Abstract
Background
Bizarre parosteal osteochondromatous proliferation (BPOP) is a benign lesion of bone, and numerous questions remain unresolved regarding its etiology, diagnosis, and treatment.
Questions/purposes
We present the Scottish Bone Tumour Registry experience of this rare lesion.
Patients and Methods
We performed a retrospective analysis of the Scottish Bone Tumour Registry records. Histologic specimens were reexamined by a musculoskeletal pathologist. Radiographs were reevaluated by a musculoskeletal radiologist.
Results
From 1983 to 2009, 13 cases (13 patients; six male, seven female) were identified. Their ages ranged from 13 to 65 years. All patients presented with localized swelling. Pain was present in five. Antecedent trauma was present in two. Nine lesions affected the hand, three the foot, and one the tibial tuberosity. Twelve lesions were excised and one was curetted. There were seven recurrences of which six were excised. One lesion recurred a second time and was excised. There were no metastases. Radiographs showed densely mineralized lesions contiguous with an uninvolved cortex. Cortical breakthrough was present in one case and scalloping in another. Histologic analysis characteristically showed hypercellular cartilage with pleomorphism and calcification/ossification without atypia, bone undergoing maturation, and a spindle cell stroma.
Conclusions
BPOP is a rare benign lesion that probably is neoplastic, with no gender predilection, and affecting patients over a wide age range. Previously trauma was considered an etiologic factor, but this no longer seems to be the case. The rate of recurrence was 50%, which may indicate a more extensive resection is required for this locally aggressive lesion. No metastases were reported. BPOP should not be mistaken for, or treated as, a malignant tumor.
Level of Evidence
Level IV, retrospective case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
BPOP is a rare benign lesion of bone originally described by Nora et al. [16] in 1983. In the original series, all lesions affected the tubular bones of the hands and feet. Subsequent reports however have identified lesions in the long bones, skull, pelvis, clavicle, maxilla, and the tibial sesamoid [1, 3, 9, 13, 19, 21, 24]. Lesions of the tubular bones of the hands and feet remain the most common, with the hand being affected four times more commonly than the foot [23]. BPOP has no gender predilection [16] and can occur at any age; however, most patients are 20 to 30 years old [8].
It has been suggested BPOP represents a proliferative reaction secondary to periosteal trauma or ischemia [2], and in 2001 Horiguchi et al. [11] identified the processes occurring in a BPOP lesion are similar to those of endochondral ossification in the normal growth plate, indicating a reactive/reparative process after periosteal injury. Dorfman and Czerniak [5] also suggested BPOP may in fact only be part of a spectrum of reactive lesions with differences being attributable to the degree of maturation, the “unitary hypothesis” [25]: florid reactive periostitis (FRP), then BPOP, and finally turret exostosis.
Against this theory is the inconsistent history of antecedent trauma [8]. It therefore was suggested by Zambrano et al. [26] that BPOP could represent a neoplastic process. Subsequent work by Nilsson et al. [15] and Endo et al. [6] supports this and suggests t(1;17) represents a distinct translocation point unique to BPOP, thereby excluding the unitary hypothesis.
Histologically, BPOP is composed of bone, cartilage, and a fibrous myxoid spindle cell stroma without cellular atypia [1, 16, 20]. The basal bony trabeculae are immature with high osteoblastic activity and uneven calcification. An irregular hypercellular cartilaginous cap containing large bizarre binucleate chondrocytes with patches of endochondral ossification that characteristically has a blue tinctorial quality covers this bone (Fig. 1). Radiographically, BPOP classically presents as a “well-marginated mass of heterotopic mineralization arising from the periosteal aspect of an intact cortex without medullary changes” [4]. The natural architecture of the bone should be preserved and there should be no cortical flaring [16]. The soft tissues also should appear normal [8, 23] (Fig. 2). Owing to its rapid growth and bizarre appearance radiographically/histologically, the differential diagnosis is wide and includes ossifying hematoma, myositis ossificans, heterotopic ossification, soft tissue chondroma, juxtacortical chondroma, osteochondroma, parosteal/periosteal osteosarcoma, and periosteal chondrosarcoma [13, 20]. For lesions with a radiographic diagnosis of BPOP, marginal resection is the preferred treatment. This however results in a recurrence rate of 50% to 55% [13, 16]. There are no reported metastases.
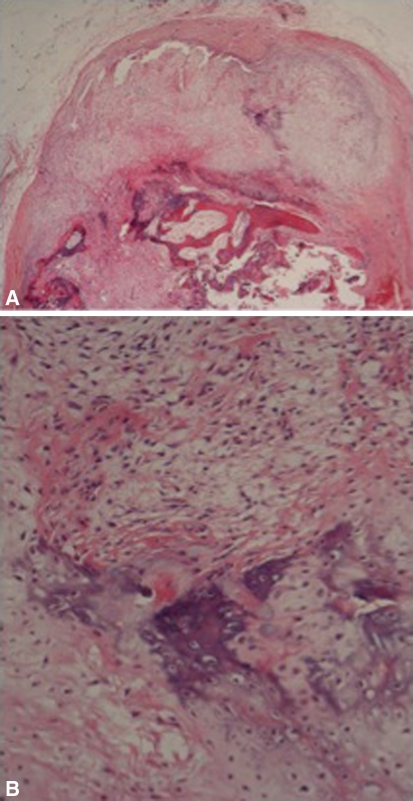
Fig. 1A–B.
