Abstract
Background
Meat intake has been positively associated with incidence and mortality of chronic diseases, including diabetes, heart disease, and several different cancers, in observational studies using self-report methods of dietary assessment; however, these dietary assessment methods are subject to measurement error. One method to circumvent such errors is the use of biomarkers of dietary intake, but currently there are no accepted biomarkers for meat intake.
Methods
We investigated four analytes (creatinine, taurine, 1-methylhistidine, and 3-methylhistidine) specifically found in meat and excreted in urine. Twenty-four hour urine samples were collected from 17 individuals on controlled diets containing varying levels of meats: vegetarian (0 g/day), low red meat (60 g/day), medium red meat (120g/day), and high red meat (420 g/day), as part of two randomized cross-over feeding studies.
Results
When compared to the low red meat diet or the vegetarian diet, the urinary levels of all four analytes were significantly higher in urine samples collected after 15 days of a high red meat diet (P<0.0001). Only urinary 1-methylhistidine and 3-methylhistidine were statistically significantly different for every diet type, increasing as the amount of meat in the diet increased (P<0.01 for 1-methylhistidine and P<0.05 for 3-methylhistidine). Furthermore, urinary excretion of 1-methylhistidine and 3-methylhistidine elevated with increasing meat intake in every individual.
Conclusion
Urinary 1-methylhistidine and 3-methylhistidine may be good biomarkers of meat intake.
Impact
To determine the public health impact of red meat on cancer risk, biomarkers are crucial to estimate true intake; these potential biomarkers should be further investigated in free-living populations.
Keywords: Biomarker, meat, methylhistidine, urine, taurine, creatinine
Introduction
Meat intake has been associated with an elevated risk of several chronic diseases, including diabetes (1), heart disease (2), and cancers of the colorectum, stomach, esophagus, prostate, breast, and pancreas (3), as well as all-cause mortality (4); although there are some inconsistencies in the data. Observational epidemiologic studies, in which diet is usually assessed by self-administered food frequency questionnaires, are associated with measurement error; therefore, the public health impact of a diet high in red and processed meat may have been underestimated. Currently, there are no available biomarkers of meat to provide a more accurate measure of intake that is independent of the subject’s memory and ability to describe foods consumed. We identified four analytes that are all present in meat and excreted in urine: creatinine (5) and 3-methyhistidine (6) are both released upon muscle or protein breakdown; and taurine (7) and 1-methylhistidine (8) are mainly from foods of animal origin and meat, respectively. In the present study, we investigated these four analytes as potential biomarkers of meat intake.
Materials and Methods
We analyzed 24-hour urine samples from two randomized, cross-over, dietary studies; detailed methodology for these feeding studies have previously been described (9). In brief, healthy male volunteers, age range of 24–74 years, were recruited to live in a metabolic suite where all food and drinks were provided. Each volunteer signed a consent form after receiving a detailed explanation of the study protocol.
In study I, a two-way cross-over study, nine volunteers were randomized to the following two diets: a 60 g/day red meat and a 120 g/day red meat diet; the protein contents of these diets were 63 and 77 g/day, respectively. In study II, a three-way cross-over study, eight volunteers were randomized to each of three diets: a 60 g/day red meat; 420 g/day red meat; and a high protein vegetarian diet (0 g/day meat) where meat was substituted with egg, peanuts, low fat cheese, kidney beans and green lentils; the protein contents of these three diets were 65, 149 and 143 g/day, respectively. Within both studies, each diet was consumed for 15 consecutive days with no washout phase between diets. All dietary components were weighed to the nearest gram per day. Furthermore, with the exception of the meat component, all diets were comprised of similar foods, including cereal, bread, milk, fruits and vegetables, and were isocaloric and matched for fat and fiber content and adjusted for the energy needs of each individual with extra bread, low fat margarine and marmalade.
