Abstract
Breast cancer metastasis to bone is frequently accompanied by pain. What remains unclear is why this pain tends to become more severe and difficult to control with disease progression. Here we test the hypothesis that with disease progression sensory nerve fibers that innervate the breast cancer bearing bone undergo a pathological sprouting and reorganization, which in other non-malignant pathologies has been shown to generate and maintain chronic pain. Injection of human breast cancer cells (MDA-MB-231-BO) into the femoral intramedullary space of female athymic nude mice induces sprouting of calcitonin gene-related peptide (CGRP+) sensory nerve fibers. Nearly all CGRP+ nerve fibers that undergo sprouting also co-express tropomyosin receptor kinase A (TrkA+) and growth associated protein-43 (GAP43+). This ectopic sprouting occurs in periosteal sensory nerve fibers that are in close proximity to breast cancer cells, tumor-associated stromal cells and remodeled cortical bone. Therapeutic treatment with an antibody that sequesters nerve growth factor (NGF), administered when the pain and bone remodeling were first observed, blocks this ectopic sprouting and attenuates cancer pain. The present data suggest that the breast cancer cells and tumor-associated stromal cells express and release NGF, which drives bone pain and the pathological reorganization of nearby CGRP+ / TrkA+ / GAP43+ sensory nerve fibers.
Keywords: periosteum, breakthrough pain, MDA-MB-231-BO, preventive analgesia, NGF
INTRODUCTION
One in eight women will be diagnosed with breast cancer in their lifetime27. Three quarters of women with advanced breast cancer will develop bone metastases as the skeleton is the most frequent site of breast cancer metastasis16. These metastases frequently cause significant bone pain and can reduce the functional status and quality of life of the patient66, 35. Unlike prostate and lung cancer, which also avidly metastasize to the skeleton but primarily affect older patients, a significant proportion of breast cancer patients are young women (20–44 years old) who are in the early or mid-stage of their careers and / or raising a family 30 57. As survival times for women with metastatic breast cancer have significantly increased 29, an important and as yet unmet objective is to develop novel analgesics that control breast cancer pain without the unwanted side effects of currently available analgesics.
Once breast cancer cells have metastasized to the skeleton, tumor-induced bone pain frequently follows8, 53, 66. Breast cancer-induced bone pain is usually described as dull in character, constant in presentation, and gradually increasing in intensity with time40, 64, 35. However, as the breast tumor grows a second type of pain known as “breakthrough pain” (because it breaks through the analgesic regimen the patient is receiving to control the ongoing pain) can occur64, 26. This incident, or breakthrough pain, is frequently divided into two types: “spontaneous pain” that occurs without any obvious precipitating event and a “movement-evoked pain” precipitated by movement of the tumor-bearing bone53, 65, 55. These breakthrough pains are generally more severe and unpredictable than ongoing cancer pain, and can be highly debilitating to the patient’s functional status and quality of life, resulting in a significant increase in health care utilization 64.
Breakthrough breast cancer-induced bone pain appears to be different from ongoing pain in terms of onset, precipitating events, and severity 64. However, a largely unanswered question is whether this pain is simply a more severe ongoing pain or whether it is largely driven by a different mechanism(s) and/or a newly formed neurological substrate not originally present when ongoing cancer pain first occurs. Previous studies have suggested that ectopic sprouting and/or pathological remodeling of sensory nerve fibers can drive difficult-to-control human pain states including painful neuroma (due to injury or transection of a peripheral nerve)44, 7 and complex regional pain syndrome (where the most common precipitating event is bone fracture)28, 17. Interestingly, our lab recently demonstrated this pathological remodeling of nerve fibers following injection of sarcoma50 or prostate cancer cells34 into the mouse femur. However, as metastatic breast cancer accounts for a large proportion of patients suffering from bone cancer pain, and induces a unique pattern of bone remodeling relative to other cancers78, there was a need to develop a tumor model that generates a pathology similar to what is observed in humans with metastatic breast cancer. The present study explores: whether human breast cancer cells can induce an active and pathological sprouting / reorganization of sensory nerve fibers, what factor(s) drives this sprouting / reorganization, and whether therapeutic intervention can attenuate this sprouting / reorganization and the accompanying pain.
