Abstract
To determine the effects of Manganese superoxide dismutase (MnSOD) plasmid liposome (PL) maternal radioprotection on fetal mice, timed pregnant female mice (E14 gestation) were irradiated to 3.0 Gy total body irradiation (TBI) dose, and the number, weight, and growth and development over 6 months after birth of newborn mice was quantitated compared to irradiated controls. Maternal MnSOD-PL treatment at E13 improved pup survival at birth (5.4 ± 0.9/litter compared to irradiated 3.0 Gy controls 4.9 ± 1.1. There was no statistically significant difference in newborn abnormalities, male to female ratio in newborn litters, or other evidence of teratogenesis in surviving newborn mice from MnSOD-PL treated compared to irradiated controls. However, E13 3Gy irradiated pups from gene therapy treated mothers showed a significant increase in both growth and overall survival over 6 months after birth (p = 0.0022).
To determine if transgene product crossed the placenta pregnant E13 mice were injected I.V. with hemagglutinin-epitope-tagged MnSOD (100 μgm plasmid in 100 μl liposomes), then 24 hours later fetal mice, placentas, and maternal tissues were removed and tested by both immunohistochemistry and RTPCR for transgene and product. There was no evidence of transgene or product in placenta or any fetal tissue while maternal liver was positive by both assays. The data provide evidence for fetal radioprotection by maternal MnSOD-PL gene therapy before irradiation which is mediated by an indirect bystander effect and is associated with a significant improvement in both survival at birth and growth and development of newborn mice.
Keywords: in utero irradiation, fetal mice, total body irradiation, MnSOD-PL gene therapy
Introduction
The teratogenetic effects of in utero ionizing irradiation on the fetus have been well documented (1-6). Irradiation-induced fetal death and teratogenesis have been demonstrated to increase with exposure at early time of gestation and are dependent upon both species and mouse strain (7-10). Growth retardation and developmental defects have been well documented in the progeny of irradiated mothers (8-11). In clinical radiotherapy, concern for radiation dose to the developing human fetus, in cases where maternal radiotherapy is indicated, include the critical need to calculate internal scatter dose as well as direct irradiation dose (12-14). Concern for irradiation teratogenic effects often enter discussions of termination of pregnancy if at early gestation, or delay in the initiation of radiotherapy to facilitate increased fetal growth, and early pre-term delivery (1, 2, 4). The effects of radiation protector drugs on fetal growth and development are a subject of significant investigation.
We have reported a significant radioprotective effect of systemic intravenous MnSOD-PL administration in adult male and female mice against both the LD 50/30 dose, 9.5 Gy, and a lower 1.0 Gy TBI dose (15). Furthermore, the increased survival after TBI of MnSOD-PL treated mice was not associated with a detectable increase in delayed irradiation effects including life shortening, carcinogenesis or neurological disorders (15).
In the present study, we determined the effect of total body irradiation on mid-gestation (E13) pregnant female C57BL/6HNsd mice with respect to irradiation effects on the fetus. The results demonstrate improvement in newborn mouse survival, as well as growth and development by maternal MnSOD-PL gene therapy. Furthermore, fetal radioprotection was independent of transgene expression in fetal tissues and was mediated through a remote effect of radioprotection.
Results
Total Body Irradiation Sensitivity of E14 Pregnant Female Mice
A dose response curve for irradiation effects on developing fetal mice was first carried out. Mid-gestation pregnant (E14) adult female C57BL/6HNsd mice were irradiated to 0, 1, 3, 5, or 7 Gy total body dose as described in the methods using 3 to 4 litters per dose group (Table 1). At 0 Gy 3 litters with 20 pups were born and all mice survived till weaning. At 1 Gy there were 3 litters with 19 pups alive and 1 dead. Four litters were born at 3 Gy with 9 pups alive and 20 pups dead. At 5 Gy there were 3 litters of 15 pups; all were dead by 48 hours after birth. At 7 Gy 3 litters were born with 19 pups, all were born dead, some in amniotic sacs, 1 fetus had no thorax or abdomen and 1 fetus was small and necrotic. Thus, the high 7 Gy irradiation dose resulted in fetal death in utero, with no live births and maternal mice receiving 5 Gy demonstrated stillborn pups. We, therefore, compared results with MnSOD-PL treated 3 Gy irradiated E14 maternal mice compared to 3 Gy irradiated controls to test the effects of radioprotective gene therapy.