Consecutive 24-hour urine collections were made on the final three days of each 15-day dietary period. Boric acid (1g/L) was added to the urine samples as a preservative and then the samples were aliquoted into 28ml tubes and frozen at −20°C. Para-amino-benzoic acid (PABA) tablets were taken by the volunteers and a colorimetric method was used to determine the completeness of collection of the 24-hour urine samples (10). Urinary nitrogen was measured in all samples by Kjeldahl analysis to determine the level of dietary compliance (11). Urine samples collected on day 15 of each dietary period were analyzed for creatinine, taurine, 1-methylhistidine, and 3-methylhistidine as potential biomarkers of meat intake. Blinded quality control samples from two different individuals on free-living diets were inserted into the batches at a level of 10%.
Creatinine was measured by an Olympus AU600 automated chemistry instrument (Olympus America, Inc. Melville, NY) at Quest Diagnostics, Baltimore, MD; the method involved a kinetic modification of the Jaffe procedure, in which creatinine reacts with picric acid at alkaline pH to form a yellow-orange complex, the rate of change in absorbance (measured at 520/800nm) is proportional to the creatinine concentration in the sample (12). Taurine, 1-methylhistidine and 3-methylhistidine were measured by an ion exchange chromatography technique involving protein precipitation with sulfosalicylic acid, separation of the amino acids on a cation-exchange column under acidic conditions, followed by treatment with ninhydrin, which reacts with the primary or secondary amino groups to form colored derivatives that are detected colorimetrically at 440/570 nm (Quest Diagnostics, San Juan Capistrano, CA).
We used mixed linear regression (PROC MIXED in SAS®, SAS institute, Cary, NC.), a type of random effects model that accounts for within-subject correlation to determine the effect of diet type on each of the potential urine biomarkers. First, we combined the data from both studies to determine whether there was an overall effect of diet on the urinary analytes, while controlling for study. Second, we compared the means across diet types within each study to determine where the differences occurred, while adjusting for multiple comparisons using the Tukey method. Two-tailed probability results ≤ 0.05 significance level were regarded as statistically significant.
Results
Of a total of 42 urine samples (9 volunteers on 2 different diets and 8 volunteers on 3 different diets), all urine samples were considered complete by the PABA-check method, in that ≥85% was recovered in the 24-hour urine collection (10). Dietary compliance, estimated by the correlation between dietary and urinary nitrogen, was also high (r = 0.99 and 0.71 for study I and II, respectively).
An overall effect of diet on the urinary excretion of all four analytes was evident (all Pvalues <0.0001), with each analyte increasing with the dose of red meat in the diet in a dose-dependent manner (all Ptrends <0.0001, Table 1). Pair-wise comparisons within study I revealed significantly higher urinary excretion of 1-methylhistidine (P <0.0001), 3-methylhistidine (P=0.0003), and creatinine, (P =0.02) when individuals consumed the 120 g compared to the 60 g/day red meat diet; taurine excretion, however, was not different when these two diets were compared (P=0.24). Pair-wise comparisons within study II revealed that urinary excretion of all four analytes was significantly higher when individuals consumed the 420 g/day red meat diet compared to the vegetarian or the 60 g/day red meat diet (P <0.0001, all comparisons). Furthermore, during the 60 g/day red meat diet, urinary excretion of 1-methylhistidine (P=0.009) and 3-methylhistidine (P =0.03) was increased compared with the vegetarian diet, but excretion of taurine (P=0.95) and creatinine (P=0.88) was not statistically different between these two diet types.
Table 1.
