Abstract
Various biodegradable hydrogels have been employed as injectable scaffolds for tissue engineering and drug delivery. We report a double crosslinking strategy of biocompatible and biodegradable hydrogels derived from aminated and oxidized hyaluronic acid (HA) with genipin (GP), a compound naturally derived from the gardenia fruit. Fast gelation is attributed to the Schiff-base reaction between amino and aldehyde groups of polysaccharide derivatives, and the subsequent crosslinking with GP results is ideal biodegradability and mechanical properties. The gelation time, morphology, equilibrium swelling, compressive modulus and degradation of double crosslinked hydrogels were examined. The double crosslinked hydrogels were examined in vivo via subcutaneous injection into mouse model. Histological results indicated favorable biocompatility as revealed by an absence of neutrophils and macrophages. These studies demonstrate that double crosslinked HA hydrogels are potentially useful as injectable, biodegradable hydrogels in tissue engineering applications.
Keywords: hyaluronic acid, Schiff-base, genipin, injectable hydrogel, soft tissue engineering
1. Introduction
Injectable hydrogels are promising biomaterials for regenerative medicine applications, and have been utilized for cell, protein and drug delivery (Tememoff et al., 2000; Anseth et al., 2002; Nicodemus et al., 2008;). The use of injectable hydrogels is clinically desired as this system allows homogeneous cell and drug encapsulation and could result in minimally invasive surgeries (Bouhadir et al., 2000; Leach et al., 2004; Balakrishnan et al., 2005). A variety of naturally-derived polymers have been widely used as injectable hydrogels for tissue engineering approaches due to excellent biocompatibility and biodegradability. Hydrogels derived from naturally occurring polysaccharides mimic many features of extracellular matrix (ECM) and thus have the potential to direct the migration and proliferation of encapsulated cells during tissue regeneration (Ameer et al., 2002; Masters et al., 2004; Wieland et al., 2007). Over the past decade, several methods have been employed for preparation of injectable polysaccharides hydrogels, including ionic interaction, photopolymerization, thermal gelation, physical self-assembly, and chemical crosslinking with agents such as glutaraldehyde, genipin, adipic dihydrazide and bis(sulfosuccinimidyl) suberate (Shu XZ et al., 2002; Motokawa et al., 2006).
More recently, the Schiff-base reaction has been utilized for rapid gelation, resulting in injectable hydrogels (Nishi et al., 2004; Jia et al., 2006). The gelation is attributed to the reaction between amino and aldehyde groups of polysaccharide derivatives. Several polysaccharides such as gum arabic, dextran, hyaluronic acid and chondroitin sulfate have been employed for possible medical applications such as drug delivery, peritoneal adhesion prevention, and tissue engineering (Ruhela et al., 2006; Ito et al., 2007). Our laboratory has recently developed an injectable polysaccharide-based hydrogel derived from water-soluble chitosan and oxidized hyaluronic acid as cell carriers for cartilage tissue engineering (Tan et al., 2009).
Hyaluronic acid (HA) is a naturally non-sulfated polysaccharide consisting of multiple repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid (Toole, 1990; Fraser et al., 1997). In the ECM, hyaluronic acid is the backbone of glycosaminoglycan (GAG) superstructure complexes, and plays an important role in many biological processes (Goa et al., 1994; Campoccia et al., 1998). By incorporating HA with other polysaccharides, it is possible to develop a more biomimetic microenvironment with improved biocompatibility and biodegradation for tissue regeneration. Hyaluronic acid has a plethora of carboxyl groups and hydroxyl groups; the carboxyl group of hyaluronic acid could react with amino group of a diamine to form amino derivatives. The vicinal hydroxyl groups could be cleaved and generate reactive aldehyde functionalities, which result in a chemical crosslinking with amino functions via the Schiff-base linkage. Although Schiff-base crosslinked polysaccharide-based hydrogels have shown excellent biocompatibility (Tan et al., 2009), other important issues regarding tissue engineering application such as biodegradable and mechanical properties remain challenging.
In this study, we report the synthesis and characterization of an injectable, double crosslinked hyaluronic acid hydrogel via the Schiff-base reaction (first crosslinking) and treatment with genipin (GP) (second crosslinking) (Figure 1). Genipin is a natural product extracted from the gardenia fruit, which overcomes the toxicity inherent in most commonly used synthetic cross-linkers (Sung et al., 1998; Tsai et al., 2000). Recent studies have identified that genipin can be utilized to crosslink functional amine groups present in natural tissues and biomaterials with very minimal cytotoxic effects compared to studies performed with glutaraldehyde, a commonly used crosslinker, resulting in materials with a deep blue color. First, the primary amine groups attacked the olefinic carbon atom at C-3 of deoxyloganin aglycone on genipin to open the dihydropyran ring. The ring-opened intermediate of the genipin derivatives can further form linked bridges. The second, slow, reaction is the nucleophilic substitution of the ester group on genipin to form a secondary amide with amine groups. Therefore, the macromolecule chains with primary amine groups could be crosslinked to form three-dimensional networks (Mi et al., 2005). The inflammatory reaction of the genipin-fixed tissues was significantly less than their glutaraldehyde-fixed counterparts, which implied that genipin could form biocompatible crosslinked products. The utilization of genipin (0.5∼3.5 wt%) to crosslink natural biocompatible polymers, such as chitosan and gelatin, to form biodegradable hydrogels has the potential to produce novel scaffolds for various tissue engineering applications. Our laboratory has recently examined the synthesis of amino-terminated multi-arm PEG based hydrogels utilizing genipin as a crosslinking agent (Tan et al., 2010).