Table 1. Maternal Total Body Irradiation Dose Dependent Fetal Mouse Death.
| Dose of Irradiation (Gy |
Number of Litters |
Number of Pups Born |
Number of Pups Born Dead |
Number of Pups Alive at Weaning |
|---|---|---|---|---|
| 0 | 3 | 20 | 0 | 20 |
| 1 | 3 | 19 | 1 | 18 (p = 0.4918) |
| 3 | 4 | 29 | 20 | 9 (p = 0.0084) |
| 5 | 3 | 15 | 15 | 0 (p = 0.0011) |
| 7 | 3 | 19 | 19 | 0 (p = 0.0011) |
C57BL/6NHsd E14 time pregnant mice were irradiated to doses of 0, 1, 3, 5 or 7 Gy whole body irradiation. The number of pups born alive or dead were determined. (p values indicate significant lethality compared to nonirradiated controls.)
MnSOD-PL Radioprotective Gene Therapy Improves Fetal Mouse Survival, Growth, and Development
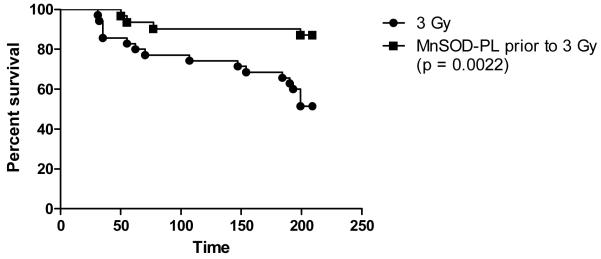
Groups of timed pregnant female mice (E13) next received (100 μg MnSOD plasmid in 100 μl liposomes) intravenously as published (15) and were irradiated 24 hours later along with noninjected control E14 pregnant mice to 0, or 3 Gy. The total number of pups was counted at birth, at day 2, and again at weaning, and the number of pups per litter was also scored (Table 2, Figure 1). Mice that were not irradiated showed no deleterious effect of MnSOD-PL treatment (Table 2, Fig. 1). The data showed a significant decrease in number of pups/litter from the pregnant mice irradiated to 3 Gy compared to control unirradiated mice. There was also a significant decrease in the weight of newborn pups from 3 Gy irradiated pregnant mice receiving MnSOD-PL compared to unirradiated mice (Table 2). There was a significant decrease in the weight at weaning of pups from 3 Gy irradiated E14 pregnant mice compared to unirradiated controls (p = .0024) (Table 2). At 6 months after birth, there was a significant decrease in weight in all pups from irradiated mothers (Table 1, Figure 1). However, pups from MnSOD-PL treated then 3 Gy irradiated E14 pregnant mice had a significant increase in survival over 220 days compared to those from 3 Gy irradiated controls (p = 0.0022) (Figure 2).
Table 2. MnSOD-PL Treatment of Pregnant Females Protects Fetal Mice from Total Body Irradiation.
| Group | Number of Pups/Litter at Weaning |
Weight at Weaning | Weight at 6 Months After Birth |
|---|---|---|---|
| 0 Gy | 7.7 ± 0.8 | 11.7 ± 0.3 | 25.8 ± 0.5 |
| 0 Gy ± MnSOD-PL | 6.7 ± 1.3 | 11.6 ± 0.2 | 30.4 ± 0.8 (p < 0.0001) |
| 3 Gy | 4.4 ± 1.1 (p = 0.0291) |
10.1 ± 0.5 (p = 0.0024) |
20.4 ± 0.7 (p < 0.0001) |
| 3 Gy ± MnSOD-PL | 5.2 ± 0.9 (p = 0.0482) |
10.6 ± 0.4 (p = 0.0202) |
20.8 ± 0.8 (p = 0.006) |
C57BL/6NHsd timed pregnant mice (E13) were injected intravenously with MnSOD-PL (100 μg plasmid DNA in 100 μl liposomes) then were irradiated 24 hrs later (E14) along with control (E14) mice to 0 or 3 Gy. At the time of weaning the number of mice per litter and weight were determined. Newborn mice were followed for 6 months then were weighed again. Statistical differences compare each irradiated group to unirradiated control mice 0 Gy.
Figure 1.
