Middlesex, UK) every 12 hours until discharge. After that, patients were given indomethacin orally for at least 4 weeks, the regimen reported in a previous study of deep venous thrombosis (DVT) in TKA [36]. No other modalities of thromboembolic prophylaxis, such as foot pumps or antiembolic stockings, were used. The mean hospital stay was similar (p = 0.332) in the two groups: 5.38 days for the control group and 5.16 days for the TEA group (Table 2). Postoperative blood loss was recorded every 2 hours for the first 8 hours, and every 8 hours thereafter, until drains were removed on the second postoperative day.
Table 2.
Postoperative data for all patients
| Parameter | Control group (n = 50) | Tranexamic acid group (n = 50) | p Value |
|---|---|---|---|
| Operative time (minutes) | 127 (16.0; 98–154) | 116 (18.5; 67–149) | 0.003 |
| Wound length (cm) | 10.4 (0.9; 8.0–12.0) | 10.1 (1.0; 8.0–12.0) | 0.125 |
| Hospital stay (days) | 5.38 (1.8; 4–10) | 5.16 (1.54–8) | 0.332 |
| Number of patients receiving transfusion (rate) | 10 (20.0%) | 2 (4%) | 0.014 |
| Mean units of transfusion (units per patient) | 0.48 (1.0) | 0.08 (0.39) | 0.013 |
| Hb (g/dL) | |||
| Day 1 | 10.9 (1.1; 8.1–13.1) | 11.5 (1.5; 7.4–16.8) | 0.050 |
| Day 2 | 9.3 (1.1; 5.6–12.8) | 10.5 (1.46; 8.1–15.2) | < 0.001 |
| Day 4 | 8.7 (1.0; 6.6–11.9) | 10.0 (1.25; 7.6–13.7) | < 0.001 |
Numbers in parenthesis are standard deviation and ranges.
Both groups followed a standard postoperative rehabilitation protocol, including continuous passive motion of the knee and muscle strengthening exercise immediately after returning to the ward. All patients were asked to get out of bed with walker support on the afternoon of the first postoperative day to decrease the incidence of DVT, as described by Pearse et al. [28].
All patients returned to the clinic 2 weeks after surgery for suture removal and clinical examinations, at 6 weeks for clinical evaluation of functional recovery, and at 12 weeks for radiographic examination of the knee. Partial weightbearing usually was allowed 4 to 6 weeks after surgery and full weightbearing usually was allowed 6 to 12 weeks after surgery.
Postoperative Hb levels were recorded on the first, second, and fourth days postoperatively. It was assumed that blood volume was normalized on the fourth postoperative day. Total Hb loss was calculated by subtracting the Hb level on the fourth postoperative day from the preoperative Hb level. The total blood loss was calculated according to the method of Nadler et al. [22], using the maximum postoperative decrease in the Hb level, adjusted for weight and height of the patient. Total blood loss consisted of the amount of blood loss, calculated from the total Hb loss and the amount of allogeneic blood transfused [7, 22]. The volume of transfused concentrated erythrocytes was calculated as 1 unit equivalent to 250 mL. An Hb level of 8.5 g/dL or less reflects blood transfusion of one unit of concentrated erythrocytes. No patients autodonated blood for transfusion. The hidden blood loss (residual blood in the joint and soft tissues and loss attributable to hemolysis) [31] was calculated by subtracting the amount of total drainage from total blood loss.
We used the principle of transfusion based on the criteria and guidelines for perioperative transfusion suggested by the National Institutes of Health Consensus Conference, which states that the decision to transfuse blood depends on clinical assessment, aided by laboratory data, indicating patients’ symptoms and signs relevant to acute anemia [24]. Therefore, our indication for blood transfusion was set at an Hb concentration of 8.5 g/dL or a postoperative Hb level between 8.5 and 9 g/dL with clinical evidence of acute anemia [1]. It may be adjusted according to patients’ cardiovascular status.
Clinical wound conditions, including hematoma, bleeding, ecchymosis, or drainage, and all signs of infection were recorded. Thigh and calf circumferences were measured at 15 cm above and below the patella if there was clinical swelling of the leg. The presence of DVT was assessed by the presence of thigh and calf swelling, or edema greater than 3 cm when compared with the contralateral leg, and calf tenderness [37] for as long as 12 weeks postoperatively. Routine screening was not used for venous thrombosis, but all clinically suspected thromboses or pulmonary embolisms were investigated using antegrade venography or CT of the chest.