(A) A histologic specimen shows a protuberance of the cellular cartilage cap with the underlying bluish stain of enchondral ossification (Stain, hematoxylin and eosin; original magnification, ×40). Deep to this, the trabecular bone (pink) is seen. (B) A histologic specimen shows the blue enchondral ossification at a higher power amid cellular cartilage and fibrous tissue (Stain, hematoxylin and eosin; original magnification, ×200).
Fig. 2.
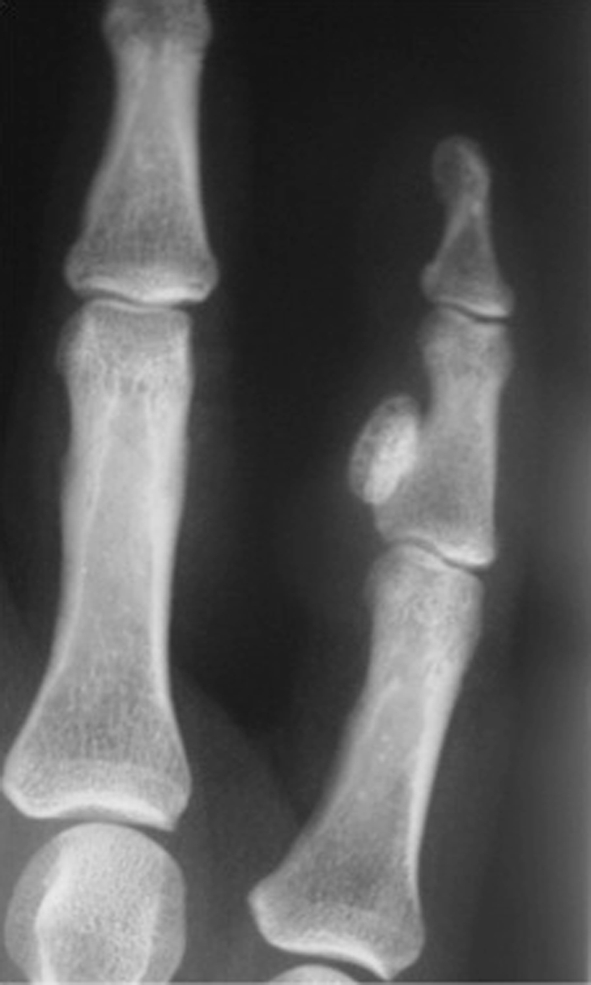
An AP radiograph shows a typical BPOP. A well-defined ossified lesion can be seen on the radiopalmer aspect of the middle phalanx of the right little finger contiguous with the cortex but without cortical/medullary infiltration or soft tissue swelling.
As BPOP is an uncommon lesion, numerous issues remain unresolved. We undertook the first UK study of BPOP in an effort to answer the following questions: (1) Is trauma important in the etiology of BPOP? (2) Does BPOP represent a neoplastic lesion and if so what are the cytogenetic aberrations? (3) Is BPOP part of a continuum of lesions? (4) Is radiographic interpretation accurate enough to diagnose BPOP or is histology required? (5) Is marginal resection the treatment of choice?
Patients and Methods
We performed a retrospective analysis of Scottish Bone Tumour Registry (SBTR) records to identify cases of histologically confirmed BPOP. Between January 1983 and February 2009, 13 cases were identified. Using this database, we were able to gather patient data regarding: age at presentation, gender, symptoms, site of lesion, presence of antecedent trauma, method of treatment, presence of recurrence, and duration of followup (Table 1).
Table 1.
Patient demographics, symptoms, treatment, and outcome
| Patient | Gender | Age at presentation (years) | Location | Symptoms | Trauma | Treatment | 1st local recurrence | Treatment of 1st recurrence | 2nd local recurrence | Treatment of 2nd recurrence | Metastasis | Followup |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 29 | Left little finger middle phalanx | Localized swelling | No | Marginal resection | 18 months | Marginal resection | No | No | 4 years | |
| 2 | Male | 41 | Left ring finger middle phalanx | Localized swelling | No | Intralesional curettage | 84 months | Marginal resection | No | No | 4 years | |
| 3 | Male | 46 | Right 2nd toe proximal phalanx | Localized swelling, pain | No | Marginal resection | 37 months | Marginal resection | No | No | 4 years | |
| 4 | Female | 47 | Right little finger middle phalanx | Localized swelling | No | Marginal resection | No | No | 5 years | |||
| 5 | Female | 23 | Left tibial tubercle | Localized swelling | Yes | Marginal resection | 20 months | Marginal resection | No | No | 2 years | |
| 6 | Female | 25 | Left little finger middle phalanx | Localized swelling, pain | No | Marginal resection | 25 months | Marginal resection | 11 months | Marginal resection | No | 2 years |
| 7 | Female | 65 | Left middle/long finger distal phalanx | Localized swelling | No | Marginal resection | 53 months | Nil | 0 | |||
| 8 | Female | 42 | Left 3rd toe proximal phalanx | Localized swelling | No | Marginal resection | No | No | 0 | |||
| 9 | Female | 39 | Right index finger metacarpal | Localized swelling | No | Marginal resection | No | No | 6 months | |||
| 10 | Male | 49 | Left index finger proximal phalanx | Localized swelling, pain | No | Marginal resection | 25 months | Wide local excision | No | No | 6 months | |
| 11 | Male | 13 | Right foot 2nd metatarsal | Localized swelling, pain, distal paresthesia | Yes | Marginal resection | No | No | 1 year | |||
| 12 | Male | 44 | Right little finger proximal phalanx | Localized swelling, pain | No | Marginal resection | No | No | 6 months | |||
| 13 | Male | 58 | Left ring finger middle phalanx | Localized swelling | No | Marginal resection | No | No | 6 months |
Six patients were male and seven were female. Nine lesions affected the hand, three the foot, and one the tibial tuberosity (Fig. 3). All patients presented with localized swelling. Pain was present in five and one patient also had paresthesia distal to the lesion. A history of trauma was apparent in two patients.