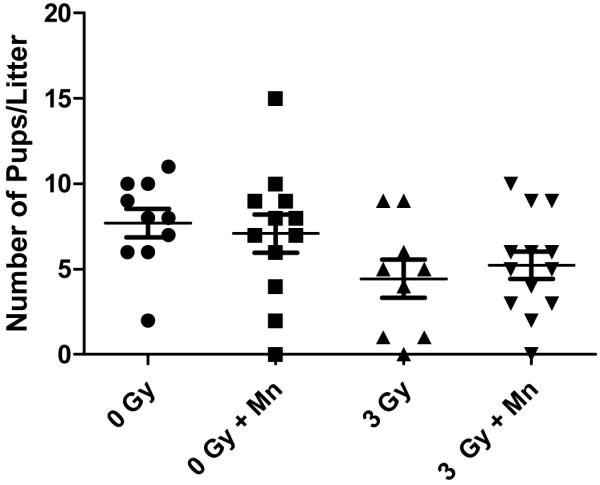
E13 pregnant mice (10-13/group) were injected with MnSOD-PL (MN) and irradiated on E14 along with non injection control mice to either 0 or 3 Gy. Pups were counted at the time of weaning and the number of pups per litter was determined. Pregnant female mice receiving 3 Gy TBI with or without MnSOD-PL had significantly decreased litter sizes compared to 0 Gy mice (p = 0.0291 or 0.0482). MnSOD-PL increased litter size in the 3Gy irradiated groups (p = 0.0799).
Figure 2.
Pups from MnSOD-PL + 3 Gy or 3 Gy pregnant mice were followed for over 200 days after birth. Pups from pregnant females that were treated with MnSOD-PL before 3 Gy TBI showed increased survival compared to pups from non injected 3 Gy irradiation pregnant mice (p = 0.0022).
MnSOD-PL Radioprotective Gene Product Does Not Detectably Cross the Placenta
To determine whether fetal radioprotective gene therapy was associated with detectable transfer of the MnSOD-Plasmid transgene or its product across the placenta, groups of E13 pregnant female mice received intravenous hemagglutinin (HA) epitope-tagged MnSOD-PL (21) and 24 hours later were euthanized according to IACUC institutional procedures. All tissues were Isolated and Tested Separately by Both In Situ Immunohistochemistry for Detection of Hemmaggluttinin epitope tag (16), and by RTPCR (17-21) for detection of MnSOD transgene transcript according to published methods. Individual fetal mice with associated umbilical cord and placenta were removed, and sagittal sections prepared for immunohistochemistry. RTPCR was carried out in each fetal sample as well as in maternal tissues.
Examination of ten individual fetal mice from three different HA-MnSOD-PL injected mothers demonstrated no detectable hemaggluttinin in placenta or in any fetal tissue (Figure 3). In contrast high levels of Ha-MnSOD-PL transgene product were detected in the maternal liver (Figure 3). RTPCR analysis of maternal and fetal tissues demonstrated a high level MnSOD-transgene in maternal liver (Figure 3). In Figure 3E, results are presented for a representative tissue from one of 10 per group including: maternal liver, liver, heart, lungs, spleen, intestine, kidney (lanes 2 – 8 respectively) and fetal whole body tissues (lanes 9-13). We also tested 5 separate placentas from each of 5 pups, removed one day after HA-MnSOD-PL injection of the mother (lanes 16-18). Examination of maternal bone marrow, skeletal muscle, and lung, showed no detectable MnSOD-transgene by RTPCR at the 24 hour time point. These results are consistent with previous publications of transgene clearance in adult mice over 24 – 48 hours after I.V. injection of MnSOD-PL and are associated with a decrease in detectable levels of MnSOD gene product over 48 – 72 hours (18).
Figure 3.
