Abstract
Background
Osteoporosis, the underlying cause of most hip fractures, is underdiagnosed and undertreated. The 2008 Joint Commission report Improving and Measuring Osteoporosis Management showed only an average of 20% of patients with low-impact fracture are ever tested or treated for osteoporosis. We developed an integrated model utilizing hospitalists and orthopaedic surgeons to improve care of osteoporosis in patients with hip fracture.
Questions/purposes
Does our integrated model combining hospitalists and orthopaedic surgeons improve the frequency of evaluation for osteoporosis, screening for secondary causes, and patients’ education on osteoporosis?
Patients and Methods
Our Hospitalist-Orthopaedic Surgeon Integrated Model of Care was implemented in September 2009. We compared the rate of evaluation and treatment of osteoporosis in 140 patients admitted with fragility hip fracture at our institution before (70 patients) and after (70 patients) implementation of the care plan.
Results
Evaluation of patients for osteoporosis was higher in the postimplementation group compared to the preimplementation group (89% versus 24%). Screening of patients for secondary causes of osteoporosis was also improved in the postimplementation group (89% versus 0%), as was the proportion of patients who received education for osteoporosis management (89% versus 0%).
Conclusion
Our model of integrated care by hospitalists and orthopaedic surgeons resulted in improvement in the evaluation for osteoporosis, screening for secondary causes of osteoporosis, and education on osteoporosis management in patients with hip fracture at our institution. This may have important implications for treatment of these patients.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteoporosis is a major public health problem. The National Osteoporosis Foundation reported, by 2010, about 12 million people older than 50 years are expected to have osteoporosis and another 40 million to have low bone mass. By 2020, these numbers are expected to increase to 14 million cases of osteoporosis and more than 47 million cases of low bone mass [10]. Such an increase could cause the number of hip fractures to double or triple by 2040 [14].
The lifetime risk of an osteoporosis-related fracture after the age of 50 years is approximately 50% for women and 25% for men [16]. Fracture incidence is usually higher for whites and lower for other ethnic groups [8]. Fracture rates increase with age for all demographic groups [2–4, 9, 12]. Older individuals have much higher risk of fracture than younger persons with the same bone mineral density (BMD) because of other factors affecting the quality of bone, such as calcium and vitamin D levels, renal insufficiency, medications that may interfere in calcium and vitamin D metabolism, and a tendency to fall [4].
Osteoporotic fracture is often presumed to be secondary to postmenopausal or age-related osteoporosis. Precise data on secondary causes of osteoporosis in patients with fragility hip fracture are unknown, but secondary causes of osteoporosis cumulatively account for considerable morbidity and mortality associated with osteoporosis and osteoporotic fracture [8]. Patients with fragility hip fracture are at higher risk of a future fracture with its associated mortality, morbidity, and healthcare cost [13].
To improve care of patients with fragility hip fracture at our institution, we developed an integrated model of care utilizing hospitalists in conjunction with orthopaedic surgeons for management of osteoporosis.
The aims of our study were to (1) evaluate the impact of this integrated model of care on the frequency of evaluation for osteoporosis and (2) examine the effect this model of care has on the frequency of screening for secondary causes of osteoporosis and on the frequency of the administration of patients’ education for osteoporosis management.