Figure 1.
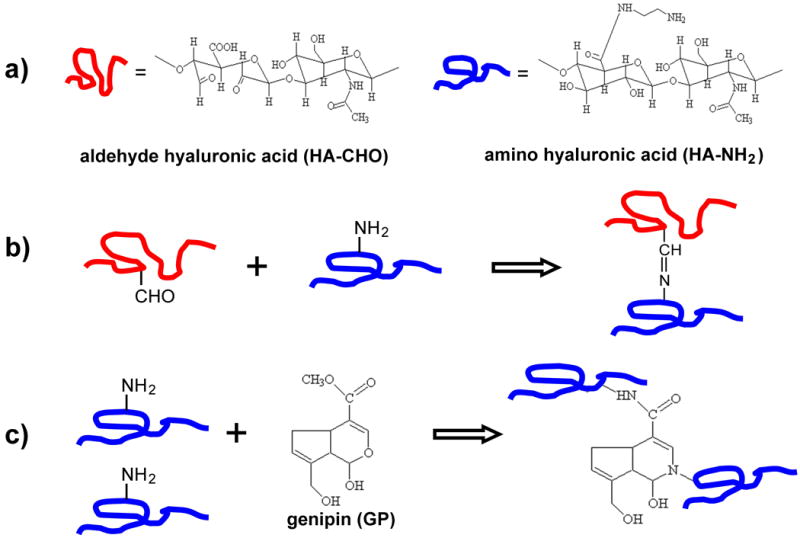
(a) Chemical structures of of aldehyde hyaluronic acid (HA-CHO) and amino hyaluronic acid (HA-NH2). (b) Scheme of HA-CHO and HA-NH2 hydrogel via Schiff-base cross-linking reaction. (c) Reaction scheme of HA-NH2 cross-linked with genipin (GP).
As such, the aim of this work was to prepare an in situ forming biodegradable hyaluronic acid hydrogel with suitable biocompatibility, biodegradation and mechanical properties for soft tissue reconstruction. The in vitro gelation, structure, morphology, equilibrium swelling, compressive modulus and degradation of double crosslinked hydrogel were examined. The efficacy of in vivo injectable feasibility was also studied using a subcutaneous injection model in the mouse. Our results demonstrate that this system has potential applications in regenerative medicine.
2. Materials and methods
2.1. Materials
Chemicals were purchased from Sigma-Aldrich unless otherwise stated. Hyaluronic acid sodium (molecular weight, ∼1.6×106), ethylenediamine, 1-hydroxybenzotriazole hydrate (HOBt), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), hyaluronidase, sodium periodate, ethylene glycol, ninhydrin and t-butyl carbazate were used as received. Genipin (GP) was purchased from Wako Pure Chemical Industries, Ltd., Japan. All other chemicals and reagents were used as received.
2.2. Synthesis of amino-HA
Amino-HA (HA-NH2) was synthesized in aqueous conditions following previously described procedures (Bulpitt P et al., 1999). Briefly, 0.5 g of HA was dissolved in 100 mL nanopure H2O to result in a 5 mg/mL solution. To this solution was added a 30-fold molar excess of ethylenediamine. The pH of the solution was adjusted to 6.8 by adding HCl. 0.8 g of EDC and 0.7 g HOBt were dissolved in DMSO/H2O (1:1 v/v, 5 mL each) and added to the reaction mixture. The reaction was stirred for 24 h at room temperature and then was exhaustively dialyzed (MWCO 10000, Spectra/Por membrane, Rancho Dominguez, CA, USA) against nanopure H2O for 3 days. NaCl was then added to produce a 5% (w/v) solution and the HA-NH2 was precipitated in ethanol. The precipitate was re-dissolved in H2O and dialyzed for 3 days to remove the salt. The purified product was freeze-dried at -50 °C (Freezone 4.5, Labconco US) and kept at 4 °C. The percentage of substitution in the HA-NH2 was quantified as 51% using a ninhydrin assay (Curotto et al., 1993).
2.3. Synthesis of aldehyde-HA
Aldehyde-HA (HA-CHO) was synthesized according to a reported procedure slightly modified (Ito et al., 2007). 1.0 g HA was dissolved in 100mL nanopure H2O at a concentration of 10 mg/ml. An aqueous solution of sodium periodate (0.5 M, 5ml) was added dropwise, and the reaction was stirred for 2 h at room temperature in the dark. 1 mL Ethylene glycol was then added to inactivate any unreacted periodate. The reaction was stirred for 1 h at ambient temperature and the solution was purified by exhaustive dialysis against H2O for 3 days, and the dry product was obtained by freeze-drying. The percentage oxidation of HA-CHO was quantified by measuring the number of aldehydes in the polymer using t-butyl carbazate (Bouhadir et al., 1999). Determination of the actual aldehyde content of HA-CHO revealed an extent of oxidation of 45%.