Immunostaining for Hemaggluttinin positive HA-MnSOD gene product in fetal and maternal tissues and RTPCR showing maternal liver positive for transgene on mRNA. A) Whole fetus (E13) negative tissues – 2 x; B) Fetal liver (E13) – negative for HA-MnSOD (20 x); C) HA-MnSOD-PL injected maternal liver – (20 x) (arrows show HA-MnSOD positive areas); D) Control uninjected maternal liver – (20 x), and E) RTPCR of maternal and fetal tissues for detection of human MnSOD transgene mRNA. Tissues were prepared as described in the Materials and Methods Section. Maternal liver positive (black arrows) all other tissues are negative. E13 pregnant females were injected intravenously with Epitope-Hemaggluttinin-Tagged MnSOD-PL (100 μg in 100 μl) and sacrificed 24 hr later. Fetal mice, placenta, and various tissues from the mother were removed and frozen in liquid nitrogen or in OCT. Samples frozen in OCT were sectioned, stained for hemaggluttinin and examined microscopically. No staining was detected in the fetal mice (n = 10) or placenta (n = 10) (2 x); or in fetal mouse liver (20 x), or control uninjected maternal liver (D) (20 x). In contrast Hemaggluttinin positive areas were detected in Ha-MnSOD treated maternal liver (C) (20 x) (red color, black arrows). RTPCR was performed on RNA extracted from maternal mouse tissues and fetal mice using primers specific for the human MnSOD transgene. Only maternal liver showed detectable expression of human MnSOD transgene.
Discussion
The present data demonstrate that intravenous MnSOD-PL administration to pregnant female mice at mid-gestations results in significant radioprotection of fetal mice that are subsequently exposed to 3 Gy total body irradiation. Previous studies have documented the radioprotective capacity of MnSOD-PL transgene product in adult male and female mice (15, 16, 18-23). In prior studies, both male and female mice receiving MnSOD-PL 24 hours prior to TBI demonstrated significant improvement in survival from the 9.5 Gy, LD 50/30 dose or a lower 1.0 Gy dose of total body irradiation (15). The known late irradiation induced life shortening in survival after TBI was not exacerbated in either male or female mice and male mice showed a surprising improvement in late irradiation induced life shortening (15). There was no increase in detectable tumors, histopathologic evidence of neurodegenerative disease in the increased number of survivors in either 9.5 Gy or 1.0 Gy, groups that received MnSOD-PL prior to irradiation (15).
The method of MnSOD-PL administration is indeed advantageous over other treatment modalities in that transgene product is produced continuously in the recipient animal for 48 – 72 hours after one transgene administration (30-38). For radioprotection, the MnSOD enzyme is not only required at the time of irradiation, but also for 24 – 48 hours after irradiation to provide continuous protection not only from the first wave of apoptosis of normal cells by irradiation, but by the secondary and tertiary waves that are induced by cytokine release, including TGFβ, TNFα, and IL-1, from irradiated tissues (30). The timing of administration of transgene, 24 hours prior to irradiation allows for transgene incorporation, mRNA transcription, and message translation (32). Continual production of transgene product in vivo for 48 to 72 hours after initial expression is the known result of a single administration (30, 37). The transgene product expression, therefore, is protracted and the advantage of gene therapy is to continually provide a “depot” of MnSOD-protein in cells and tissues where targeting the mitochondria can occur continually. In contrast, administration of MnSOD protein has been shown to be ineffective even in multiple administrations (36).
In the present studies, pregnant female mice were treated to a dose of 3 Gy TBI that was associated with early post partum newborn death (30-38). Pregnant female mice receiving 3.0 Gy demonstrated a significant decrease in the number of live births, and newborn mice showed decreased growth and development compared to non-irradiated controls. Irradiation induced decrease in growth and development of fetal mice has been documented previously (8-10). In the present studies, MnSOD-PL administration 24 hours prior to 3.0 Gy irradiation did not significantly change the number of pups/litter or weight at weaning. There was also no change in weight at six months after birth. However, there was an increase in overall survival of pups from pregnant mice that received MnSOD-PL 24 hour before 3 Gy total body irradiation compared to pups from pregnant mice irradiated to 3 Gy only. These results are consistent with a radioprotective effect of MnSOD-PL gene therapy on the mid gestation mouse fetus (16, 18-23). The mechanism of irradiated newborn mouse defects may be through delayed growth of the newborn mice, placental defects – maternal or fetal, or both mechanisms.
The teratogenic effects of ionizing irradiation are well documented (1-6). In rodent models, as well as larger mammals, total body irradiation of pregnant females has been demonstrated to result in fetal death, growth abnormalities, and multi-generational transmission of genetic mutations as well as chromosome abnormalities. In murine model systems, teratogenetic abnormalities are highest, early in the developmental process, and decreases with gestational age of the fetus (24). We saw no effect of 3 Gy or MnSOD-PL on teratogenesis in E13 mice. Studies on earlier gestation fetal mice may be required to detect such differences.