Patients and Methods
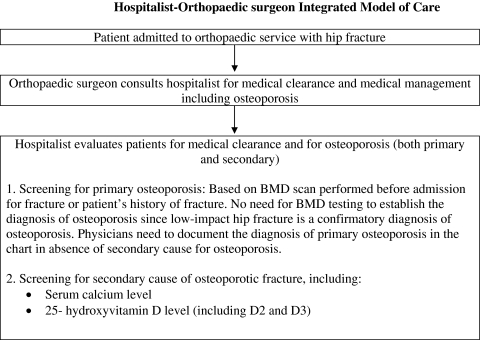
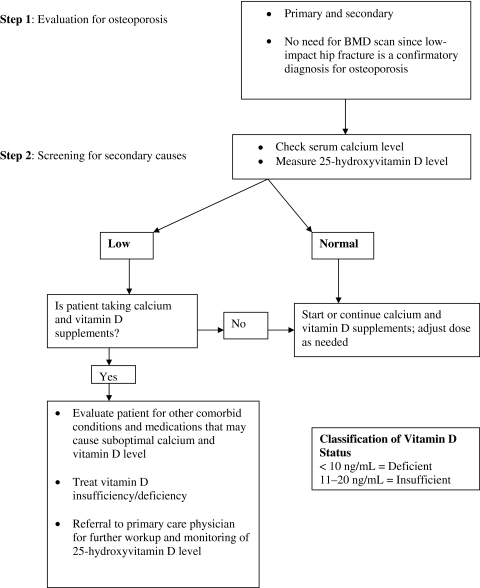
Our Hospitalist-Orthopaedic Surgeon Integrated Model of Care was implemented in September 2009. In this model, orthopaedic surgeons consulted the hospitalist for osteoporosis management for patients admitted with fragility hip fracture along with medical clearance for hip surgery (Fig. 1). The hospitalist evaluated the patient not only for medical clearance, but also for secondary causes of osteoporosis. We developed a standardized protocol for evidence-based diagnostic testing for secondary causes of osteoporosis (Fig. 2). For the diagnosis of primary osteoporosis, information on BMD testing was extracted from patients’ electronic medical records. Serum calcium and 25-hydroxyvitamin D levels were measured in all patients for evaluation of secondary causes, along with renal function (Fig. 2). In patients who had no secondary cause for osteoporosis, the diagnosis of primary osteoporosis was made even if BMD testing showed no evidence of osteoporosis or a prior BMD test had not been performed; in these patients, the low-impact hip fracture confirmed the diagnosis of osteoporosis.
Fig. 1.
A flowchart shows the algorithm for our Hospitalist-Orthopaedic Integrated Model of Care. BMD = bone mineral density.
Fig. 2.
A flowchart shows our protocol for screening for osteoporosis in patients with hip fracture in acute care settings. BMD = bone mineral density.
We studied 140 patients, 70 patients seen before implementation of our integrated model of care (preimplementation group) and 70 patients seen after implementation (postimplementation group). These were sequential cohorts with patients excluded from the study only if they had a diagnosis of cancer or were on systemic corticosteroids. For each patient, characteristics and comorbidity information were collected regarding age, sex, race, hypertension, coronary artery disease, atrial fibrillation, congestive heart failure, diabetes, thyroid disease, gastroesophageal reflux disease (GERD), colitis, stroke, chronic obstructive pulmonary disease, chronic kidney disease, and liver or kidney transplant (Table 1). Patient medications associated with low bone mass and for treatment of osteoporosis on admission were recorded (Table 2). We compared the patient characteristics, comorbid conditions, and medications between the preimplementation and postimplementation groups. Hypertension was more common (p < 0.001) in the preimplementation group (93% versus 63%), and GERD was more common (p = 0.042) in the postimplementation group (24% versus 10%). We believed these differences were not important relative to the end points of the study.
Table 1.