We determined differences in distribution of demographics and preoperative clinical data, including BMI, PT-INR, platelet count, APTT-INR, Hb level, and Hct between groups using the Wilcoxon rank sum test. We determined differences in operative time, wound length, hospital stay, drainage blood loss, total blood loss, hidden blood loss, postoperative Hb level, and Hb change level between the control and the TEA groups using the Mann-Whitney test. Descriptive data including blood transfusion risk and average volume were compared through the chi square test. All statistical comparisons were made using Statistical Package for Social Science (SPSS) software (Version 13; Chicago, IL, USA).
Results
The total blood loss was less (p < 0.001) in the TEA group than in the control group (833 versus 1453 mL). The hidden blood loss was less (p < 0.001) in the TEA group than in the control group (354 versus 897 mL) (Table 3). There was a reduction (p = 0.014) in transfusion rates in the TEA group compared with the control group (4% versus 20%) (Table 2). Ten patients in the control group received allogenic red cell transfusion (two patients received 2 units and eight patients received 1 unit), whereas in the TEA group, two patients received allogenic red cell transfusion (1 unit each). The mean units of transfusion per patient were less (p = 0.013) in the TEA group than in the control group (0.08 versus 0.48 units) (Table 2). The amounts of drainage from postoperative 0 to 2 hours, 2 to 4 hours, and 4 to 6 hours were less in the TEA group than in the control group (Table 3), but the total drainage was similar. The Hb level on the first postoperative day was similar (p = 0.05) in the two groups. It was greater in the TEA group than in the control group on the second and fourth postoperative days (Table 2).
Table 3.
Postoperative drainage and total blood loss
| Parameter | Control group (n = 50) | Tranexamic acid group (n = 50) | p Value |
|---|---|---|---|
| Drainage (mL) | |||
| 0–2 hours | 96.6 (91.8) | 45 (34.0) | 0.002 |
| 2–4 hours | 75.0 (49.0) | 37 (30.3) | < 0.001 |
| 4–6 hours | 59.5 (39.2) | 35 (22.7) | < 0001 |
| 8–24 hours | 186 (89.8) | 228 (132.1) | 0.119 |
| > 24 hours | 93.0 (63.4) | 88 (49.2) | 0.989 |
| Total drainage | 556.0 (248; 57–1090) | 478 (166; 128–1015) | 0.211 |
| Estimated total blood loss (mL) | 1453 (383;733–2537) | 833 (144; 374–1014) | < 0.001 |
| Hidden blood loss (mL) | 897 (357; 65–1759) | 354 (194.5; 161–768) | < 0.001 |
Numbers in parenthesis are standard deviation and ranges.
Two patients in the TEA group had superficial skin necrosis, and one of them had a wound infection develop. The superficial skin necrosis was treated with wound care using iodine and no additional débridement or skin graft was necessary. One patient in the control group had a superficial wound infection. All infections resolved after administration of oral antibiotics and wound care for approximately 1 week. Postoperative hematomas were observed in 11 patients (22%) in the TEA group and in 18 patients (36%) in the control group. The hematomas in both groups were minor and all resolved with rest and use of ice packs for approximately 1 week. Postoperatively, six patients (12%) in the control group had ecchymosis over the thigh and/or leg in the operative limb, but ecchymosis of the operative limb was not observed in the TEA group. Other complications, such as deep infection or fat embolism, were not observed in either group. Two patients in each group had venography of the legs and one patient in each group (2%) had radiographically confirmed DVT in the leg. The symptoms were relieved in both patients after warfarin therapy for 3 months. No other patients had clinical evidence of DVT postoperatively and no further investigations were performed. No patient had clinical evidence of pulmonary embolism, and there were no fatalities from any cause.
Discussion
We are aware that the use of antifibrinolytics such as TEA is effective for reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing conventional TKAs [5, 13, 26, 32]. The dose protocols of TEA reported in the literature included one intraoperative dose of 10 mg/kg [5], one intraoperative dose of 15 mg/kg [26], or two intraoperative doses of 10 mg/kg, given 3 hours apart [11]. There have been no reports regarding the use of TEA as chemoprophylaxis for perioperative bleeding during MIS-TKA. The measurement of blood loss after MIS-TKA reported in the literature was based on intraoperative bleeding and postoperative drainage [18, 35] that do not reflect actual blood loss. We therefore asked whether one intraoperative injection protocol of TEA would reduce postoperative blood loss and blood transfusion requirements after MIS-TKA.