The fetal protective effects of MnSOD-PL were not associated with detectable MnSOD gene product in the fetal tissues by measure of HA-MnSOD, epitope-tagged gene product, or transgene transcript in fetal tissues as measured by RTPCR. The results indicate that the fetal radioprotective effect was mediated by a remote effect of radioprotection. Ionizing irradiation increases inflammatory cytokines in irradiated tissues, as well as levels of inducible nitric oxide synthase (20, 23, 26-27). These small molecules that are transmitted across the placenta from maternal tissues may have been reduced in MnSOD-PL treated mice, and this reduced level may have decreased secondary cytokine elevations in the fetus. However, since fetal tissues were irradiated and the same inflammatory proteins would have been expected to be elevated as part of the inflammatory response to irradiation in utero it is likely that the transmission of a protective mediator(s) was delivered across the placenta as has been demonstrated in other systems (25). Further studies will be required to document the mechanism of indirect protection of the fetus by maternal MnSOD-PL.
The present data may be useful in clinical radiotherapy where normal tissue radioprotection by localized MnSOD-PL gene therapy is being evaluated in clinical trials with respect to safe use in pregnant women (17). The development of radiation counter measures particularly those applicable to a large populations, also requires consideration of the effect of a radiation protector or radiation mitigator drug on the developing fetus (28). The present evidence suggests that MnSOD-PL administration to pregnant females should not be deleterious to the fetus and may be indirectly protective by a beneficial remote effect.
Materials and Methods
Pregnant Mice
Timed pregnant E13 female C57BL/6HNsd mice were derived according to published methods (29). Three 6 – 8 week old mice were placed in the cage with 1 comparably aged male mouse and females observed daily for detection of the vaginal plug according to published methods (29). Once vaginal plugs were detected, females were placed 1 per cage and held for 13 days. The 13 day mid-gestational time point was chosen as teratogenic effects of irradiation in this gestational period have been well documented. Mice received intravenously MnSOD-PL (100 μg plasmid DNA) on E-13. Subgroups of mice were irradiated on E14. All pups were followed for liter size, pup weight at weaning and six months after birth, and pup survival for 200 days after birth.
Total Body Irradiation
Mice were irradiated to varying doses using a Cesium Gamma cell irradiator dose rate 70 Gy/minute (15). Mice were irradiated according to published procedures (15).
MnSOD-PL Radioprotective Gene Therapy
The method for preparation of MnSOD-Plasmid Liposomes has been published previously. Briefly, the small pNVL3-MnSOD-plasmid was prepared according to published methods, and mixed with lipofectmine liposomes according to published methods (20). Pregnant female mice received 100 microgram of plasmid at 100 microliter of liposome intravenously 24 hours prior to total body irradiation.
Epitope-Hemaggluttinin-Tagged MnSOD-Plasmid Liposomes
Epitope-tagged MnSOD construct has been described previously (16). Cells transfected with HA-MnSOD-PL have been demonstrated to produce epitope labeled MnSOD which has biological radioprotective capacity identical to that of native plasmid liposomes, but has detectable hemaggluttinin by in situ immunohistochemistry scoring.
Tissue sections from explanted embryos, placenta, and adult female mouse organs, were prepared for immunohistochemical staining according to published methods. Five sections from each adult mouse organ and ten sections from each explanted fetus and attached umbilical cord and placenta were analyzed for percent of hemaggluttinin positive cells reporting at least 1000 cells per slide.
Reverse Transcriptase Polymerase Chain Reaction
The methods for RTPCR, quantitating MnSOD transgene transcript has been published previously (17). A DNA ladder utilizing MnSOD-plasmid as cDNA was used as positive control. Under these conditions, a lowest concentration of blank plasmid was detectable by adding positive control plasmid cDNA to tissue preparation of control mouse liver. Organs from adult female tissues, as well as fetal tissues were prepared for RTPCR according to published methods.
Statistics
Student T-test was used to compare different treatment groups as previously described (20). A Log-rank (Mantel-Cox) test was performed to compare survival of mice for 200 days after birth (15).
Acknowledgements
This project was supported by award number U19A1068021by the National Institute of Allergy and Infectious Diseases.
Footnotes
Conflicts of Interest: All authors have no conflicts of interest to report.