MATERIALS AND METHODS
Mice
Experiments were performed using 66 adult female athymic nude mice (8 weeks old, Jackson Laboratories, Bar Harbor, ME, USA), weighing 20–32g. The mice were housed in accordance with the NIH guidelines under specific pathogen-free conditions in autoclaved cages maintained at 22ºC with a 12 hr alternating light and dark cycle and were given autoclaved food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the VA Medical Center (Minneapolis, MN) and the University of Arizona (Tucson, AZ).
Culture and injection of tumor cells
In the current report, we present a new model of bone cancer pain using human breast cancer cells (MDA-MB-231-BO, a kind gift from Dr. Setsuko Chambers of the Arizona Cancer Center, University of Arizona) and immunodeficient female mice. Whereas the parental cell line (MDA-MB-231) has been used extensively in breast cancer studies, the cell line used in the present study is a bone-seeking cell line82. These cells predominantly induce osteolytic lesions, yielding a pathology that is reflective of what is seen in the majority of human patients with breast cancer metastases to bone78. A total of 66 mice were evaluated in this study, 34 of which were injected with MDA-MB-231-BO human breast cancer cells into the femoral intramedullary space (left femur) according to previously described protocols72, 71. Following induction of general anesthesia with ketamine/xylazine (100/5 mg/kg, i.p.), an arthrotomy was performed exposing the condyles of the distal femur. The bone was cored with a 30 gauge needle inserted at the level of the intercondylar notch. The coring needle was then replaced with a 29 gauge hypodermic needle used to inject either Hank’s buffered sterile saline as the control (HBSS, Sigma, 20μl) or HBSS containing 105 MDA-MB-231-BO cells (20μl) into the intramedullary space. In order to prevent cell reflux following injection, the injection site was sealed with dental grade amalgam (Dentsply) using an endodontic messing gun (Union Broach), followed by copious irrigation with sterile filtered water (hypotonic solution). Wound closure was achieved using a single 7mm auto wound clip (Becton Dickinson). Day 42 after cell injection was chosen as the date of sacrifice, as by this time there was extensive cancer-induced bone resorption and significant pain behaviors. All efforts were made to minimize the suffering and number of animals used.
Anti-NGF treatment
The anti-nerve growth factor (NGF) sequestering antibody (mAb 911), kindly provided by Dr. David Shelton (Rinat/Pfizer), is effective in blocking the binding of NGF to both TrkA (tropomyosin receptor kinase A) and p75 receptors and inhibiting TrkA autophosphorylation23. The anti-NGF antibody possesses a plasma half-life of approximately 5–6 days in the mouse and it does not appreciably cross the blood brain barrier73. The dose used (10 mg/kg) was based on previous studies22, 71, 34 and delivered by intraperitoneal injection (i.p.) on days 14, 19, 24, 29, 34, and 39 post cell injection. Tumor-bearing mice were divided into 2 groups: MDA-MB-231-BO + vehicle (0.9% saline solution) and MDA-MB-231-BO + anti-NGF treatment.
Radiographic analysis of tumor-induced bone destruction
In order to assess disease progression, mice were lightly anesthetized (2% isoflurane) and digital radiographs (MX20 DC12, Faxitron XRay) of lower extremities were obtained following behavioral analysis on days 16, 21, 28, 35, and 42 post cell injection. Radiograph images of the medial-lateral plane of both femurs were used to evaluate tumor-induced bone destruction as previously performed in an osteolytic bone cancer model24. Radiographs of tumor-bearing femora, were used to evaluate bone destruction and were assigned scores of 0–4: 0, normal bone with no signs of destruction; 1, small radiolucent lesions indicative of bone destruction (one to three lesions); 2, increased number of lesions (three to six lesions) and loss of medullary bone; 3, loss of medullary bone and erosion of cortical bone; 4, full-thickness unicortical bone loss. Analysis was performed in a blinded fashion.