We recognize limitations to our study. First, our sample size (100 patients) is too small to address some questions. However, our calculation of the study size was according to a minimum difference of blood loss of 400 mL between groups, obtaining a power of 0.85, and an alpha value of 0.05. Second, our study design was nonblinded and nonrandomized and can be regarded only as a prospective comparative trial. Third, our selection of patients was limited to those with no history of thromboembolism, a preoperative Hb of 10 g/dL or greater, and no previous surgery of the affected knee. Fourth, there was no conventional TKA group for comparison.
We found the use of TEA reduces total blood loss and transfusion requirements in MIS-TKA. The total mean blood loss in the control group for MIS-TKA in our study was 1453 mL, which is similar to reported rates for conventional TKAs using intraoperative blood loss and postoperative drainage or by maximum loss of Hb level as methods of calculation [5, 9, 11, 13, 16, 17, 20, 31] (Table 4). The blood-saving effect of TEA in postoperative blood loss after conventional TKA was 45% to 48% in two studies [5, 13]. In our study, the total mean blood loss in the TEA group was 833 mL. The blood-saving effect was 43%, which is similar to effects reported by previous investigators for patients who underwent conventional TKA [5, 13]. As a result of conserving blood, the mean Hb level was greater in the TEA group than in the control group on the second and fourth postoperative days. On the first postoperative day, the mean Hb was 11.5 g/dL in the TEA group and 10.9 g/dL in the control group. This may be attributable to the small sample size of our study and inadequate fluid resuscitation on the first postoperative day.
Table 4.
Reports of perioperative blood loss after TKA without tranexamic acid prophylaxis
| Study | Study design | Number of patients | VTE chemoprophylaxis | Method of blood loss calculation | Perioperative blood loss | Conventional or MIS TKA |
|---|---|---|---|---|---|---|
| Lotke et al. [20] (1991) | Nonblinded RCT | 121 | Aspirin | Formula by maximum Hb decrease | 1518 mL | Conventional |
| Hiippala et al. [13] (1995) | Double-blinded RCT | 29 | Enoxaparin | Intraoperative blood loss and postoperative drainage | 1549 mL | Conventional |
| Benoni and Fredin [5] (1996) | Double-blinded RCT | 86 | Enoxaparin | Intraoperative blood loss and postoperative drainage | 1410 mL | Conventional |
| Keating et al. [17] (1998) | Retrospective study | 280 | Heparin sodium | Formula by maximum Hb decrease | 1925 mL | Conventional |
| Good et al. [11] (2003) | Double-blinded RCT | 51 | Dalteparin | Formula by maximum Hb decrease | 1426 mL | Conventional |
| Sehat et al. [31] (2004) | Prospective study | 101 | None | Formula by maximum Hb decrease | 1498 mL | Conventional |
| Kalairajah et al. [16] (2005) | RCT | 60 | Aspirin | Formula by maximum Hb decrease | 1747 mL | Conventional |
| Camarasa et al. [9] (2006) | Double-blinded RCT | 127 | Dalteparin | Formula by maximum Hb decrease | 1784 mL | Conventional |
| Current study | Prospective comparative study | 50 | Enoxaparin | Formula by maximum Hb decrease | 1453 mL | MIS |
VTE = venous thromboembolic event; RCT = randomized control trial; TKA = total knee arthroplasty; MIS = minimally invasive surgery; Hb = hemoglobin.
The amount of postoperative drainage that occurred within 6 hours was substantially greater in the TEA group than in the control group. However, after 6 hours, the amount of drainage was similar in both groups. This time-related change of postoperative blood loss is similar to that reported by Yamasaki et al. [38], who reported lower blood loss in the TEA group compared with the control group only up to 4 hours after cementless THA. This result may be related to the minimum therapeutic duration of approximately 3 hours after intravenous injection of tranexamic acid [4].
The operative time for the control group was longer than for the TEA group. However, the mean total blood loss was less for the TEA group than for the control group. The operative time in our study did not reflect the complexity of the procedure because, as a local teaching hospital, many of the wound closure procedures were performed by young surgeons. In that case, a prolonged operative time is expected. Furthermore, a longer operative time does not imply a greater chance of postoperative bleeding. Kolisek et al. [18] reported a longer operative time for a MIS-TKA than for a standard TKA. However, the blood loss in both groups was similar. There were more hematomas in the knee and more cases of skin ecchymosis of the lower limb in the control group than in the TEA group in our study. With the use of a tourniquet during TKA, the early posttraumatic fibrinolysis is further augmented [10]. The expected postoperative blood loss within 8 hours will be 60% of the total blood loss in the TEA group and 80% in the control group [4, 5]. This explains our results of more hematomas and skin ecchymosis in the control group.