Distribution of urinary analytes for each diet type
| Urinary analyte | Vegetarian n = 8 (all from study II) |
60 g/d red meat n = 17 (9 from study I and 8 from study II) |
120 g/d red meat n = 9 (all from study I) |
420 g/d red meat n = 8 (all from study II) |
Ptrend* |
|---|---|---|---|---|---|
| Creatinine (mmol/day) | |||||
| Mean (standard deviation) | 14.5 (1.6) | 14.2 (2.2) | 15.9 (1.0) | 21.0 (3.8) | < 0.0001 |
| Median (inter-quartile range) | 14.4 (13.2–16.0) | 14.5 (13.0–16.1) | 16.0 (15.3–16.5) | 19.8 (18.7–21.6 | |
| Taurine (µmol/day) | |||||
| Mean (standard deviation) | 543.1 (235.6) | 544.8 (339.0) | 671.9 (383.2) | 1675.5 (621.4) | < 0.0001 |
| Median (inter-quartile range) | 517.3 (389.0–740.8) | 482.9 (346.0–719.0) | 603.8 (469.9–729.3) | 1695.1 (1187.6–1887.8) | |
| 1-methylhistidine (µmol/day) | |||||
| Mean (standard deviation) | 37.8 (11.5) | 91.3 (13.3) | 166.1 (15.0) | 457.2 (56.2) | < 0.0001 |
| Median (inter-quartile range) | 34.0 (31.2–40.5) | 95.1 (82.6–98.5) | 166.7 (155.0–172.7) | 449.2 (425.6–473.7) | |
| 3-methylhistidine (µmol/day) | |||||
| Mean (standard deviation) | 184.1 (21.8) | 238.0 (40.0) | 306.4 (21.3) | 552.8 (86.9) | < 0.0001 |
| Median (inter-quartile range) | 184.7 (166.6–199.2) | 248.8 (217.2–262.6) | 305.4 (295.5–320.8) | 529.2 (497.8–576.4) |
Ptrend test
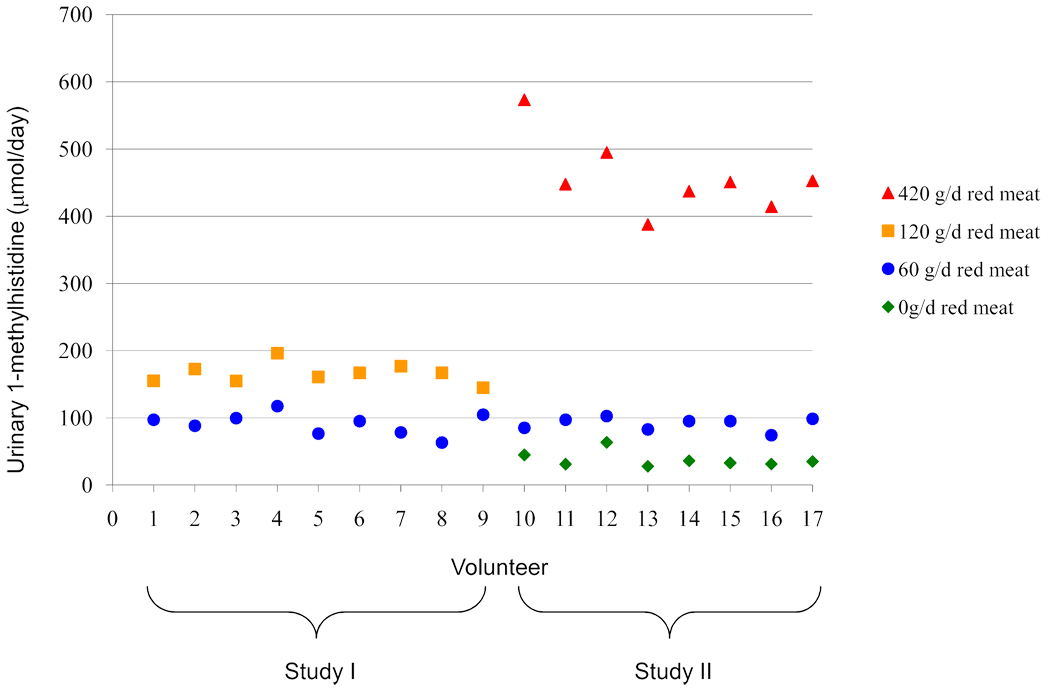
Although there was individual variation, urinary excretion of 1-methylhistidine and 3-methylhistidine was lowest on the vegetarian diet and increased in every individual with increasing doses of red meat (Figure 1 shows individual data for 1-methylhistidine). All individuals were assigned to a 60 g/day red meat as part of study I and study II, and the urinary excretion of 1-methylhistidine in all 17 of these people was at a similar level when they were consuming this diet (Figure 1). When both studies were combined, the median urinary excretion of 1-methylhistidine and 3-methylhistidine during the 60 g/day red meat diet was 95.1 µmol/day (inter-quartile range, IQR: 82.6–98.5) and 248.8 µmol/day (IQR: 217.2–262.6), respectively (Table 1). Analyzing this data by study, revealed the median excretion of 1-methylhistidine was consistent between study I (95.1 µmol/day, IQR: 78.4–99.7) and study II (95.1 µmol/day, IQR: 83.9–97.8), as was 3-methylhistidine between study I (230.4 µmol/day, IQR: 217.2–258.0) and study II (254.1 µmol/day, IQR: 228.3–279.1).
Figure 1.
Urinary 1-methylhistidine for each volunteer according to diet type
Discussion
All four urinary analytes examined were able to distinguish between the diets with the extreme levels of meat, but only urinary 1-methylhistidine and 3-methylhistidine was statistically different for each dose of meat in the diet. Urinary 1-methylhistidine excretion in our study increased with increasing dietary red meat, these differences were statistically significant for each of the four diet types. The majority of 1-methylhistidine excreted in the urine is from dietary sources, particularly