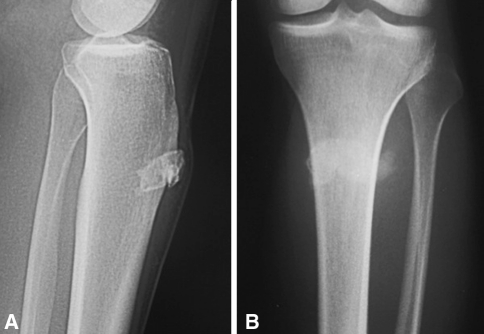
Fig. 3A–B.
(A) Lateral and (B) AP radiographs show a BPOP lesion of the tibial tuberosity.
Marginal resection was the primary mode of treatment in 12 cases. In one case, the lesion was curetted as the definitive method of treatment.
There were seven local recurrences at a median of 2 years (range, 6 months–7 years). Six of the recurrent lesions were treated with aggressive local resection (five marginal and one wide local). One lesion was asymptomatic and so the patient declined further surgery. One lesion recurred a second time 14 months after revision surgery. This was treated with further aggressive marginal resection. There was no recurrence after the second resection. There were no reported metastatic lesions.
All radiographic images were reevaluated by a consultant musculoskeletal radiologist (DR) specifically looking for the site of the lesion, cortical involvement, periosteal reaction, intramedullary change, and soft tissue involvement (Table 2). Radiographs also were analyzed to identify whether they fit with the classic description of a BPOP as described by Dhondt et al. [4].
Table 2.
Patient radiology
| Patient | Date of imaging | Imaging | Description | Typical/Atypical |
|---|---|---|---|---|
| 1 | 08/31/87 | Radiograph | Partially mineralized parosteal lesion (3 × 4 mm) ulnopalmar aspect middle phalanx left little finger, separate from cortex | Typical |
| 02/06/89 | Radiograph | Partially mineralized recurrent lesion (7 × 6 × 6 mm) ulnopalmar aspect of middle phalanx of left little finger, contiguous with cortex but no cortical/medullary infiltration | Typical | |
| 2 | 11/16/92 | Radiograph | Predominantly ossified lesion (13 × 12 × 10 mm) palmar aspect of middle phalanx of left ring finger, unable to exclude intramedullary involvement owing to poor quality radiographs | Typical |
| 3 | 07/14/95 | Radiograph | Ossified parosteal lesion (22 × 15 × 9 mm) dorsomedial aspect of proximal phalanx right 2nd toe, contiguous with cortex but no cortical/medullary infiltration | Typical |
| 11/09/98 | Radiograph | Partially mineralized recurrent lesion (14 × 10 × 8 mm) dorsomedial aspect of proximal phalanx of right 2nd toe; no cortical/medullary infiltration | Typical | |
| 4 | 02/15/94 | Radiograph | Well-defined ossified lesion (9 × 7 × 4 mm) radiopalmar aspect of middle phalanx of right little finger, contiguous with cortex but no cortical/medullary infiltration | Typical |
| 5 | 09/08/94 | Radiograph | Well-defined ossified parosteal lesion (37 × 21 × 9 mm) anterior aspect of proximal tibia just below tibial tuberosity, contiguous with cortex; deep extension could not be ruled out; DD: BPOP, myositis ossificans, ossified hematoma osteochondroma, periosteal/soft tissue chondroma/chondrosarcoma, parosteal osteosarcoma | Typical appearance but atypical sight |
| 6 | 08/04/98 | Radiograph | Ill-defined partially mineralized lesion (6 × 4 mm) palmar aspect of middle phalanx of left little finger, contiguous with cortex, some irregularity of cortex; no medullary infiltration; DD: BPOP, periosteal chondroma/chondrosarcoma, parosteal osteosarcoma | Atypical owing to cortical irregularity |
| 7 | 04/12/00 | Radiograph | Irregular mineralized parosteal and paraarticular mass contiguous with distal phalanx of left little finger and DIPJ osteophytes; origin thought to be bone surface; DD: periosteal chondroma/chondrosarcoma, parosteal osteosarcoma, arthropathy | Atypical as contiguous with DIPJ osteophytes |
| 8 | 07/28/00 | Radiograph | Ossified surface lesion (15 × 9 mm) dorsomedial aspect of left 3rd toe, ? phalanx; cortex ill-defined; poor-quality radiograph | Atypical owing to ill-defined cortex |
| 9 | 01/25/02 | Radiograph | Soft tissue swelling dorsal aspect of 2nd MCPJ; no mineralization; inhomogeneous lesion (19 × 10 × 10 mm) dorsal aspect of 2nd MC contiguous with cortex; cortical surface irregularity. | Atypical owing to cortical surface irregularity |
| 06/18/02 | MRI | Inhomogeneous lesion (19 × 10 × 10 mm) dorsal aspect of 2nd MC contiguous with cortex; cortical surface irregularity | ||