Patient characteristics and comorbid conditions
| Variable | Preimplementation (n = 70) | Postimplementation (n = 70) | p Value* |
|---|---|---|---|
| Age (years)† | 85 (53, 102) | 83 (51, 97) | 0.54 |
| Sex (male) | 15 (21%) | 17 (24%) | 0.84 |
| Race (white) | 70 (100%) | 70 (100%) | 1.00 |
| Hypertension | 65 (93%) | 44 (63%) | < 0.001 |
| Coronary artery disease | 12 (17%) | 19 (27%) | 0.22 |
| Atrial fibrillation | 5 (7%) | 13 (19%) | 0.075 |
| Congestive heart failure | 9 (13%) | 8 (11%) | 1.00 |
| Diabetes | 12 (17%) | 13 (19%) | 1.00 |
| Thyroid disease | 17 (24%) | 19 (27%) | 0.85 |
| Gastroesophageal reflux disease | 7 (10%) | 17 (24%) | 0.042 |
| Colitis | 2 (3%) | 6 (9%) | 0.27 |
| Stroke/transient ischemic attack | 3 (4%) | 5 (7%) | 0.72 |
| Chronic obstructive pulmonary disease | 7 (10%) | 7 (10%) | 1.00 |
| Chronic kidney disease | 0.12 | ||
| Normal | 42 (60%) | 51 (73%) | |
| Stage 1 | 23 (33%) | 18 (26%) | |
| Stage 2 | 5 (7%) | 1 (1%) | |
| Liver or kidney transplant | 1 (1%) | 1 (1%) | 1.00 |
| Creatinine (mg/dL)† | 0.8 (0.4, 4.1) | 0.7 (0.5, 3.9) | 0.65 |
| Bone mineral density scan | 21 (30%) | 28 (40%) | 0.29 |
* p Values result from a Wilcoxon rank sum test or Fisher’s exact test; †values are expressed as sample median, with minimum and maximum in parentheses; all other values are expressed as number of patients, with percentage in parentheses.
Table 2.
Patient medications and treatments
| Variable | Preimplementation (n = 70) | Postimplementation (n = 70) | p Value* |
|---|---|---|---|
| Medications associated with low bone mass | |||
| Proton pump inhibitors† | 17 (24%) | 17 (24%) | 1.00 |
| Thyroxin | 15 (21%) | 18 (26%) | 0.69 |
| Anticonvulsants‡ | 2 (3%) | 3 (4%) | 1.00 |
| Selective serotonin reuptake inhibitors§ | 12 (17%) | 6 (9%) | 0.21 |
| Furosemide/diuretics | 18 (26%) | 17 (24%) | 1.00 |
| Cyclosporin | 4 (6%) | 0 (0%) | 0.12 |
| Low-molecular-weight heparin | 1 (1%) | 1 (1%) | 1.00 |
| Tamoxifen | 1 (1%) | 2 (3%) | 1.00 |
| Gonadotropin-releasing hormone antagonist (luprolide) | 1 (1%) | 0 (0%) | 1.00 |
| Medications associated with treatment of osteoporosis | |||
| Bisphosphonate therapy | 16 (23%) | 16 (23%) | 1.00 |
| Calcium supplements | 18 (26%) | 27 (39%) | 0.15 |
| Vitamin D supplements | 21 (30%) | 30 (43%) | 0.16 |
Values are expressed as number of patients, with percentage in parentheses; * p Values result from Fisher’s exact test; †proton pump inhibitors: omeprazole, lansoprazole, esmoprazole, and pantoprazole; ‡anticonvulsants: phenobarbitone and phenytoin; §selective serotonin reuptake inhibitors: citalopram, escitalopram, fluoxetine, paroxetine, and sertraline.
The primary outcomes measured were evaluation and documentation of osteoporosis, serum calcium and 25-hydroxyvitamin D levels, and whether patients were given specific education regarding osteoporosis. This study was approved by our institution review board.
Numerical variables are expressed as the sample median, minimum, and maximum. Categorical variables are expressed as number and percentage. Patient characteristics, comorbid conditions, and medication information were compared between patients in the preimplementation and postimplementation groups using a Wilcoxon rank sum test (continuous and ordinal variables) or Fisher’s exact test (nominal variables). The primary end point of the study (screening for osteoporosis) and secondary end points (screening for secondary cases of osteoporosis, administration of education for osteoporosis management) were examined before and after implementation of our model of care using Fisher’s exact test. The proportion of patients who experienced each end point was determined separately in the preimplementation and postimplementation groups along with 95% confidence intervals. Statistical analyses were performed using S-PLUS® (Version 8.0.1; Insightful Corp, Seattle, WA).
Results
Implementation of an integrated model of care resulted in an improvement in the care of patients with hip fracture at our institution. Our primary end point, evaluation of patients with hip fracture for osteoporosis, improved (p < 0.001) from 24% in the preimplementation group to 89% in the postimplementation group.