References
- 1.Naumburg E, Bellocco R, Cnattingius S, Hall P, Boice JD, Jr., Ekbom A. Intrauterine exposure to diagnostic x rays and risk of childhood leukemia subtypes. Radiat Res. 2001;156:718–723. doi: 10.1667/0033-7587(2001)156[0718:ietdxr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima E. Relationship of five anthropometric measurements at age 18 to radiation dose among atomic bomb survivors exposed in utero. Radiat Res. 1994;138:121–126. [PubMed] [Google Scholar]
- 3.Benjamin A, Lee AC, Angleton GM, Saunders WJ, Keefe TJ, Mallinckrodt CH. Mortality in beagles irradiated during prenatal and postnatal development. I. Contribution of non-neoplastic diseases. Radiat Res. 1998;150:316–329. [PubMed] [Google Scholar]
- 4.Ohtaki K, Kodama Y, Nakano M, Itoh M, Awa AA, Cologne J, et al. Human fetuses do not register chromosome damage inflicted by radiation exposure in lymphoid precursor cells except for a small but significant effect at low doses. Radiat Res. 2004;161:373–379. doi: 10.1667/3147. [DOI] [PubMed] [Google Scholar]
- 5.Nakano M, Kodama Y, Ohtaki K, Nakashima E, Niwa O, Toyoshima M, et al. Chromosome aberrations do not persist in the lymphocytes or bone marrow cells of mice irradiated in utero or soon after birth. Radiat Res. 2007;167:693–702. doi: 10.1667/RR0718.1. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima E, Carter RL, Neriishi K, Tanaka S, Funamoto S. Height reduction among prenatally exposed atomic-bomb survivors: a longitudinal study of growth. Health Phys. 1995;68:766–772. doi: 10.1097/00004032-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Anderson LM. Predictive values of traditional animal bioassay studies for human perinatal carcinogenesis risk determination. Toxicology and Applied Pharmacology. 2004;199:162–174. doi: 10.1016/j.taap.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Devi PU, Satyamitra M. Tracing radiation induced genomic instability in vivo in the haemopoietic cells from fetus to adult mouse. British J of Radiology. 2005;78:928–933. doi: 10.1259/bjr/18119329. [DOI] [PubMed] [Google Scholar]
- 9.Hossain M, Devi PU, Bisht KS. Effect of prenatal gamma irradiation during the late fetal period on the postnatal development of the mouse. Teratology. 1999;59:133–138. doi: 10.1002/(SICI)1096-9926(199903)59:3<133::AID-TERA4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Devi PU, Hossain M. Effect of early fetal irradiation on the postnatal development of the mouse. Teratology. 2001;64:45–50. doi: 10.1002/tera.1046. [DOI] [PubMed] [Google Scholar]
- 11.Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidente in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 2008;100:428–436. doi: 10.1093/jnci/djn045. [DOI] [PubMed] [Google Scholar]
- 12.Merianos DJ, Tiblad E, Santote MT, Todorow CA, Laje P, Endo M, Zoltick PW, Flake AW. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest. 2009;119:2590–2598. doi: 10.1172/JCI38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatsukawa Y, Nakashima E, Yamada M, Funamoto S, Hida A, Akahoshi M, et al. Cardiovascular disease risk among atomic bomb survivors exposed in utero, 1978-2003. Radiat Res. 2008;170:269–274. doi: 10.1667/RR1434.1. [DOI] [PubMed] [Google Scholar]
- 15.Epperly MW, Smith T, Wang H, Schlesselman J, Franicola D, Greenberger JS. Modulation of total body irradiation induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Radiat Res. 2008;170:437–444. doi: 10.1667/rr1286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperly MW, Guo HL, Jefferson M, Wong S, Gretton J, Bernarding M, et al. Cell phenotype specific duration of expression of epitope-tagged HA-MnSOD in cells of the murine lung following intratracheal plasmid liposome gene therapy. Gene Ther. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 17.Tarhini AA, Belani C, Luketich JD, Ramalingam SS, Argiris A, Gooding W, et al. A phase I study of concurrent chemotherapy (Paclitaxel and Carboplatin) and thoracic radiotherapy with swallowed manganese superoxide dismutase (MnSOD) plasmid liposome (PL) protection in patients with locally advanced stage III non-small cell lung cancer. Human Gene Therapy. doi: 10.1089/hum.2010.078. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, et al. Radioprotective gene therapy. Current Gene Therapy. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 19.Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-plasmid liposome (SOD2-PL) intratracheal gene therapy. Radiat Res. 2000;154:365–374. doi: 10.1667/0033-7587(2000)154[0365:dprrom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Epperly MW, Gretton JA, DeFilippi SJ, Sikora CA, Liggitt D, Koe G, et al. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Epperly MW, Guo HL, Bernarding M, Gretton J, Jefferson M, Greenberger JS. Delayed intratracheal injection of manganese superoxide dismutase (MnSOD)-plasmid/liposomes provides suboptimal protection against irradiation-induced pulmonary injury compared to treatment before irradiation. Gene Ther Mol Biol. 2003;7:61–68. [Google Scholar]