Behavioral analysis
Mice were behaviorally tested at days 16, 21, 28, 35, and 42 post-cell or HBSS (vehicle) injection to assess ongoing (spontaneous) and movement-evoked pain behaviors as previously described46, 72. Briefly, mice were placed in a clear plastic observation box with a wire mesh floor and allowed to habituate for a period of 15 min. After acclimation, the number of spontaneous flinches and the time spent guarding the tumor-bearing limb were recorded over a 2 minute period. Flinches were defined as the number of times the animal raised its hindpaw aloft while not ambulatory. Guarding was defined as the time the hindpaw was held aloft while ambulatory. Pain due to ambulation was evaluated using previously validated tests46. Limb use during normal ambulation was scored on a scale of 5 to 0: (5) normal use and (0) complete lack of limb use. These behaviors were selected because they reflect what is observed in patients who protect or suspend their afflicted limb79. The investigator was blinded as to the experimental condition of the animals.
Euthanasia
At day 42, mice were deeply anesthetized using CO2 delivered from a compressed gas cylinder and perfused intracardially with 20 ml of 0.1M phosphate buffered saline (PBS, pH=7.4 at 4°C) followed by 30 ml of 4% formaldehyde /12.5% picric acid solution in 0.1M PBS (pH=6.9 at 4°C). After sacrifice and perfusion, mouse femurs were removed, postfixed for 24 hours in the perfusion fixative and placed in PBS solution at 4°C.
Micro-computed tomography (μCT) analysis
In order to characterize the breast cancer-induced changes in mineralized bone micro-architecture at day 42 post cell injection, femurs were analyzed with an eXplore Locus SP micro-computed tomographer (μCT, GE Healthcare). This conebeam μCT scanner uses a 2300 × 2300 CCD detector with current and voltage set at 80 μA and 80 KVp, respectively. Specimens were scanned in 1080 views through 360° with a 2100 ms integration time. Scans were then reconstructed at 16-μm3 resolution using Reconstruction Utility software (GE Healthcare), and volume renderings were generated using the Microview software (GE Healthcare).
Immunohistochemistry
Following μCT analysis, femurs were prepared for processing as either frozen sections (longitudinal / cross sectional view) or periosteal whole mounts (bird’s eye view). Of the 66 mice evaluated in the present report, 10 were processed as whole mounts for visualization purposes.
For frozen sections, mouse femurs were gently decalcified in 10% EDTA for ~2 weeks. Decalcification was monitored radiographically to ensure that the femurs received the minimum amount of exposure to EDTA in an effort to maximize immunohistochemical antigenicity. After complete decalcification the femurs were then cryoprotected by immersion in a 30% sucrose solution for 48 hours, and serially sectioned using a cryostat along the longitudinal axis at a thickness of 20μm. Sections were thaw-mounted on gelatin-coated slides for processing. Sections of the femur were dried at room temperature (RT) for 30 min and washed in 0.1M PBS three times for 10 min each (3X10). Then sections were blocked with 3% normal donkey serum and 0.3% triton X-100 for 1 hr and incubated with primary antibodies overnight at RT. Peptide-rich primary afferent sensory neurons were labeled with a polyclonal antibody that was raised in rabbit against rat CGRP conjugated to keyhole limpet hemocyanin 38, 52, 60 (1:10,000; Sigma Chemical Co., St. Louis, MO, USA; Cat #: C8198). Sprouting nerve fibers were labeled with an antibody against growth associated protein-43 (GAP43). This GAP43 polyclonal antibody was raised in rabbit against the entire rat-derived GAP43 protein 75 (rabbit anti-GAP43, Millipore, Cat # AB5220). Nerve fibers expressing the tropomyosin receptor kinase A (TrkA) were labeled with an antibody against TrkA (1:50, R&D Systems, Cat. # AF1056). The goat anti-TrkA antibody was generated against the NS0-derived recombinant rat TrkA extracellular domain 31, 84, 42. Preparations were then washed three times in PBS and incubated in secondary antibodies for three hours. For double-immunofluorescence, preparations were incubated with a mixture of primary antibodies followed by a mixture of Cy2 (1:200) and Cy3 (1:600) conjugated secondary antibodies (Jackson Immunolab, West Grove, PA). While we and previously cited authors believe the antibodies used in the present study represent staining of the specific antigen being targeted, we use the (+) convention (i.e. TrkA+), which we define as like-immunoreactivity.