| 10 | 12/13/00 | Radiograph | Predominantly mineralized lesion (28 × 18 × 25 mm) ulnar aspect proximal phalanx of left index finger with early cortical scalloping; DD: periosteal chondroma/chondrosarcoma, parosteal osteosarcoma | Atypical owing to cortical scalloping |
| 11/14/02 | Radiograph | Recurrent lesion at same site and similar size but more lucent with sclerotic shell attached to cortex; no medullary infiltration | ||
| 11 | 08/27/06 | Radiograph | Mineralized lesion (12 × 9 mm) dorsal aspect of 2nd/3rd toe interspace contiguous with 2nd MT metaphysis | Typical |
| 01/30/07 | Radiograph | Lesion increased in size (25 × 14 mm) with periosteal reaction of 2nd MT shaft | Atypical owing to periosteal reaction | |
| 01/30/07 | MRI | Lesion (20 × 15 × 14 mm) dorsal aspect of 2nd MT neck with 5-mm cortical breach with underlying medullary edema and periosteal reaction | Atypical owing to periosteal reaction/cortical breach/medullary edema | |
| 12 | 12/10/07 | Radiograph | Completely mineralized lesion (10 × 11 × 12 mm) radiopalmar aspect of proximal phalanx of left little finger contiguous with cortex but no infiltration of cortex or medulla | Typical |
| 13 | 12/15/07 | Radiograph | Predominantly mineralized lesion (4.5 × 3.5 × 3.5 mm radial aspect of middle phalanx of left ring finger contiguously fused with cortex but no cortical infiltration; small central areas of nonmineralization | Typical |
DD = differential diagnosis; BPOP = bizarre parosteal osteochondromatous proliferation; DIPJ = distal interphalangeal joint; MCPJ = metacarpophalangeal joint; MC = metacarpal; MT = metatarsal.
All pathologic specimens were reevaluated by a consultant pathologist specializing in musculoskeletal oncology (EM). Histology specimens were sectioned at 3 μm and standard hematoxylin and eosin stains were used. Histology characteristically showed hypercellular cartilage with pleomorphism and calcification/ossification without atypia, bone undergoing maturation, and a spindle cell stroma. All specimens were confirmed as BPOP on histologic reevaluation (Table 3). Cytogenetic analysis was performed on one lesion as a routine part of the patients’ care. This involved culture of cells from the histologic specimen. Twenty-two G banded metaphases were available for light microscopy evaluation of chromosomes.
Table 3.
Patient histology
| Patient | Initial report | Diagnosis on reevaluation |
|---|---|---|
| 1 | (1) Multiple chondroid nodules with focal calcification in areas and ossification; poorly formed connective tissue capsule | BPOP |
| (2) Bone with adjacent fibrous tissue and chondroid material with pleomorphism and occasional binucleate cells with focal calcification and an irregular bone/cartilage interface | BPOP | |
| 2 | (1) Haphazardly arranged nodules of cartilage, bone, and calcified tissue around fibrous connective tissue capsule | Synovial chondromatosis (reported abroad and unable to get specimen) |
| (2) Connective tissue with masses of fibroblastic tissue and cartilage with focal calcification/ossification | BPOP | |
| 3 | (1) Outgrowth of mature lamellar bone and woven bone merging with irregular cartilage cap; cellular island of cartilage toward surface with binucleate chondrocytes and some pleomorphism | BPOP |
| (2) Chondroid component active and pushing into adjacent soft tissues; slightly worrying although not infiltrative | BPOP | |
| 4 | Fragment of cartilaginous tissue with focal calcification and ossification at periphery; chondrocytes are disorganized and degree of pleomorphism; no mitoses | BPOP |
| 5 | (1) Thick proliferative cartilage cap over cancellous bone | BPOP |
| (2) Well-circumscribed nodular osteochondroid nodule in which there is a background myxochondroid/hypercellular periphery surrounding central osteoid component of lamellar and interconnected woven bone; focal heavy calcification; chondroid background is myxoid with hypercellularity but no atypia | BPOP | |
| 6 | (1) Cartilage with irregular margin with bone and large amount of spindle cell tissue with chondroid matrix, mitotic activity, and mild pleomorphism in spindle matrix; clear margin with adjacent dense fibrous tissue | BPOP |
| (2) Cap of moderate cellular cartilage and fibrous tissue underlying irregular enchondral ossification | BPOP | |