2.4. Hydrogel fabrication
HA-CHO and HA-NH2 were dissolved in PBS separately at a concentration of 20 mg/mL. The HA-NH2/HA-CHO hydrogels (HA-HA) were formed by mixing of polysaccharide solutions at various volumes ratio of 1/9, 3/7, 5/5, 7/3 and 9/1 at room temperature. For preparation of the double crosslinked hydrogel (HA-NH2/HA-CHO with genipin, HA-HA/GP), genipin was dissolved in the HA-CHO solution at the desired concentrations. Double crosslinked hydrogels were formed by mixing of HA-NH2 and HA-CHO/genipin solutions with various volumes ratio. The gelation rate of composite hydrogels was monitored under sealed conditions at room temperature. 600 μL of the HA mixtures was injected into 2 mL polypropylene test tube. The gelation time was recorded when the HA solution mixtures solidified and lost its fluidity under shaking.
2.5. Characterization of hydrogels
2.5.1. Enzymatic degradation in vitro
Degradation of HA-HA and HA-HA/GP hydrogels was examined with respect to weight loss under aqueous conditions in the presence of hyaluronidase. Hyaluronidase was dissolved in PBS to result in a 100 U/mL enzyme solution. Weight loss of initially weighed hydrogels (W0) was monitored as a function of incubation time in PBS at 37 °C. At specified time intervals, hydrogels were removed from the PBS and weighed (Wt). The weight loss ratio was defined as 100%×(W0–Wt)/W0. The weight remaining ratio was defined as 1–100%×(W0–Wt)/W0.
2.5.2. Morphology
Surface morphology of HA-HA and HA-HA/GP hydrogels were characterized by utilizing scanning electron microscopy (SEM) after gelation. The hydrogels were freeze-dried and then gold-coated using a Cressington 108 Auto (Cressington, Watford UK). The surface and cross-sectional morphologies were viewed using a JSM-6330F SEM (JEOL, Peabody, MA) operated at 10kV accelerating.
2.5.3. Equilibrium swelling
The known weights of freeze-dried HA-HA and HA-HA/GP hydrogels were immersed in DMEM/F12/10%FBS and PBS, respectively, and kept at 37 °C for 2 h until equilibrium of swelling had been reached. The swollen hydrogels were removed and immediately weighed with a microbalance after the excess of water lying on the surfaces was absorbed with a filter paper. The equilibrium swelling ratio (ESR) was calculated using the following equation:
ESR = (Ws − Wd)/Wd
where Ws and Wd are the weights of the hydrogels at the equilibrium swelling state and at the dry state, respectively.
2.5.4. Compressive modulus
Mixtures of HA-HA and HA-HA/GP solutions described above were injected into a 12-well culture plate to obtain columned hydrogels (22 mm diameter, 7 mm height). Compressive modulus of elasticity was measured in the elastic region of hydrogel using a dynamic mechanical analyzer (ELF3200, Endura TEC) in unconfined compression up to 20% strain at room temperature.
2.6. In vivo model
Following the guidelines of the University of Pittsburgh Institutional of Animal Care and Use Committee, HA-HA and HA-HA/GP hydrogels was administered by bilateral dorsal subcutaneous injections in male Balb-C mice (n = 6, 6 week-old, Harlan, Indianapolis, Ind.) to evaluate biocompatibility in vivo. Each injection was 1 ml in volume and performed through hypodermic syringe. For histological evaluation, after 24h and 5 days, animals were sacrificed and excised gel-implants were rinsed in PBS and fixed in 10% buffered formalin at 37 °C. The gel-explants and surrounding tissues were dehydrated in a graded series of alcohols at 37 °C, and embedded in paraffin. The transverse paraffin was sectioned and histologically processed using hematoxylin and eosin (H & E) stains.
2.7. Statistical analysis
The experimental data from all the studies were analyzed using Analysis of Variance (ANOVA). Statistical significance was set to p value ≤ 0.05. Results are presented as mean ± standard deviation.
3. Results and discussion
3.1. Hydrogel gelation
The chemical structure of HA-CHO and HA-NH2 are shown in Figure 1a. Aldehyde groups were introduced to hyaluronic acid by reaction with sodium periodate, which oxidizes the vicinal hydroxyl groups to dialdehydes, thereby opening the sugar ring to form dialdehyde derivatives. HA-NH2 was synthesized by coupling ethylenediamine to hyaluronic acid through amide bond linkages via water soluble carbodiimide chemistry. Both of HA-CHO and HA-NH2 are water soluble at various pHs.
The gelation rate of HA-HA hydrogels was monitored at room temperature (Figure 2). Ratios of HA-CHO and HA-NH2 significantly influenced the gelation time. When HA-NH2 and HA-CHO were mixed with five different volume ratios, gelation occurred within 25 min. The gelation of 3/7, 5/5, and 7/3 gels occurred within 12 min, which were significantly quicker than the two others (p < 0.05). The quick gelation rate was attributed to the Schiff-base reaction between amino and aldehyde groups of polysaccharide derivatives (Figure 1b). Since the reaction time of genipin was significantly slower than that of Schiff-base, addition of genipin did not significantly influence on the gelation time. The ratio of 1:1 was chosen for the remaining studies due to the suitable gelation time.