from meat (8), and since 1-methylhistidine is not a marker of muscle catabolism and there is minimal endogenous formation (13), this could be a good potential biomarker of meat intake. Measuring 1-methylhistidine in urine was sufficient to predict vegetarian status in a study of 126 individuals (14). Although a study of five individuals found a strong linear association between intake of chicken, pork or plaice and urinary excretion of both 1-methylhistidine and 3-methylhistidine (8), a study comparing meat eaters to lactovegetarians reported a more pronounced effect on urinary 1-methylhistidine than 3-methylhistidine (15).
Our study noted significantly elevated urinary excretion of 3-methylhistidine with increasing red meat consumption; however, there was considerable variation in 3-methylhistidine excretion between individuals on the same diet, perhaps because it also reflects muscle catabolism and muscle mass (16). Meat contains 3-methylhistidine in a soluble form as well as bound to the muscle proteins actin and myosin (6), breakdown of these proteins consequently results in urinary excretion of 3-methylhistidine (17, 18). Several studies have reported an increase in urinary 3-methylhistidine after meat consumption (6, 15, 16, 19). A small study found an approximate quantitative relationship between ingested 3-methylhistidine and that excreted in the urine in five subjects (19). Furthermore, 3-methylhistidine has been correlated with meat intake reported by dietary recall one year previously (r = 0.77) in free-living individuals (20).
Creatinine is a muscle breakdown product and previous studies have reported higher urine creatinine levels in individuals who consume a diet high in red meat (5). Our study noted statistically significant differences for creatinine only between the extreme doses of meat. Furthermore, there are caveats of creatinine as a biomarker of meat intake, which include the associations of urinary creatinine with an individual’s muscle mass (21) and renal function, since the kidney filters creatinine.
Even though a small amount may be synthesized in humans (22, 23), the main source of taurine is from ingestion of foods of animal origin (7), this is reflected in the low levels of taurine excretion in vegans (22). However, vegans can excrete more taurine than they consume, which suggests that endogenous taurine synthesis can result in urinary excretion even when dietary intake is low (22). A previous study demonstrated that increasing taurine intake leads to an equivalent rise in urinary taurine excretion (24). Although we did observe higher urinary taurine excretion when individuals were consuming the high red meat diet, compared to the low red meat diet, excretion during the vegetarian diet was not lower than excretion during the low red meat diet. Furthermore, the consumption of specialized supplements and energy drinks, which sometimes contain taurine, could preclude its potential as a good biomarker of meat intake in free-living individuals.