Owing to the antifibrinolytic effects, the risk of increasing venous thromboembolism by using TEA is a cause for concern [2, 3, 27]. We excluded patients with a history of thromboembolic disease, and we routinely used low-molecular-weight heparin postoperatively for thromboembolic prophylaxis. We diagnosed only one patient (2%) in each group with DVT and there was no clinical evidence of pulmonary embolism. TEA does not influence fibrinolytic activity in vein walls [3]. Therefore, neither our study nor previous studies [5, 14, 15, 32] observed a greater incidence of venous thrombosis in patients treated with TEA. However, the detection of DVT in our patients was based on clinical observations (thigh/calf swelling > 3 cm and calf tenderness), followed by positive findings of antegrade venography which did not reveal the true incidence of DVT. Further investigations using thromboembolism screening tests such as radioisotope venography may be required [32].
Our prospective comparative study showed one intraoperative injection of TEA is effective in reducing total blood loss and the requirement of blood transfusion after MIS-TKA for patients without a history of thromboembolic disease. We found the total blood loss after MIS-TKA not as low as expected.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity of interest, patient/licensing arrangement, etc) that might pose a conflict of interest in connection with submitted article.
One or more of the authors (PCL, CHH, WSC, JWW) has received funding from research grant from Chang Gung Memorial Hospital (CMRPG870741).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in research was obtained.
References
- 1.Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86:970–973. doi: 10.1302/0301-620X.86B7.14682. [DOI] [PubMed] [Google Scholar]
- 2.Arnljots B, Wieslander JB, Dougan P, Salemark L. Importance of fibrinolysis in limiting thrombus formation following severe microarterial trauma: an experimental study in the rabbit. Microsurgery. 1991;12:332–339. doi: 10.1002/micr.1920120504. [DOI] [PubMed] [Google Scholar]
- 3.Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol. 1978;67:203–205. [PubMed] [Google Scholar]
- 4.Benoni G, Carlsson A, Petersson C, Fredin H. Does tranexamic acid reduce blood loss in knee arthroplasty? Am J Knee Surg. 1995;8:88–92. [PubMed] [Google Scholar]
- 5.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–440. [PubMed] [Google Scholar]
- 6.Bonutti PM, Mont MA, McMahon M, Ragland PS, Kester M. Minimally invasive total knee arthroplasty. J Bone Joint Surg Am. 2004;86(suppl 2):26–32. doi: 10.2106/00004623-200412002-00005. [DOI] [PubMed] [Google Scholar]
- 7.Brecher ME, Monk T, Goodnough LT. A standardized method for calculating blood loss. Transfusion. 1997;37:1070–1074. doi: 10.1046/j.1537-2995.1997.371098016448.x. [DOI] [PubMed] [Google Scholar]
- 8.Callaghan JJ, O’Rourke MR, Liu SS. Blood management: issues and options. J Arthroplasty. 2005;20((4 suppl 2)):51–54. doi: 10.1016/j.arth.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Camarasa MA, Ollé G, Serra-Prat M, Martin A, Sánchez M, Ricós P, Pérez A, Opisso L. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576–582. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 10.Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63:461–465. [PubMed] [Google Scholar]
- 11.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 12.Haas SB, Cook S, Beksac B. Minimally invasive total knee replacement through a mini midvastus approach: a comparative study. Clin Orthop Relat Res. 2004;428:68–73. doi: 10.1097/01.blo.0000147649.82883.ca. [DOI] [PubMed] [Google Scholar]
- 13.Hiippala S, Strid L, Wennerstrand M, Arvela V, Mäntylä S, Ylinen J, Niemelä H. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74:534–537. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 14.Husted H, Blønd L, Sonne-Holm S, Holm G, Jacobsen TW, Gebuhr P. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand. 2003;74:665–669. doi: 10.1080/00016470310018171. [DOI] [PubMed] [Google Scholar]
- 15.Ido K, Neo M, Asada Y, Kondo K, Morita T, Sakamoto T, Hayashi R, Kuriyama S. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120:518–520. doi: 10.1007/s004029900132. [DOI] [PubMed] [Google Scholar]
- 16.Kalairajah Y, Simpson D, Cossey AJ, Verrall GM, Spriggins AJ. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg Br. 2005;87:1480–1482. doi: 10.1302/0301-620X.87B11.16474. [DOI] [PubMed] [Google Scholar]