| (3) Bone and fragments of proliferating cartilage undergoing irregular enchondral ossification | BPOP | |
| 7 | Multiple nodules of hyaline chondroid tissue partly enclosed by lamellar bone and partly by hyalinized soft tissue; chondroid islands are hypercellular with slight pleomorphism and slightly myxoid background; widespread enchondral ossification and calcification | BPOP |
| 8 | Florid and cellular superficial fibrous component that looks active; deep tissue is chondroid with irregular enchondral ossification | BPOP |
| 9 | Lobulated mass largely of cartilage; cartilage cap has abrupt transition to trabecular bone that has varying calcification; mature and immature bone trabeculae; interspersed loose woven tissue of bland spindle cells without atypia; osteoblastic activity; hypercellular cartilage with increased cytoplasm and open chromatin; well-defined with surrounding dense fibrous tissue | BPOP |
| 10 | (1) Benign osteocartilaginous lesion with bony proliferation | BPOP |
| (2) Proliferating fibrous lesion with cartilage cap and underlying trabecular bone separated by fibrous tissue | BPOP | |
| 11 | (1) Osteocartilaginous lesion with thick cartilage cap in areas of chondrocytes with hypertrophic appearance lying in loose hyaline cartilage; enchondral ossification at base of cartilage that is part typical and part basophilic and irregular; periphery is cellular fibrous tissue with intramembranous ossification with basophilic irregular bony trabeculae; fibrous tissue between irregular bony trabeculae is cellular without atypia | BPOP |
| 12 | Mixture of bone and cellular fibrous tissue; transition between fibroblastic proliferation, mature cartilage, and underlying cortical bone | BPOP |
| 13 | Proliferating spindle cell component cartilage with marked basophilic calcification and enchondral ossification | BPOP |
BPOP = bizarre parosteal osteochondromatous proliferation.
Patients were followed up for a median of 1 year (range, 0–5 years) after the last surgical resection.
An extensive literature review was performed using PubMed, MEDLINE, and Google Scholar. Search terms included “bizarre parosteal osteochondromatous proliferation” and “Nora lesion.” Exclusion criteria included no English translation available or no full text available.
Results
Antecedent trauma was identified in two patients. In both patients, the reported trauma was minor and therefore unlikely to induce periosteal reaction. We believe trauma is not significant in the etiology of BPOP.
Cytogenetic analysis of one of our lesions showed chromosomal abnormalities at chromosome 6 and chromosome 7 in 12 cells (pericentric inversion of Ch 6 between bands 6p25 and 6q15 and paracentric inversion of Ch 7 at bands 7q22.1 and 7q31.3). The remaining cells had a normal karyotype. This favors a neoplastic etiology, but as only one lesion had cytogenetic evaluation, our results are not conclusive.
None of the original or recurrent lesions showed evidence of progression from FRP to BPOP. All presented or represented as BPOP, suggesting BPOP is not part of a continuum of lesions.
Of the 13 lesions, seven fit the classic description of a BPOP and five did not. One lesion could not be diagnosed radiographically as the imaging was of poor quality and the cortex was ill-defined. Another lesion presented with no mineralization; however, calcification and cortical irregularity were present on a subsequent MR image. One lesion was present in an arthritic joint and this made radiographic diagnosis difficult. One lesion had cortical scalloping, again preventing a definitive radiographic diagnosis (Fig. 4). One lesion initially appeared typical of BPOP; however, there was subsequent periosteal reaction on radiographs and an MRI scan showed cortical destruction and medullary edema, giving an atypical radiographic appearance (Fig. 5). One lesion was present on the tibial tuberosity, and although it appeared typical, it was present in a site not previously identified in the literature.
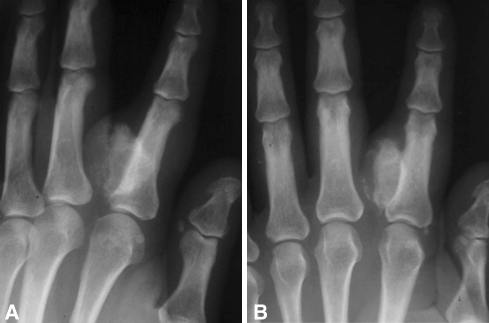
Fig. 4A–B.
(A) Oblique and (B) AP radiographs show an atypical BPOP lesion with cortical scalloping.