Figure 2.
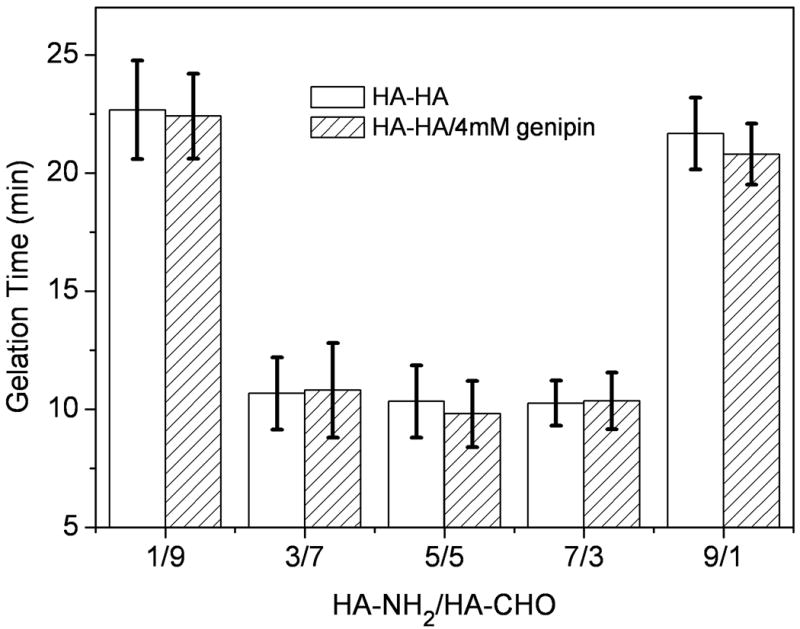
Gelation time of HA-HA and HA-HA/GP hydrogels as a function of HA-NH2/HA-CHO volume ratio at room temperature. The total polysaccharide concentration was 20 mg/mL. Values reported are an average n = 3, ± standard deviation.
3.2. Hydrogel in vitro degradation
Degradation of HA-HA and HA-HA/GP hydrogels was examined as a function of incubation time in PBS at 37 °C, as shown in Figure 3. The HA-HA hydrogels showed a faster weight loss than the double crosslinked hydrogels, which fully degraded in 10 days. The HA-HA/GP hydrogels showed a slower degradation rate than the HA-HA hydrogels due to the sufficient cross-linking. The crosslinked hydrogels with a higher GP concentration of 8 mM demonstrated a slower weight loss than a 4 mM GP concentration; however, no significant difference was found (p > 0.05). At day 28, the weight remaining ratio of hydrogel with 8 mM and 4 mM of GP were 56.1% and 68.6%, respectively. Since HA is an enzymatically degradable polysaccharide, the addition of hyaluronidase resulted in a faster weight loss. The 8 mM GP crosslinked hydrogel showed a significantly slower weight losing rate than 4 mM GP of gel (p < 0.05). The HA-HA/GP hydrogels completely hydrolyzed in 4 weeks under hyaluronidase conditions.
Figure 3.
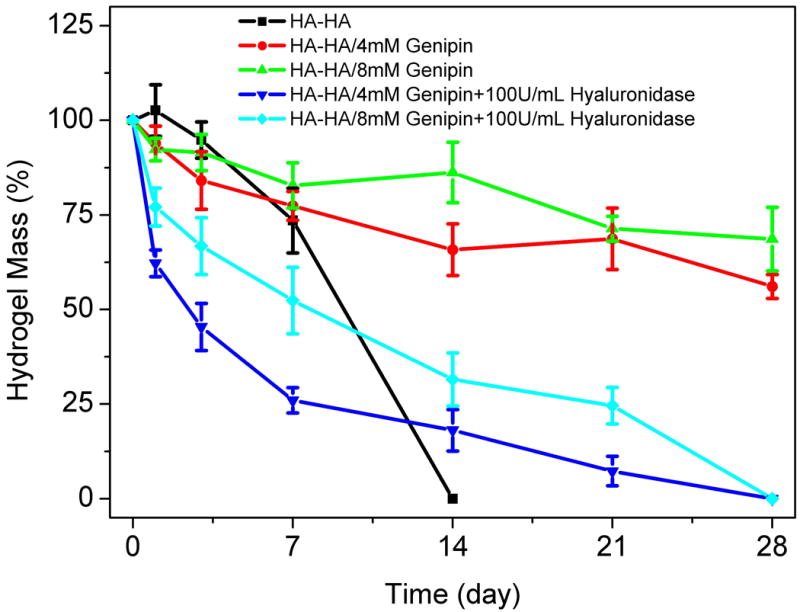
Degradation of HA-HA and HA-HA/GP hydrogel in PBS and 100 U/ml hyaluronidase/PBS at 37 °C with respect to weight loss. GP concentrations were fixed with 4 mM and 8 mM, respectively. Values reported are an average n = 5, ± standard deviation.