Bone sections were then washed with PBS three times and counterstained with DAPI (4’, 6-diamidino-2-phenyl-indole, dihydrochloride, 1:30,000, Molecular Probes, OR, USA) for 5 minutes and washed with PBS. Finally, tissue was dehydrated through an alcohol gradient (70, 80, 90, and 100%), cleared in xylene, and coverslipped with din-butylphthalate-polystyrene-xylene (Sigma). Bone sections were allowed to dry at room temperature for 12 hours before imaging.
As previously described, the periosteum from the diaphyseal shaft was removed as a whole mount preparation10 and processed for immunohistochemistry as previously reported 21, 33, 50. Briefly, excess muscle was removed from the femur using surgical scissors (Cat # 14004110, Fine Science Tools Inc, Foster City, CA, USA) without disturbing the bone and attached periosteum. The periosteum was harvested from the distal growth plate region to immediately below the third trochanter. The periosteum was removed from the bone by tracing the lower and upper limits of the desired area with a stainless steel surgical blade No. 11 (Feather Safety Razor, Co, Kita-Ku, Osaka, Japan) and a vertical cut was then performed along the posterior surface of the bone. Under a dissecting microscope, the periosteum was removed by gently scraping against the bone using the edge of a forceps (Dumont # 5145, Fine Science Tools Inc, Foster City, CA, USA) 10. During the periosteal removal, femurs were continually irrigated with PBS to prevent tissue dehydration. The size of the periosteal whole mount preparation and its attached thin muscle layer used for immunohistochemistry was approximately: width=6mm, length=6mm, and thickness=0.5mm. Whole mount preparations were then processed and stained with the same methodology and antibodies used for frozen sections listed above.
Additional tissue was processed for histological analysis as follows. Decalcified femora were pressure-infiltrated and embedded in paraffin. Femoral sections 7μm-thick were then cut longitudinally and stained with hematoxylin and eosin (H&E) to visualize normal bone marrow elements and breast cancer cells.
Laser confocal microscopy
Images used for illustration purposes were acquired with an Olympus Fluoview FV1000 system equipped with different lasers (Multiline Argon (458 nm, 488 nm, 515 nm), Green HeNe (543 nm), Red HeNe (633 nm), and Blue Diode (405 nm)) and multiple excitation and emission fluorescence filters. Sequential acquisition mode was used to reduce bleed-through. Confocal images of frozen sections were acquired from sections 20 μm in thickness and were projected from 80 optical sections at 0.5 μm intervals with a 40× objective. For illustration purposes, confocal images of whole mount periosteum preparations were acquired with a 40× objective, projected from 40 optical sections at 0.5μm intervals. Camera-lucida images were rendered using the ‘trace’ function in Image Pro Plus Version 3.0. Images were then processed with Adobe Photoshop CS4 and assembled into figures with Adobe Illustrator CS4.
Quantification of nerve density / unit volume
For quantification, frozen sections were used as the cross sections allow visualization of the bone’s landmarks (such as the growth plate), which enable the observer to locate the same anatomical area when quantifying different animals. The number of animals used for frozen section analysis was: n=20 naïve, n=21 for sham + vehicle, n=9 for MBA-MB-231-BO + vehicle, and n=6 for MBA-MB-231-BO + anti-NGF.
Approximately 30 separate frozen sections were obtained from each femur, each section cut at a thickness of 20 μm. For each given marker, 3 images were obtained per animal. The area evaluated was within a 1 mm-long segment initiated 1 mm from to the distal or proximal growth plate, with images taken from different sections at least 0.1 mm apart. The volume of periosteum that was analyzed was an average 418 μm (length), 70 μm (width), 20 μm (depth). The Z-stacked images were analyzed with Image-Pro Plus v. 3.0 (Media Cybernetics) and nerve fibers were manually traced to determine the length of nerve fibers. Nerve sprouting was reported as density of nerve fibers per volume of periosteum81.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Immunohistochemical and pain behavioral data were analyzed across treatment groups using a Kruskal-Wallis nonparametric analysis of variance. Significant main effects of groups were followed by Mann-Whitney nonparametric t-tests with Bonferroni adjustment for multiple comparisons.