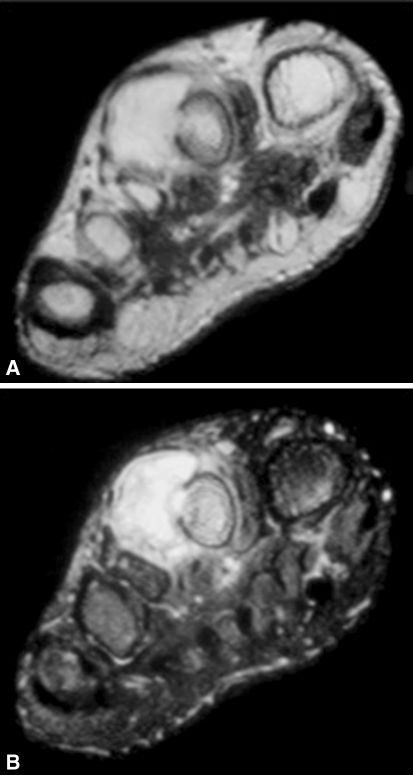
Fig. 5A–B.
Axial (A) T1- and (B) T2-weighted MR images of an atypical BPOP lesion show cortical breach and medullary inflammation.
In our series, there were seven local recurrences (54%), including six lesions treated with marginal resection and one lesion curetted as the primary mode of treatment. Of the seven local recurrences, five were treated with an additional, more extensive marginal resection and one, which had cortical scalloping, was treated with wide local excision (Fig. 6). One lesion recurred a second time after marginal resection and this again was treated with an extensive marginal resection. The recurrent lesion with cortical scalloping did not subsequently recur. Marginal resection remains the preferred treatment for primary lesions; however, recurrent lesions may require more extensive resection.
Fig. 6.
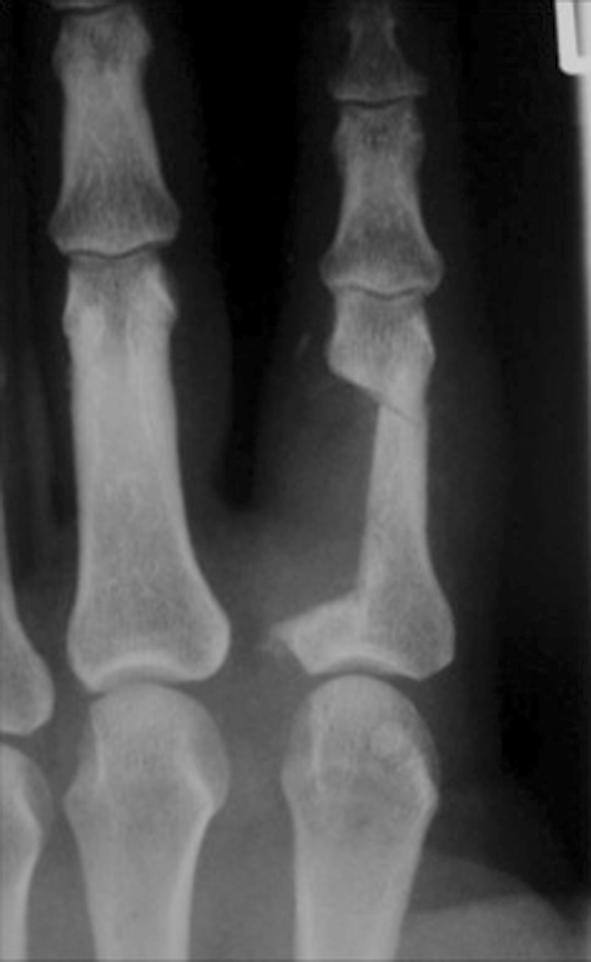
An AP radiograph shows wide local excision of the BPOP lesion that had cortical scalloping.
We did not find any gender predilection and lesions were found to occur over a wide age range. The tubular bones of the hand represented the most common site.
Discussion
BPOP is a rare lesion, and as a result, there are numerous questions remaining unresolved regarding etiology, diagnosis, and treatment. We therefore retrospectively reviewed SBTR records from 1983 to 2009 to determine whether we could answer some of these questions. Apart from the original series by Nora et al. [16] (35 cases), a subsequent series by Meneses et al. [13] (65 cases), and a radiology-based series by Dhondt et al. [4] (24 cases), our study of 13 patients is the largest in the literature.
The major limitation of this study is that it is a retrospective case series; however, owing to the rarity of this lesion, it would be difficult to perform a prospective study. Another limitation of this study is that the SBTR relies on individual hospitals referring patients with a suspected primary bone tumor to this central registry. SBTR records therefore are dependent on local hospitals identifying such lesions and referring them appropriately.
Although Ly et al. [12] reported the case of a radiographically confirmed BPOP developing after injury, we do not believe trauma is an etiologic factor. In the original series of Nora et al. [16], trauma was not identified in any patient. In our series, antecedent trauma was an inconsistent finding (< 20%), and when present, it was minor and therefore unlikely to cause periosteal reaction.
We believe BPOP represents a neoplastic rather than reactive lesion. This is supported by Zambrano et al. [26], who identified an abnormal karyotype in one lesion (Ch 12 additional ring chromosomes, Ch 7 trisomy) and nonclonal abnormalities of chromosomes 2, 8, and 14 in another. The finding of a distinct t(1;17)(q32;q21) balanced translocation by Nilsson et al. [15] and a t(1;17)(q42;p23) translocation by Endo et al. [6] also supports this theory. Only one of our lesions was evaluated with genetic testing and this revealed karyotypic abnormalities at chromosomes 6 and 7. This, we believe, favors a neoplastic etiology, although as trauma was present in this case, a reactive lesion inducing genetic abnormalities cannot be excluded. It has been suggested t(1;17) represents a distinct translocation point in BPOP [6]. As only one lesion in our series had cytogenetic studies performed, we are not able to comment further on the significance of our findings regarding a specific genetic abnormality characteristic of BPOP, but the abnormal karyotype we identified has not been reported previously, indicating BPOP may not result from one pathogenetically significant aberration, as suggested by Nilsson et al. [15], or t(1;17) is not the only distinct translocation point, as suggested by Endo et al. [6].