3.3. Hydrogel morphology
The gross view of HA-HA and HA-HA/GP (4 mM) gels is shown in Figure 4. The HA-HA gel was transparent, while the HA-HA/GP gel had the expected blue appearance and a better maintenance of shape. SEM images were obtained to characterize the microstructure morphologies of freeze-dried hydrogels (Figure 5). The surface images of HA-HA and HA-HA/GP hydrogels are presented in Figure 5a-b. A thin polymer layer can be observed, which is likely related to the collapse of surface pores by the freeze-drying process. According to cross-sectional SEM images (Figure 5c-d), both HA-HA and HA-HA/GP hydrogels displayed a continuous and porous structure resembling other natural macromolecular hydrogel system structures with pores diameter in the range of 10∼100 μm, whereas HA-HA/GP hydrogel showed a more compact structure with smaller pore sizes. The SEM results demonstrated that a GP crosslinking results in the formation of tighter network structure in HA-HA hydrogels, which is likely due to the comparatively sufficient crosslinking.
Figure 4.
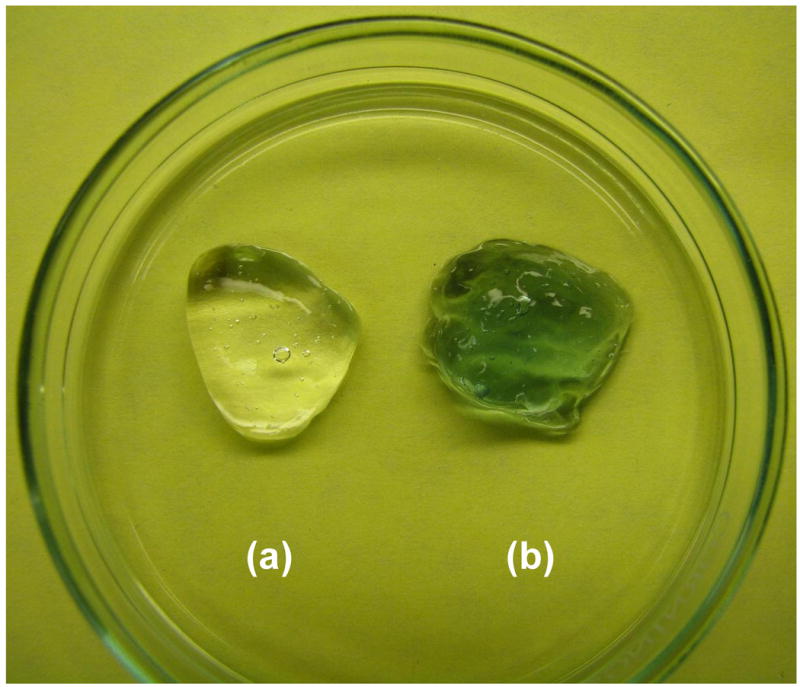
Photograph of HA-HA (a) and HA-HA/GP (4 mM) (b) hydrogels.
Figure 5.
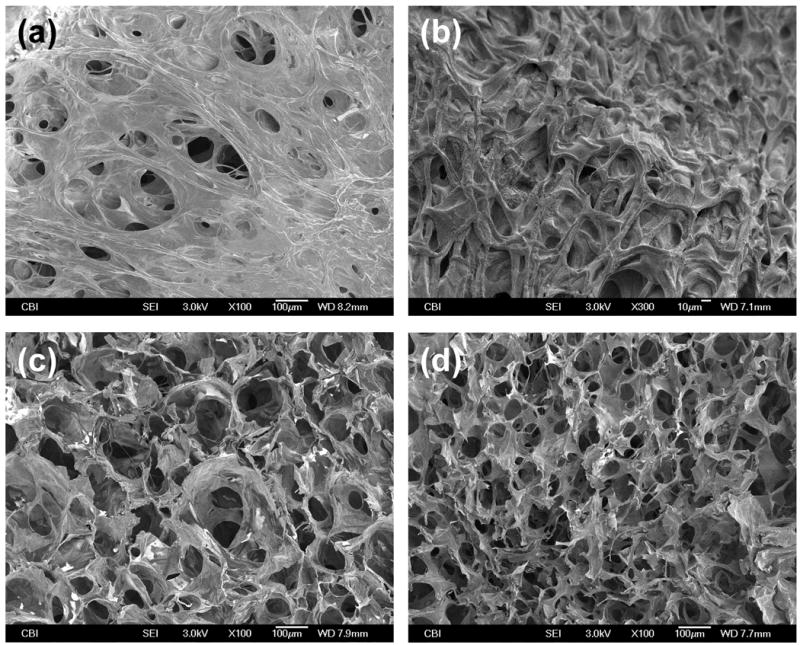
SEM photographs of freeze-dried hydrogels: (a) & (c) surface and cross-sectional morphologies of HA-HA hydrogel; (b) & (d) surface and cross-sectional morphologies of HA-HA/GP (4 mM) hydrogel.