RESULTS
Human breast cancer cells grow in bone and induce bone destruction
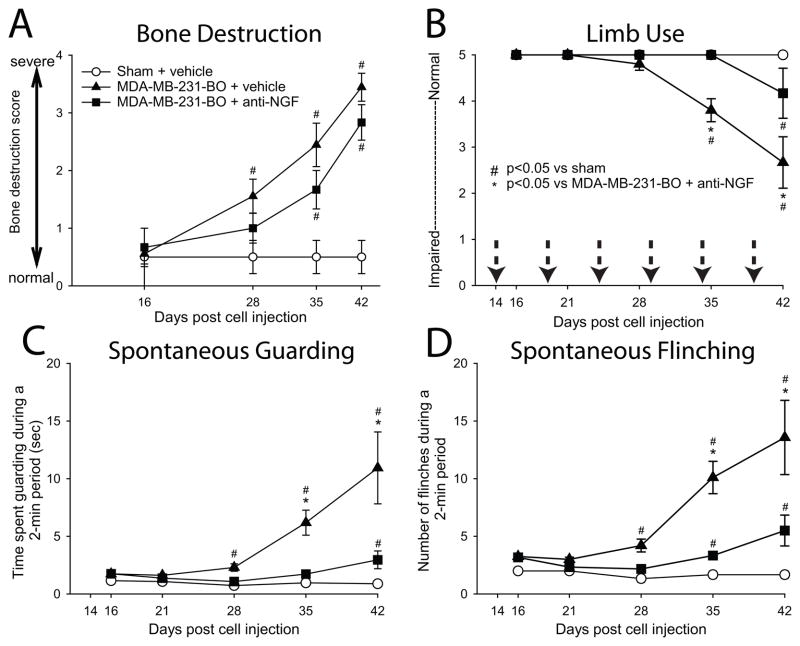
In the present study we developed a new model of breast cancer-induced bone pain that involves injecting and confining MDA-MB-231-BO human breast cancer cells into the intramedullary space of female nude mice. Radiograph and μCT analyses revealed that control animals receiving a sham injection did not exhibit significant bone remodeling when compared to naïve animals (Fig 1A–B, D–E). Twenty-eight days following injection of MDA-MB-231-BO breast cancer cells into the intramedullary space of the mouse femur, we observed significant bone resorption and the first signs of osteolytic lesions in the proximal and distal metaphyses. These lesions appear as radiolucent spots that grow in size and abundance with advancing disease (data not shown). At day 42, the mean bone destruction score for sham-treated animals was 0.5±0.2, whereas animals injected with MDA-MB-231-BO cells + vehicle had a significantly higher mean bone destruction score of 3.4±0.2. At this time, cancer cells had escaped from the intramedullary space, likely through the nutrient foramen or tumor-induced erosion of cortical bone (Fig 1C, F). Histological analysis (Fig 1G, H) showed that in the MDA-MB-231-BO injected animals there were cancer cells in the marrow space and growing between the surface of the cortical bone and the overlying periosteum (Fig 1H).
Figure 1. Human breast cancer cells produce osteolytic lesions when injected into the femur of an immunocompromised mouse.

Radiograph and μCT images from naïve (A, D), sham + vehicle (B, E), or MDA-MB-231-BO + vehicle mice (C, F) at 42 days post-injection showing the difference in bone remodeling in the distal femur following the different experimental procedures. Note that the sham-injected femur does not display a significant amount of bone destruction when compared to the naïve bone. However, when MDA-MB-231-BO cells are injected into the femur, they produce osteolytic lesions in the cortical bone. These indices of bone destruction provide the tumor with an avenue to escape from the intramedullary space and invade the periosteum. Serial sections (7μm-thick) stained with H&E demonstrate this phenomenon as well as the difference in morphology observed between normal marrow (G) versus cancerous tissue (H) and the apparent loss of trabecular bone in the tumor-bearing femur.