The finding of cytogenetic aberrations distinct to BPOP also supports the view that BPOP is not part of a spectrum of lesions, as suggested by Dorfman and Czerniak [5]. In contrast to this, Dhondt et al. [4] and Sundaram et al. [22] claim to have identified lesions that have progressed from FRP to BPOP radiographically, thus supporting the unitary hypothesis [25]. Histologic confirmation of the progression from FRP to BPOP is not present in either of these reports [4, 22], and so we maintain BPOP does represent a distinct clinicopathologic entity. However, we are not able to prove or disprove this, as all patients presented and underwent excision biopsy as part of their investigation/treatment at the BPOP stage.
In agreement with Dhondt et al. [4] and Torreggiani et al. [23], we believe radiology alone is sufficient to diagnose BPOP in typical cases (typical radiographic appearance and typical clinical findings). There are however radiographic reports in the literature of corticomedullary continuity [18], cortical destruction [10], and intramedullary inflammation [17] in histologically confirmed BPOP. We would recommend, if the clinical and radiographic appearances are typical and the site also is typical, then radiographic diagnosis is satisfactory. It may be the case that cross-sectional imaging such as CT scanning or MRI is required in cases where radiographs cannot exclude cortical involvement. If the lesion appears atypical on radiographs, we would, in agreement with Flint and McKay [7], recommend cross-sectional imaging and histologic confirmation.
For symptomatic lesions with a presumptive diagnosis of BPOP, marginal resection is the preferred treatment. With such treatment, the recurrence rate is high at 50% to 55% [13, 16]. This prompted Michelsen et al. [14] to suggest a more aggressive resection, including the pseudocapsule, periosteum, and decortication of underlying abnormal host bone. They believe this is responsible for their low recurrence rate of 10%. With a wide resection, segmental amputation may occur owing to the location of the lesion in the hands and feet [8], and as this is a benign lesion, a balance needs to be struck between resection margin and function. Therefore, we would recommend a marginal resection as the first-line treatment for primary lesions. We subsequently would suggest a more aggressive resection in keeping with that suggested by Michelsen et al. [14] for recurrent lesions. Our more aggressive approach with recurrent lesions may explain our low rate of secondary recurrence after revision marginal resection (20%).
BPOP is a rare benign mass lesion of probable neoplastic origin that primarily affects the tubular bones of the hands and feet. A combination of radiographic and histologic diagnosis remains the gold standard. Current treatment involves marginal resection; however, patients should be counseled about the high likelihood of recurrence.
Acknowledgment
We acknowledge the contribution to this study by Anand Pillai regarding collection of patient data.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Glasgow Royal Infirmary and Glasgow Western Infirmary.
References
- 1.Abramovici L, Steiner GC. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion): a retrospective study of 12 cases, 2 arising in long bones. Hum Pathol. 2002;33:1205–1210. doi: 10.1053/hupa.2002.130103. [DOI] [PubMed] [Google Scholar]
- 2.Boudova L, Michal M. Atypical decubital fibroplasia associated with bizarre parosteal osteochondromatous proliferation (Nora’s reaction) Pathol Res Pract. 1999;195:99–103. doi: 10.1016/S0344-0338(99)80079-X. [DOI] [PubMed] [Google Scholar]
- 3.Bush JB, Reith JD, Meyer MS. Bizarre parosteal osteochondromatous proliferation of the proximal humerus: case report. Skeletal Radiol. 2007;36:535–540. doi: 10.1007/s00256-006-0236-8. [DOI] [PubMed] [Google Scholar]
- 4.Dhondt E, Oudenhoven L, Khan S, Kroon HM, Hogendoorn PC, Nieborg A, Bloem JL, Schepper A. Nora’s lesion: a distinct radiological entity? Skeletal Radiol. 2006;35:497–502. doi: 10.1007/s00256-005-0041-9. [DOI] [PubMed] [Google Scholar]
- 5.Dorfman HD, Czerniak B. Bone Tumors. St Louis, MO: Mosby; 1998. [Google Scholar]
- 6.Endo M, Hasegawa T, Tashiro T, Yamaguchi U, Morimoto Y, Nakatani F, Shimoda T. Bizarre parosteal osteochondromatous proliferation with a t(1;17) translocation. Virchows Arch. 2005;447:99–102. doi: 10.1007/s00428-005-1266-7. [DOI] [PubMed] [Google Scholar]