3.4. Equilibrium swelling
Figure 6 indicates the equilibrium swelling ratio of HA-HA and HA-HA/GP hydrogels determined in PBS and DMEM/F12/10%FBS. The swelling ratio of HA-HA hydrogel was slightly higher than that of HA-HA/GP hydrogel, withno difference found (p > 0.05). A GP concentration of 4 mM didn't result in a significant influence on the equilibrium swelling properties of gels. The swelling ratio of HA-HA and HA-HA/GP hydrogels in DMEM/F12/10%FBS was slightly higher than those of PBS, whereas no difference was found (p > 0.05). In the case of DMEM/F12/10%FBS medium, the swelling value was 56.8 for HA-HA hydrogels, and 49.1 for HA-HA/GP hydrogels. The GP cross-linking slightly decreased the equilibrium swelling ratio of double crosslinked hydrogels, which didn't significantly affect swelling properties.
Figure 6.
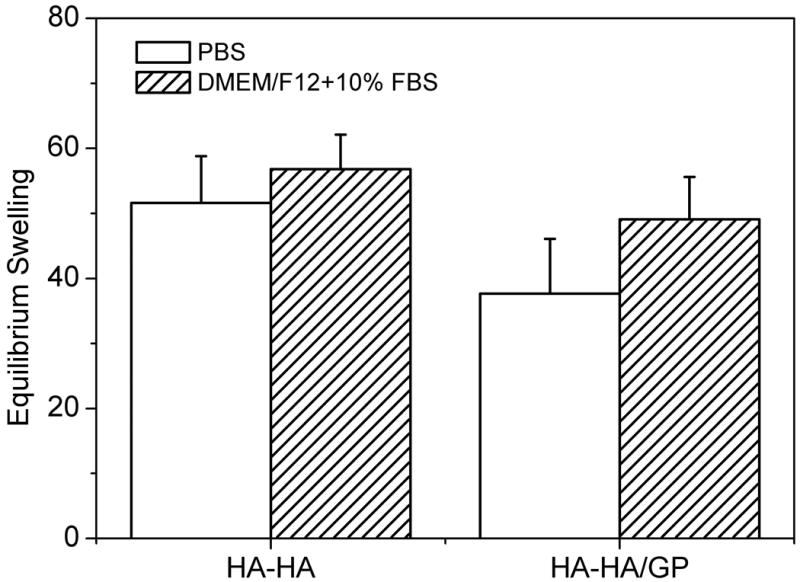
Equilibrium swelling ratio of HA-HA and HA-HA/GP (4 mM) hydrogels in PBS and DMEM/F12/10%FBS at 37 °C. Values reported are an average n = 5, ± standard deviation.
3.5. Compressive modulus
Compressive modulus of the HA-HA and HA-HA/GP hydrogels are showed in Figure 7. The HA-HA/GP hydrogels demonstrated a significantly higher compressive modulus than the HA-HA hydrogel (p < 0.05), which were 9.3 and 22.6 kPa, respectively. The mechanical properties of injectable hydrogels are particularly important for soft tissue engineering applications, such as repair after trauma or tumor resection. The additional GP crosslinking results in a increase in crosslinking density, which influences many of the macroscopic properties of hydrogels, such as compact microstructure, less water content, slower mass loss and higher compressive modulus.
Figure 7.
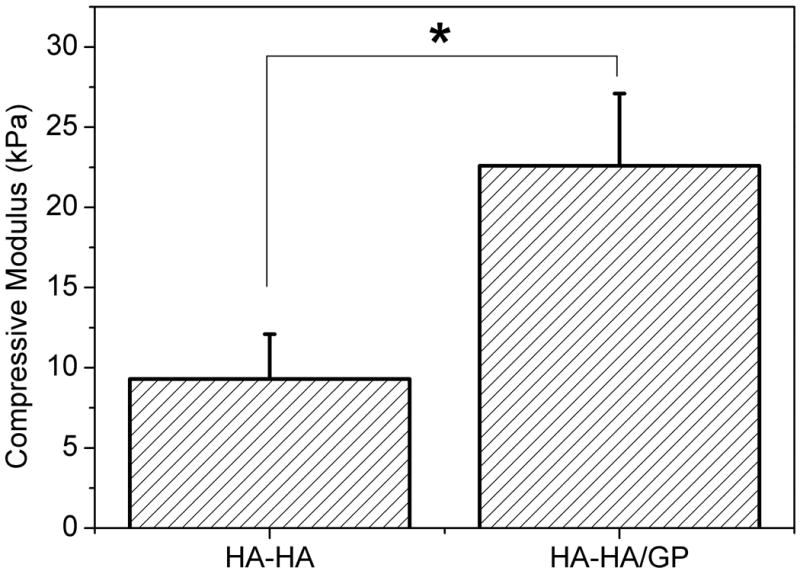
Compressive modulus of HA-HA and HA-HA/GP (4 mM) hydrogels at room temperature. Values reported are an average n = 3, ± standard deviation.