Based on our findings, both 1-methylhistidine and 3-methylhistidine may be potentially good biomarkers of meat intake; although 1-methylhistidine may be superior since it is independent of muscle mass and catabolism. We observed a dose-dependent effect of red meat in the diet on urinary excretion of both 1-methylhistidine and 3-methylhistidine. In addition to a targeted biomarker approach, some studies have reported data from metabolomics analyses in order to identify potential biomarkers of meat intake; however, this approach generally identifies patterns (or profiles) that distinguish between diet types rather than excretion levels of a particular metabolite. Nevertheless, a recent review on biomarkers and metabolomics (25) noted two studies that had reported trimethylamine-N- oxide as a urinary metabolite that differed according to meat in the diet; therefore, this potential biomarker should also be investigated in future studies.
Our data is from randomized, highly controlled, cross-over studies where individuals were their own controls, the optimum study design for investigating dietary biomarkers. However, our study design did not consider the potential of these analytes as long-term biomarkers of intake, especially if meat is episodically consumed. It is likely that 1-methylhistidine and 3-methylhistidine may be good short-term biomarkers of meat intake, and that a single urine sample may not be sufficient to assess ‘usual’ intake; a very small study reported that the half-lives of 1-methylhistidine and 3-methylhistidine were 11.7 and 12.6 hours, respectively (8). Nevertheless, these biomarkers could be used in studies where repeat samples are available and in calibration studies for measurement error correction. In addition, good short-term biomarkers of intake can be used in conjunction with existing self-reported questionnaires to improve the accuracy of exposure assessment (26). Since our results are from a controlled feeding study, they must be replicated in free-living individuals to determine whether the excretion of these compounds can categorize individuals according to their usual meat intake irrespective of other components of the diet. Despite tight control of the diets in our study, there remained a degree of variation in urinary excretion of all the analytes between individuals on the same diet, which may indicate that this variation would be even greater in free-living populations.
References
- 1.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–2287. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 2.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WCRF/AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, D.C: 2007. World Cancer Research Fund / American Institute for Cancer Research. [Google Scholar]
- 4.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169:562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lykken GI, Jacob RA, Munoz JM, Sandstead HH. A mathematical model of creatine metabolism in normal males--comparison between theory and experiment. Am J Clin Nutr. 1980;33:2674–2685. doi: 10.1093/ajcn/33.12.2674. [DOI] [PubMed] [Google Scholar]
- 6.Huszar G, Golenwsky G, Maiocco J, Davis E. Urinary 3-methylhistidine excretion in man: the role of protein-bound and soluble 3-methylhistidine. Br J Nutr. 1983;49:287–294. doi: 10.1079/bjn19830037. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968;48:424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- 8.Sjolin J, Hjort G, Friman G, Hambraeus L. Urinary excretion of 1-methylhistidine: a qualitative indicator of exogenous 3-methylhistidine and intake of meats from various sources. Metabolism. 1987;36:1175–1184. doi: 10.1016/0026-0495(87)90245-9. [DOI] [PubMed] [Google Scholar]