Our secondary end points also showed improvement. Screening for secondary causes of osteoporosis improved (p < 0.001) from 0% in the preimplementation group to 89% in the postimplementation group. Similarly, the proportion of patients who received education for osteoporosis improved (p < 0.001) from 0% in the preimplementation group to 89% in the postimplementation group (Table 3). In the preimplementation group, the percentages of women (14 of 55, 25%) and men (three of 15, 20%) screened for osteoporosis were similar (p = 1.00). The percentages of women (47 of 53, 89%) and men (15 of 17, 88%) screened for osteoporosis in the postimplementation group were also similar (p = 1.00).
Table 3.
Comparison of osteoporosis screening outcomes before and after implementation
| Outcome | Preimplementation (n = 70) | Postimplementation (n = 70) | p Value* | ||
|---|---|---|---|---|---|
| Proportion (%) | 95% CI | Proportion (%) | 95% CI | ||
| Primary outcome | |||||
| Screening for osteoporosis | 17/70 (24%) | 15%–36% | 62/70 (89%) | 79%–95% | < 0.001 |
| Secondary outcomes | |||||
| Screening for secondary causes of osteoporosis | 0/70 (0%) | 0%–5% | 62/70 (89%) | 79%–95% | < 0.001 |
| Education for osteoporosis management | 0/70 (0%) | 0%–5% | 62/70 (89%) | 79%–95% | < 0.001 |
* p Values result from Fisher’s exact test; CI = confidence interval.
While not an end point of this study, we identified 33 of 62 (53%) patients with 25-hydroxyvitamin D levels of less than 20 ng/mL in our study, allowing these patients to be treated appropriately for vitamin D insufficiency. We also identified the majority of patients with low 25-hydroxyvitamin D levels also had a suboptimal serum calcium level (serum calcium < 8.9 mg/dL).
Discussion
Evaluation of patients with hip fracture for osteoporosis is often not performed. The 2008 Joint Commission report Improving and Measuring Osteoporosis Management showed, on average, only 20% of patients with fragility fracture were ever tested for or treated for osteoporosis [15]. We developed an integrated model utilizing hospitalists and orthopaedic surgeons to improve care of osteoporosis in patients with hip fracture. In this study, we answered the following questions: Does our integrated model combining hospitalists and orthopaedic surgeons improve the frequency of evaluation for osteoporosis, screening for secondary causes, and patients’ education on osteoporosis?
Our study has some limitations. First, this is a nonrandomized interventional trial. Second, we collected some information from patients’ electronic medical records and extracted several data points by chart review. Thus, the accuracy and completeness of data are dependent on the extent of documentation in the chart. Lastly, we did not include patients with cancer and patients who were on systemic corticosteroids in our study, limiting the applicability of our results to these populations.
Through a quality improvement project, we developed an integrated model utilizing hospitalists in conjunction with orthopaedic surgeons for the management of osteoporosis in patients with hip fracture in the acute care setting (Fig. 1). We developed a standardized protocol for evaluation of osteoporosis and for diagnostic testing for secondary causes (Fig. 2). Before this model, any evaluation of patients with hip fracture for osteoporosis was performed by the orthopaedic surgeon or the medical consultant who was involved in evaluating the patients for operative clearance.
Our integrated model of care resulted in an improvement in evaluation of patients for osteoporosis with hip fracture at our institution. In our preimplementation group, only 24% of patients with hip fracture were diagnosed with osteoporosis during their hospital admission for hip fracture. This percentage increased to 89% in the postimplementation group.
Osteoporosis, the underlying cause of most hip fractures, is often assumed by physicians to be primary osteoporosis. Further testing to determine whether secondary causes are present is usually not performed. Secondary causes of osteoporosis are numerous and are cumulatively associated with substantial mortality and morbidity [7]. In our preimplementation group, no patients were screened for secondary causes of osteoporosis. In our postimplementation group, this percentage increased to 89%, as did the proportion of patients receiving education regarding osteoporosis.