- 7.Flint JH, McKay PL. Bizarre parosteal osteochondromatous proliferation and periosteal chondroma: a comparative report and review of the literature. J Hand Surg Am. 2007;32:893–898. doi: 10.1016/j.jhsa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Gruber G, Giessauf C, Leithner A, Zacherl M, Clar H, Bodo K, Windhager R. Bizarre parosteal osteochondromatous proliferation (Nora lesion): a report of 3 cases and a review of the literature. Can J Surg. 2008;51:486–489. [PMC free article] [PubMed] [Google Scholar]
- 9.Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21:408–412. doi: 10.1177/107110070002100509. [DOI] [PubMed] [Google Scholar]
- 10.Helliwell TR, O’Connor MA, Ritchie DA, Feldberg L, Stilwell JH, Jane MJ. Bizarre parosteal osteochondromatous proliferation with cortical invasion. Skeletal Radiol. 2001;30:282–285. doi: 10.1007/s002560100347. [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi H, Sakane M, Matsui M, Wadano Y. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the foot. Pathol Int. 2001;51:816–823. doi: 10.1046/j.1440-1827.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 12.Ly JQ, Bui-Mansfield LT, Taylor DC. Radiologic demonstration of temporal development of bizarre parosteal osteochondromatous proliferation. Clin Imaging. 2004;28:216–218. doi: 10.1016/S0899-7071(03)00235-3. [DOI] [PubMed] [Google Scholar]
- 13.Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion) Am J Surg Pathol. 1993;17:691–697. doi: 10.1097/00000478-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Michelsen H, Abramovici L, Steiner G, Posner MA. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) in the hand. J Hand Surg Am. 2004;29:520–525. doi: 10.1016/j.jhsa.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson M, Domanski HA, Mertens F, Mandahl N. Molecular cytogenetic characterization of recurrent translocation breakpoints in bizarre parosteal osteochondromatous proliferation (Nora’s lesion) Hum Pathol. 2004;35:1063–1069. doi: 10.1016/j.humpath.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hands and feet. Am J Surg Pathol. 1983;7:245–250. doi: 10.1097/00000478-198304000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Orui H, Ishikawa A, Tsuchiya T, Ogino T. Magnetic resonance imaging characteristics of bizarre parosteal osteochondromatous proliferation of the hand: a case report. J Hand Surg Am. 2002;27:1104–1108. doi: 10.1053/jhsu.2002.36526. [DOI] [PubMed] [Google Scholar]
- 18.Rybak LD, Abramovici L, Kenan S, Posner MA, Bonar F, Steiner GC. Cortico-medullary continuity in bizarre parosteal osteochondromatous proliferation mimicking osteochondroma on imaging. Skeletal Radiol. 2007;36:829–834. doi: 10.1007/s00256-007-0300-z. [DOI] [PubMed] [Google Scholar]
- 19.Shankly PE, Hill FJ, Sloan P, Thakker NS. Bizarre parosteal osteochondromatous proliferation in the anterior maxilla: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:351–356. doi: 10.1016/S1079-2104(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 20.Smith NC, Ellis AM, McCarthy S, McNaught P. Bizarre parosteal osteochondromatous proliferation: a review of seven cases. Aust N Z J Surg. 1996;66:694–697. doi: 10.1111/j.1445-2197.1996.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 21.Soubeyrand M, Pinieu G, Biau D, Anract P, Tomeno B. [Bizarre parosteal osteochondromatous proliferation (Nora’s lesion): two cases] [in French] Rev Chir Orthop Reparatrice Appar Mot. 2007;93:494–500. doi: 10.1016/s0035-1040(07)90332-3. [DOI] [PubMed] [Google Scholar]
- 22.Sundaram M, Wang L, Rotman M, Howard R, Saboeiro AP. Florid reactive periostitis and bizarre parosteal osteochondromatous proliferation: pre-biopsy imaging evolution, treatment and outcome. Skeletal Radiol. 2001;30:192–198. doi: 10.1007/s002560100343. [DOI] [PubMed] [Google Scholar]
- 23.Torreggiani WC, Munk PL, Al-Ismail K, O’Connell JX, Nicolaou S, Lee MJ, Masri BA. MR imaging features of bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion) Eur J Radiol. 2001;40:224–231. doi: 10.1016/S0720-048X(01)00362-X. [DOI] [PubMed] [Google Scholar]
- 24.Vlychou M, Gibbons CL, Rigopoulou A, Ostlere SJ, Athanasou NA. Bizarre parosteal osteochondromatous proliferation of the clavicle. J Shoulder Elbow Surg. 2008;17:e18–e20. doi: 10.1016/j.jse.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Yuen M, Friedman L, Orr W, Cockshott WP. Proliferative periosteal processes of phalanges: a unitary hypothesis. Skeletal Radiol. 1992;21:301–303. doi: 10.1007/BF00241768. [DOI] [PubMed] [Google Scholar]
- 26.Zambrano E, Nose V, Perez-Atayde AR, Gebhardt M, Hresko MT, Kleinman P, Richkind KE, Kozakewich HP. Distinct chromosomal rearrangements in subungal (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion) Am J Surg Pathol. 2004;28:1033–1039. doi: 10.1097/01.pas.0000126642.61690.d6. [DOI] [PubMed] [Google Scholar]