Additionally, in agreement with previous in vivo studies, chronic administration of anti-NGF therapy did not have a statistically significant affect on disease progression as measured by tumor growth within or outside the marrow space (data not shown), or tumor-induced bone destruction / remodeling (Fig. 2A)22, 71.
Figure 2. Breast cancer-induced pain behaviors increase with disease progression, and are reduced with sustained anti-NGF therapy.
Injection of MDA-MB-231-BO breast cancer cells into the intramedullary space of the femur results in significant osteolytic bone destruction (A) and bone pain-related behaviors (B–D). Anti-NGF therapy (10 mg/kg, i.p.) appeared to slow the bone destruction induced by the tumor; however, this effect was not statistically significant. Tumor-bearing mice treated with vehicle exhibited significantly greater pain behaviors compared to sham mice from day 28 until day 42 post cell injection (B, C, D). Whereas anti-NGF administration did not have a statistically significant effect on tumor-induced bone destruction, the therapy did significantly reduce cancer pain behaviors in the mid to late stages of disease. Each bar represents the mean ± SEM. The number of animals used for this analysis was n≥5 for each experimental group. Dotted arrows (B) indicate the dates of anti-NGF therapy administration.
Human breast cancer cells induce pain-related behaviors that are attenuated by anti-NGF therapy
Ongoing (spontaneous guarding and flinching) and movement-evoked pain-related behaviors (limb use) were analyzed in naïve, sham + vehicle, MDA-MB-231-BO + vehicle, and MDA-MB-231-BO + anti-NGF treated mice. Sham-treated mice displayed baseline pain behaviors that were not significantly different from the behavior of naïve mice (data not shown). However, mice injected with MDA-MB-231-BO cells + vehicle displayed cancer-induced pain behaviors (Fig 2B, C, D), which increased with disease progression. Interestingly, cancer-induced pain behaviors were only first observable at day 28 post cell injection, the same timepoint we first observed the presence of osteolytic lesions, and thereafter continued to increase in severity until the date of sacrifice (Day 42). When MDA-MB-231-BO-injected animals were treated with anti-NGF, cancer-induced pain behaviors were significantly reduced at every time point after day 28 compared to MDA-MB-231-BO + vehicle-treated animals.
Human breast cancer cells induce sprouting of CGRP+, GAP43+, and TrkA+ nerve fibers in the periosteum
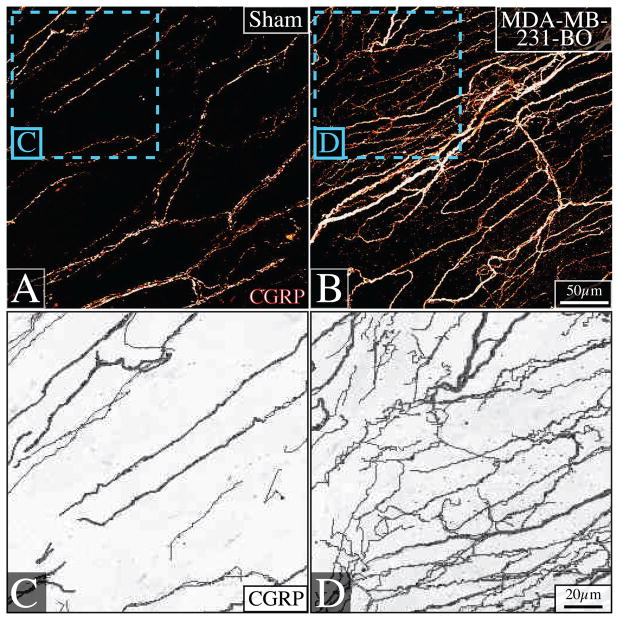
In order to initially characterize the morphology and density of nerve fibers innervating the periosteum of sham-injected vs. cancer-injected mice, the tissue was processed as whole mount preparations. In naïve periosteum, CGRP+ nerve fibers formed a net-like meshwork (Fig 3A). However, in tumor-bearing animals harvested at day 42 post-cancer cell injection, there was a dramatic change in morphology and increase in the density of CGRP+ sensory nerves (Fig 3B). Camera-lucida techniques demonstrated that it is the thin sprouting fibers that account for the difference in innervation between sham-injected (Fig 3C) and tumor-bearing femur periosteum (Fig 3D). These thin sprouts branch and extend from the main axon in an irregular, nonlinear fashion.