Identification of patients with low serum 25-hydroxyvitamin D allows such patients to be treated to optimize their vitamin D status. We identified 53% of patients with vitamin D deficiency/insufficiency (vitamin D level < 20 ng/mL) in our study, allowing these patients to be treated appropriately. Recently published literature shows lower serum 25-hydroxyvitamin D concentration increases the risk of hip fracture [5]. Other published literature also supports the association between vitamin D deficiency and the risk of fall and fracture [1, 5]. Therefore, identification of patients with low 25-hydroxyvitamin D level and optimization of 25-hydroxyvitamin D level is important for prevention of future fracture and fall in these high-risk patients.
Evaluation of serum calcium levels in patients with hip fracture is also critical for evaluation and treatment of osteoporosis. Of note, low 25-hydroxyvitamin D levels are commonly associated with low serum calcium levels [5]. Our results are consistent with this, as the majority of patients with low 25-hydroxyvitamin D levels also had a suboptimal serum calcium level. Inadequate calcium intake is generally considered a common cause of calcium deficiency. In our study, only 26% and 39% of patients in the preimplementation and postimplementation groups, respectively, were taking calcium supplements before hip fracture, supporting nutritional deficiency as a common cause. Furthermore, many comorbid conditions and medication may affect calcium and vitamin D levels [11]. Therefore, monitoring and correction of calcium and 25-hydroxyvitamin D levels are important to optimize care of osteoporosis. Optimization of calcium and 25-hydroxyvitamin D level is recommended before initiation of bisphosphonate therapy for treatment of osteoporosis [6].
Noncompliance with medication is a common concern in many patients, especially the elderly. Patients with poor understanding about their disease are more likely to be noncompliant with their treatment. To improve their compliance with treatment, we educated patients regarding osteoporosis and its treatment. As a first step, the hospitalists provided education to all patients. In addition, the nursing staff is intimately involved in this effort. They provide face-to-face education, provide patients with educational materials, and document these activities in the electronic medical record. The hospitalists have continued to remain involved in all aspects of medical care of these patients, including diagnosis, evaluation, and management of osteoporosis.
In conclusion, an integrated model of care between orthopaedic surgeons and hospitalists improves osteoporosis care provided to patients with hip fracture. Hospitalists specifically evaluated patients for primary and secondary osteoporosis and calcium and vitamin D deficiency and educated patients on the management of osteoporosis. This study may have important implications in the design of healthcare delivery models for these patients.
Footnotes
One or more of the authors (MIO) is a paid consultant for Zimmer, Inc (Warsaw, IN); a board member of the American Association of Hip and Knee Surgeons and the Association of Bone and Joint Surgeons; and a member of the Board of Trustees of Clinical Orthopaedics and Related Research.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 3.George A, Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18:2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP. Bone mass, bone loss, and osteoporosis prophylaxis. Ann Intern Med. 1998;128:313–314. doi: 10.7326/0003-4819-128-4-199802150-00014. [DOI] [PubMed] [Google Scholar]
- 5.Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008;149:751–760. doi: 10.7326/0003-4819-149-10-200811180-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc. 2009;84:632–637. doi: 10.4065/84.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kok C, Sambrook PN. Secondary osteoporosis in patients with an osteoporotic fracture. Best Pract Res Clin Rheumatol. 2009;23:769–779. doi: 10.1016/j.berh.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol. 1997;146:502–509. doi: 10.1093/oxfordjournals.aje.a009304. [DOI] [PubMed] [Google Scholar]
- 9.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 10.America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002. [Google Scholar]
- 11.Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]
- 12.Nelson DA, Jacobsen G, Barondess DA, Parfitt AM. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 13.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:99–111. doi: 10.7326/0003-4819-153-2-201007200-00262. [DOI] [PubMed] [Google Scholar]
- 14.Schneider EL, Guralnik JM. The aging of America: impact on health care costs. JAMA. 1990;263:2335–2340. doi: 10.1001/jama.263.17.2335. [DOI] [PubMed] [Google Scholar]
- 15.Improving and Measuring Osteoporosis Management. Oakbrook Terrace, IL: The Joint Commission; 2007. [Google Scholar]
- 16.US Department of Health and Human Services, Office of the Surgeon General. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004.