- 22.Guo HL, Seixas-Silva JA, Epperly MW, Gretton JE, Shin DM, Greenberger JS. Prevention of irradiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Res. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Guo HL, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, et al. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic lewis lung carcinomas. In Vivo. 2003;17:13–22. [PubMed] [Google Scholar]
- 24.Hall EJ, Gacca A. Radiobiology for the Radiologist. 6th Edition. Lippincott, William & Wilkins; New York: 2008. New York. [Google Scholar]
- 25.Streffer C. Bystander effects, adaptive response and genomic instability induced by prenatal irradiation. Mutat Res. 2004;568:79–87. doi: 10.1016/j.mrfmmm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Greenberger JS. Perspectives/opinion “Radioprotection”. In Vivo. 2009;23:323–336. [PMC free article] [PubMed] [Google Scholar]
- 27.Derradji H, Bekaert S, De Meyer T, Jacques P, Abou-El-Ardat K, Garrid M, et al. Ionizing radiation-induced gene modulations, cytokine content changes and telomere shortening in mouse fetuses exhibiting forelimb defects. Dev Biol. 2008;322:302–313. doi: 10.1016/j.ydbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan MS, Stone B, Rwigema JC, Salimi U, Epperly MW, Goff J, et al. Total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (NOS-1−/−) mice. Rad Res. in press. [Google Scholar]
- 29.Mader SL, Libal NL, Pritchett-Corning K, Yang R, Murphy SJ. Refining timed pregnancies in two strains of genetically engineered mice. Lab Animal. 2009;38(9):305–310. doi: 10.1038/laban0909-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epperly MW, Travis EL, Sikora C, Greenberger JS. Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-α, and TGF-β correlates with delay of organizing alveolitis/fibrosis. Biology of Blood Marrow Transplant. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 31.Epperly MW, Bray JA, Esocobar P, Bigbee WL, Watkins S, Greenberger JS. Overexpression of the human MnSOD transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat Onco. Inves. Clinical & Basic Res. 1999;7:331–342. doi: 10.1002/(SICI)1520-6823(1999)7:6<331::AID-ROI3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Stickle RL, Epperly MW, Klein E, Bray JA, Greenberger JS. Prevention of irradiation-induced esophagitis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Oncol Invest Clinical & Basic Res. 1999;7(6):204–217. doi: 10.1002/(SICI)1520-6823(1999)7:4<204::AID-ROI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend K, Greenberger JS. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 2000;7(12):1011–1018. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- 34.Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese Superoxide dismutase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiat Res. 2000;154(4):365–374. doi: 10.1667/0033-7587(2000)154[0365:dprrom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Epperly MW, Travis EL, Whitsett JA, Epstein CJ, Greenberger JS. Overexpression of manganese superoxide dismutase (MnSOD) in whole lung or alveolar type II (AT-II) cells of MnSOD transgenic mice does not provide intrinsic lung irradiation protection. Radiat Oncol Invest. 2001;96:11–21. doi: 10.1002/1097-0215(20010220)96:1<11::aid-ijc2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 36.Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, et al. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated irradiation. Int J Cancer (Radiat Oncol Invest) 2001;96(4):221–233. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- 37.Epperly MW, Sikora CA, Defilippi SJ, Gretton JE, Bar-Sagi D, Carlos T, et al. Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by MnSOD-PL gene therapy. Biol Blood Bone Marrow Transplant. 2002;8(4):175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 38.Epperly MW, Sikora C, Defilippi S, Gretton J, Zhan Q, Kufe DW, et al. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and Jnk1 translocation. Radiat Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]