Figure 3. Sprouting of sensory nerve fibers in the periosteum of the tumor-bearing bone.
Representative confocal images of non-decalcified whole-mount preparations of periosteum immunostained with calcitonin gene-related peptide (CGRP) (A, B), and their accompanying higher-power camera-lucida renderings (C, D), show that there is a striking change in the density and morphology of CGRP+ nerve fibers innervating the periosteum of naïve (A, C) vs. tumor-bearing femurs (B, D) 42 days post-tumor injection. In the presence of cancer, very thin CGRP+ nerve fibers are apparent which have a more disorganized and non-linear pattern. Confocal images were acquired with a 40× objective, projected from 80 optical sections at 0.5μm intervals. Camera-lucida renderings were created using the ‘trace’ function in Image Pro Plus Version 3.0.
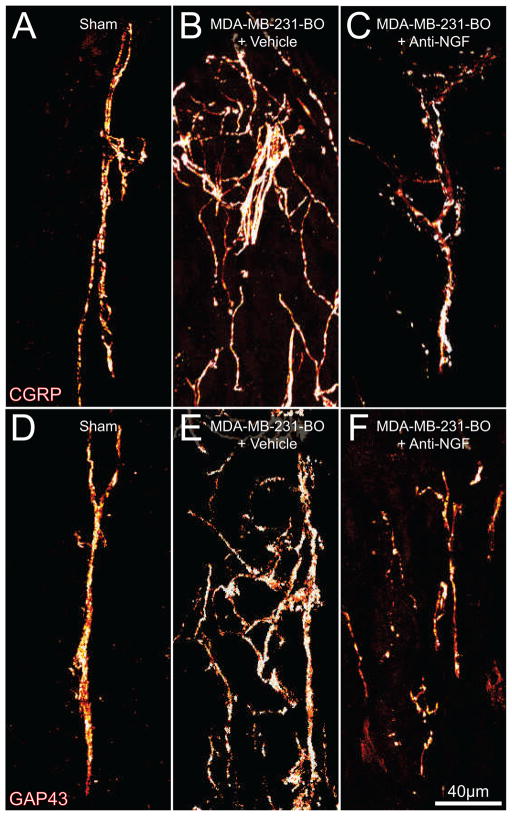
In order to visualize the periosteum in another plane and quantify the density of nerve fibers innervating the periosteum of the tumor-bearing femur, frozen sections of the tissue were processed for analysis. There did not appear to be any differences in the organization or density of CGRP+ and GAP43+ periosteal nerve fibers that innervate the femur of naïve vs. sham-injected mice (data not shown). These fibers take on a mostly linear morphology, when viewed sectionally, and have a homogenous density within the periosteum (Fig 4A, D). Immunohistochemical analysis revealed that there was a significant sprouting by CGRP+ (Fig 4B) and GAP43+ (Fig 4E) nerve fibers in tumor-bearing periosteum at day 42 post-cancer cell injection. The density of CGRP+ and GAP43+ nerve fibers increase in the tumor-bearing tissue as compared to the density of sham mice, and these fibers assume a disorganized morphology that is never observed in the periosteum of naïve or sham-injected mice (Fig 5).
Figure 4. Human breast cancer cells induce nerve sprouting in the periosteum, and this sprouting is blocked by the administration of anti-NGF.