3.6. Hydrogel in vivo biocompatibility
The ability of HA-HA and HA-HA/GP gels to be efficiently injected were studied using a mice model. HA-HA and HA-HA/GP hydrogels were injected bilaterally into the dorsal subcutaneous region of Balb-C mice to investigate initial in vivo biocompatibility. The injection experiments confirmed the availability of in situ gel formation after subcutaneous injection (Figure 8). Figure 8a and 8b display the representative macroscopic images of HA-HA and HA-HA/GP hydrogels immediately following, and after 24 h injection, respectively. The preliminary results show that the injected solutions could in situ form hydrogels (Figure 8c and 8d), and the gross appearance of gels was similar to the in vitro results (Figure 4). The contour of the pockets of HA-HA were more clearly demarcated than those of HA-HA/GP as seen through the skin, and in subcutaneous tissue upon dissection. While HA-HA/GP gels were somewhat cohesive, these gels were more solid than HA-HA gels and had adhered to tissue planes. Histological cross sections of the implanted hydrogel and nearby tissue are presented in Figure 9. No infections or inflammatory reactions were observed in the skin and subcutaneous tissues at 24 h and 5 day. The tissues at the gel–tissue interface showed an absence of infiltration by neutrophils and macrophages, as indicated by the minimal formation of a fibrous capsule.
Figure 8.
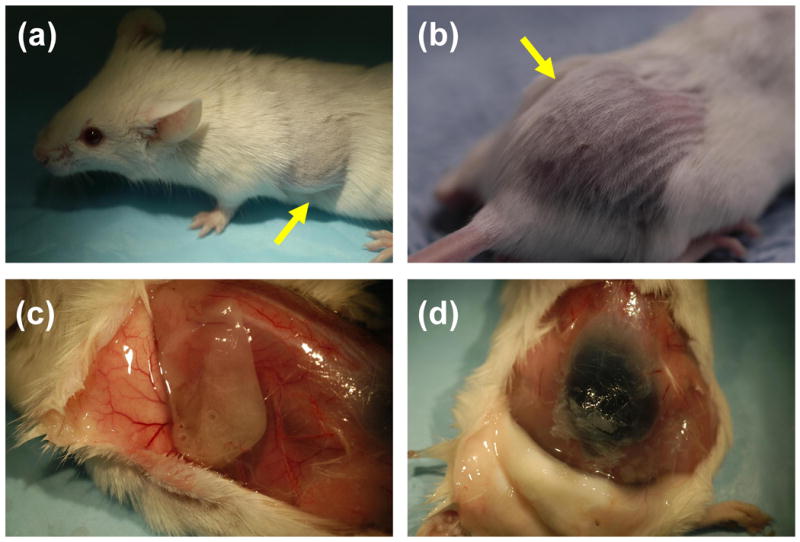
Subcutaneous injection and gross appearance of HA-HA (a, c) and HA-HA/GP (4 mM) (b, d) hydrogel implants in Balb-C mice after 2 h (a, b) and 24 h (c, d).
Figure 9.
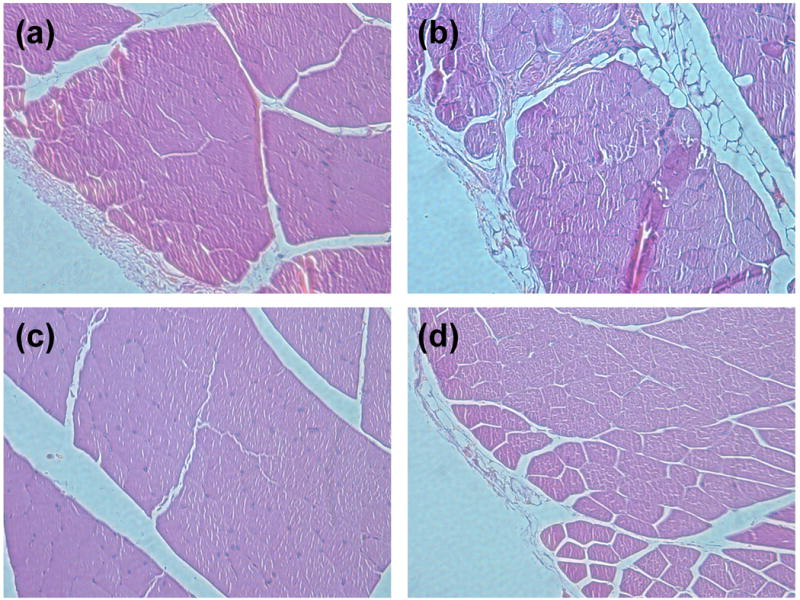
Representative histology of injection site of HA-HA (a, c) and HA-HA/GP (4 mM) (b, d) hydrogels after 24 h (a, b) and 5 d (c, d) of implantation.
4. Conclusions
Double crosslinked in situ forming HA-NH2 and HA-CHO hydrogels were prepared via Schiff-base reaction and genipin incorporation. The gelation time, gel morphologies, equilibrium swelling, degradation and compressive modulus in vitro were dependent upon structure and crosslinking density of hydrogels. An additional genipin crosslinking strategy consequently results in more compact microstructure, slower mass loss and higher compressive modulus of hydrogels, and doesn't significantly influence the water content. The murine experiments confirmed the ability of in situ gel formation after subcutaneous injection. The histological results showed HA-HA/GP hydrogel are initially biocompatible. While longer term pre-clinical studies are needed, our studies indicate that the double cross-linked hyaluronic acid hydrogel may have potential use as an injectable scaffold in soft tissue engineering.