- 17.Keating EM, Meding JB, Faris PM, Ritter MA. Predictors of transfusion risk in elective knee surgery. Clin Orthop Relat Res. 1998;357:50–59. doi: 10.1097/00003086-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kolisek FR, Bonutti PM, Hozack WJ, Purtill J, Sharkey PF, Zelicof SB, Ragland PS, Kester M, Mont MA, Rothman RH. Clinical experience using a minimally invasive surgical approach for total knee arthroplasty: early results of a prospective randomized study compared to a standard approach. J Arthroplasty. 2007;22:8–13. doi: 10.1016/j.arth.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Laskin RS, Beksac B, Phongjunakorn A, Pittors K, Davis J, Shim JC, Pavlov H, Petersen M. Minimally invasive total knee replacement through a mini-midvastus incision: an outcome study. Clin Orthop Relat Res. 2004;428:74–81. doi: 10.1097/01.blo.0000148582.86102.47. [DOI] [PubMed] [Google Scholar]
- 20.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML. Blood loss after total knee replacement: effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am. 1991;73:1037–1040. [PubMed] [Google Scholar]
- 21.Mylod AG, Jr, France MP, Muser DE, Parsons JR. Perioperative blood loss associated with total knee arthroplasty: a comparison of procedures performed with and without cementing. J Bone Joint Surg Am. 1990;72:1010–1012. [PubMed] [Google Scholar]
- 22.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 23.Nakahara M, Sakahashi H. Effect of application of a tourniquet on bleeding factors in dogs. J Bone Joint Surg Am. 1967;49:1345–1351. [PubMed] [Google Scholar]
- 24.National Institutes of Health Consensus Conference Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703. doi: 10.1001/jama.260.18.2700. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol) 1980;14:41–47. [PMC free article] [PubMed] [Google Scholar]
- 26.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13:106–110. doi: 10.1016/j.knee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Øvrum E, Am Holen E, Abdelnoor M, Oystese R, Ringdal ML. Tranexamic acid (Cyklokapron) is not necessary to reduce blood loss after coronary artery bypass operations. J Thorac Cardiovasc Surg. 1993;105:78–83. [PubMed] [Google Scholar]
- 28.Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of postoperative venous thromboembolism. J Bone Joint Surg Br. 2007;89:316–322. doi: 10.1302/0301-620X.89B3.18196. [DOI] [PubMed] [Google Scholar]
- 29.Petäjä J, Myllynen P, Myllylä G, Vahtera E. Fibrinolysis after application of a pneumatic tourniquet. Acta Chir Scand. 1987;153:647–651. [PubMed] [Google Scholar]
- 30.Risberg B. The response of the fibrinolytic system in trauma. Acta Chir Scand Suppl. 1985;522:245–271. [PubMed] [Google Scholar]
- 31.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty: correct management of blood loss should take hidden blood loss into account. J Bone Joint Surg Br. 2004;86:561–565. [PubMed] [Google Scholar]
- 32.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83:702–705. doi: 10.1302/0301-620X.83B5.11745. [DOI] [PubMed] [Google Scholar]
- 33.Tashiro Y, Miura H, Matsuda S, Okazaki K, Iwamoto Y. Minimally invasive versus standard approach in total knee arthroplasty. Clin Orthop Relat Res. 2007;463:144–150. [PubMed] [Google Scholar]
- 34.Tenholder M, Clarke HD, Scuderi GR. Minimal-incision total knee arthroplasty: the early clinical experience. Clin Orthop Relat Res. 2005;440:67–76. doi: 10.1097/01.blo.0000185450.89364.10. [DOI] [PubMed] [Google Scholar]
- 35.Tria AJ, Jr, Coon TM. Minimal incision total knee arthroplasty: early experience. Clin Orthop Relat Res. 2003;416:185–190. doi: 10.1097/01.blo.0000093030.56370.d9. [DOI] [PubMed] [Google Scholar]
- 36.Wang CJ, Wang JW, Weng LH, Hsu CC, Huang CC, Yu PC. Prevention of deep-vein thrombosis after total knee arthroplasty in Asian patients: comparison of low-molecular-weight heparin and indomethacin. J Bone Joint Surg Am. 2004;86:136–140. doi: 10.2106/00004623-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, Clement C, Robinson KS, Lewandowski B. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki S, Masuhara K, Fuji T. tranexamic acid reduces postoperative blood loss in cementless total hip arthroplasty. J Bone Joint Surg Am. 2005;87:766–770. doi: 10.2106/JBJS.D.02046. [DOI] [PubMed] [Google Scholar]