- 9.Cross AJ, Pollock JR, Bingham SA. Haem, not Protein or Inorganic Iron, Is Responsible for Endogenous Intestinal N-Nitrosation Arising from Red Meat. Cancer Res. 2003;63:2358–2360. [PubMed] [Google Scholar]
- 10.Bingham S, Cummings JH. The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci. 1983;64:629–635. doi: 10.1042/cs0640629. [DOI] [PubMed] [Google Scholar]
- 11.Bingham SA, Cummings JH. Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr. 1985;42:1276–1289. doi: 10.1093/ajcn/42.6.1276. [DOI] [PubMed] [Google Scholar]
- 12.Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- 13.Datta SP, Harris H. Dietary origin of urinary methylhistidine. Nature. 1951;168:296–297. doi: 10.1038/168296a0. [DOI] [PubMed] [Google Scholar]
- 14.Myint T, Fraser GE, Lindsted KD, Knutsen SF, Hubbard RW, Bennett HW. Urinary 1- methylhistidine is a marker of meat consumption in Black and in White California Seventh-day Adventists. Am J Epidemiol. 2000;152:752–755. doi: 10.1093/aje/152.8.752. [DOI] [PubMed] [Google Scholar]
- 15.Block WD, Hubbard RW, Steele BF. Excretion of Histidine and Histidine Derivatives by Human Subjects Ingesting Protein from Different Sources. J Nutr. 1965;85:419–425. doi: 10.1093/jn/85.4.419. [DOI] [PubMed] [Google Scholar]
- 16.Lukaski H, Mendez J. Relationship between fat-free weight and urinary 3-methythistidine excretion in man. Metabolism. 1980;29:758–761. doi: 10.1016/0026-0495(80)90199-7. [DOI] [PubMed] [Google Scholar]
- 17.Long CL, Haverberg LN, Young VR, Kinney JM, Munro HN, Geiger JW. Metabolism of 3-methylhistidine in man. Metabolism. 1975;24:929–935. doi: 10.1016/0026-0495(75)90084-0. [DOI] [PubMed] [Google Scholar]
- 18.Marliss EB, Wei CN, Dietrich LL. The short-term effects of protein intake on 3- methylhistidine excretion. Am J Clin Nutr. 1979;32:1617–1621. doi: 10.1093/ajcn/32.8.1617. [DOI] [PubMed] [Google Scholar]
- 19.Elia M, Carter A, Bacon S, Smith R. The effect of 3-methylhistidine in food on its urinary excretion in man. Clin Sci (Lond) 1980;59:509–511. doi: 10.1042/cs0590509. [DOI] [PubMed] [Google Scholar]
- 20.McKeown-Eyssen GE, Yeung KS, Bright-See E. Assessment of past diet in epidemiologic studies. Am J Epidemiol. 1986;124:94–103. doi: 10.1093/oxfordjournals.aje.a114374. [DOI] [PubMed] [Google Scholar]
- 21.Fuller L, Rich AJ. An index of lean body mass from 24-hour urinary creatinine excretion. Proc Nutr Soc. 1982;41:A104. [Google Scholar]
- 22.Laidlaw SA, Shultz TD, Cecchino JT, Kopple JD. Plasma and urine taurine levels in vegans. Am J Clin Nutr. 1988;47:660–663. doi: 10.1093/ajcn/47.4.660. [DOI] [PubMed] [Google Scholar]
- 23.Irving CS, Marks L, Klein PD, Foster N, Gadde PL, Chase TN, et al. New evidence for taurine biosynthesis in man obtained from 18O2 inhalation studies. Life Sci. 1986;38:491–495. doi: 10.1016/0024-3205(86)90027-5. [DOI] [PubMed] [Google Scholar]
- 24.Thompson DE, Vivian VM. Dietary-induced variations in urinary taurine levels of college women. J Nutr. 1977;107:673–679. doi: 10.1093/jn/107.4.673. [DOI] [PubMed] [Google Scholar]
- 25.Dragsted LO. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010;84:301–307. doi: 10.1016/j.meatsci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Freedman LS, Tasevska N, Kipnis V, Schatzkin A, Mares J, Tinker L, et al. Gains in statistical power from using a dietary biomarker in combination with self-reported intake to strengthen the analysis of a diet-disease association: an example from CAREDS. Am J Epidemiol. 2010;172:836–842. doi: 10.1093/aje/kwq194. [DOI] [PMC free article] [PubMed] [Google Scholar]