Acknowledgments
We thankfully acknowledge the Center for Biologic Imaging for SEM analysis, Professor Steven Abramowitch and Andrew Feola for assistance with mechanical testing, NIH R01CA114246-01A1 (JPR), and DOD W81XWH-08-2-0032 (JPR and KGM).
References
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Tememoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–2412. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- Anseth KS, Metters AT, Bryant SJ, et al. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Rel. 2002;78:199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue Engineering applications. Tissue Eng. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou QP, De Bank PA, Shakesheff KM. Injectable scaffolds for tissue regeneration. J Mater Chem. 2004;14:1915–1923. [Google Scholar]
- Bouhadir KH, Kruger GM, Lee KY, et al. Sustained and controlled release of daunomycin from cross-linked poly(aldehyde guluronate) hydrogels. J Pharm Sci. 2000;89:910–919. doi: 10.1002/1520-6017(200007)89:7<910::AID-JPS8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Tan H, Marra KG. Injectable, biodegradable hydrogels for tissue engineering applications. Materials. 2010;3:1746–1767. [Google Scholar]
- Elisseeff J, McIntosh W, Anseth K, et al. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Leach JB, Bivens KA, Collins CN, et al. Development of photocrosslinkable hyaluronic acid polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res. 2004;70A:74–82. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26:3941–3951. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Holland TA, Tessmar JK, Tabata Y, et al. Transforming growth factor-beta1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Rel. 2004;94:101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Wieland JA, Houchin-Ray TL, Shea LD. Non-viral vector delivery from PEG-hyaluronic acid hydrogels. J Control Rel. 2007;120:233–241. doi: 10.1016/j.jconrel.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Ramirez CM, Miljkovic N, et al. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30:6844–6853. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi FL, Tan YC, Liang HC, et al. In vitro evaluation of a chitosan membrane cross-linked with genipin. J Biomater Sci Polym Ed. 2001;12:835–850. doi: 10.1163/156856201753113051. [DOI] [PubMed] [Google Scholar]
- Ameer GA, Mahmood TA, Langer R. A biodegradable composite scaffold for cell transplantation. J Orthop Res. 2002;20:16–19. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- Masters KS, Shah DN, Walker G, et al. Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J Biomed Mater Res. 2004;71A:172–180. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- Leach JB, Bivens KA, Collins CN, et al. Development of photocrosslinkable hyaluronic acid polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res. 2004;70A:74–82. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- Shu XZ, Liu YC, Luo Y, et al. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jia X, Yeo Y, Clifton RJ, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- Motokawa K, Hahn SK, Nakamura T, et al. Selectively crosslinked hyaluronic acid hydrogels for sustained release formulation of erythropoietin. J Biomed Mater Res. 2006;78A:459–465. doi: 10.1002/jbm.a.30759. [DOI] [PubMed] [Google Scholar]
- Maia J, Ferreira L, Carvalho R, et al. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer. 2005;46:9604–9614. [Google Scholar]
- Nishi KK, Jayakrishnan A. Preparation and in vitro evaluation of primaquine-conjugated gum arabic microspheres. Biomacromolecules. 2004;5:1489–1495. doi: 10.1021/bm0499435. [DOI] [PubMed] [Google Scholar]
- Yu H, Lu J, Xiao C. Preparation and properties of novel hydrogels from oxidized Konjac Glucomannan cross-linked chitosan for in vitro drug delivery. Macromol Biosci. 2007;7:1100–1111. doi: 10.1002/mabi.200700035. [DOI] [PubMed] [Google Scholar]
- Ruhela D, Riviere K, Szoka FC. Efficient synthesis of an aldehyde functionalized hyaluronic acid and its application in the preparation of hyaluronan-lipid conjugates. Bioconjugate Chem. 2006;17:1360–1363. doi: 10.1021/bc0600721. [DOI] [PubMed] [Google Scholar]
- Ito T, Yeo Y, Highley CB, et al. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. 2007;28:975–983. doi: 10.1016/j.biomaterials.2006.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhadir KH, Hausman DS, Mooney DJ. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer. 1999;40:3575–3584. [Google Scholar]
- Tan H, Chu CR, Payne KA, et al. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990;2:839–844. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- Goa KL, Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47:536–566. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- Campoccia D, Doherty P, Radice M, et al. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;23:2101–2127. doi: 10.1016/s0142-9612(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Sung HW, Huang RN, Huang LLH, et al. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42:560–567. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Huang RN, Sung HW, et al. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J Biomed Mater Res. 2000;52:58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Mi FL, Shyu SS, Peng CK. Characterization of ring-opening polymerization of genipin and pH-dependent cross-linking reactions between chitosan and genipin. J Polym Sci Part A: Polym Chem. 2005;43:1985–2000. [Google Scholar]
- Tan H, DeFail AJ, Rubin JP, et al. Novel multiarm PEG-based hydrogels for tissue engineering. J Biomed Mater Res. 2010;92A:979–987. doi: 10.1002/jbm.a.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto E, Aros F. Quantitative determination of chitosan and the percentage of free amino groups. Anal Biochem. 1993;211:240–241. doi: 10.1006/abio.1993.1263. [DOI] [PubMed] [Google Scholar]