Representative confocal images of femoral sections from sham + vehicle (A, D), MDA-MB-231-BO + vehicle (B, E), and MDA-MB-231-BO + anti-NGF (C, F) treated mice. Decalcified bone sections were immunostained with an antibody against CGRP (A, B, C) or antibody against GAP43 (D, E, F). Note that at day 42 post-injection there is a greater density of CGRP+ (B) and GAP43+ (E) nerve fibers in MDA-MB-231-BO + vehicle mice and the nerve fibers have a disorganized morphology as compared to nerve fibers innervating sham bones (A, D). Anti-NGF therapy (10 mg/kg; i.p., given at days 14, 19, 24, 29, 34, and 39 post cell injection) significantly reduces this pathological tumor-induced reorganization of CGRP+ and GAP43+ nerve fibers (C, F). Confocal images were acquired in the distal metaphyseal periosteum (~2 mm distal from the growth plate, 20μm in thickness) using sequential acquisition mode to reduce bleed-through and were projected from 40 optical sections at 0.5μm intervals with a 40× objective.
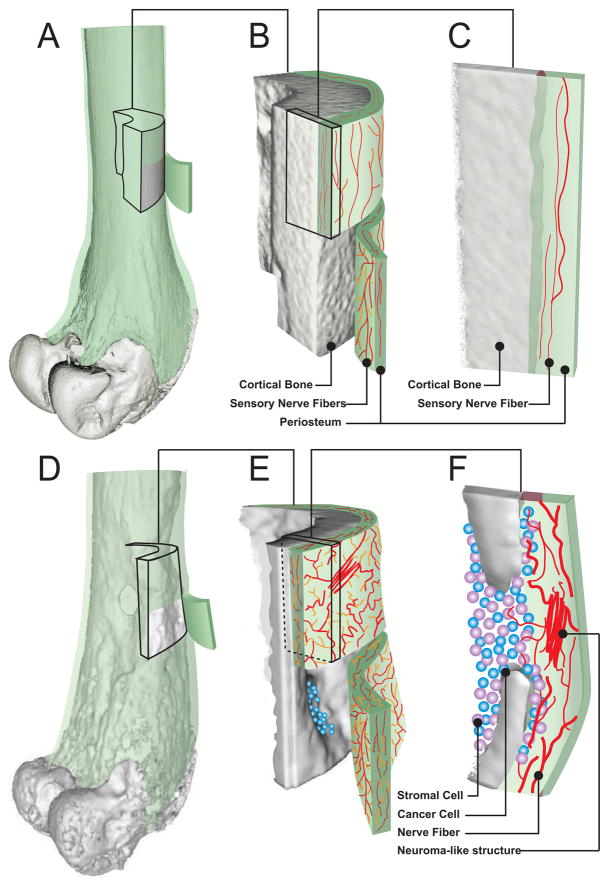
Figure 5. Schematic depicting how breast cancer cells induce sprouting of CGRP+ and GAP43+ nerve fibers in the bone.
The periosteum of the bone is a thin cellular and fibrous sheath that envelops the entire outer surface of bone but is absent from areas of articular cartilage. The periosteum is innervated by and sensory and sympathetic nerve fibers and is sensitive to distortion of the underlying cortical bone (A). Sensory nerve fibers that innervate the normal periosteum have a net-like morphology (B) and appear linear when viewed in cross-section (C). As breast cancer colonizes and remodels the bone, extensive osteoclast-driven bone resorption occurs (D), forming lesions in the cortical bone that allows cancer cells to escape into the periosteum (E, F) where these cancer and their associated stromal cells induce sprouting of CGRP+ and GAP43+ nerve fibers (E, F).
To further define what factor(s) might drive the sprouting of CGRP+ and GAP43+ nerve fibers, we used double-label immunohistochemistry with an additional antibody to TrkA, the cognate receptor for NGF. Nearly all of the sprouted CGRP+ nerve fibers expressed TrkA in the periosteum (Fig 6A, B, C) and the great majority of TrkA+ fibers were also GAP43+ (Fig 6D, E, F).
Figure 6. The majority of CGRP+ and GAP43+ sensory nerve fibers in the tumor bearing periosteum co-express the neurotrophin receptor TrkA (yellow, C, F).
Confocal images of tumor-bearing (day 42 post-cell injection) bone sections that were immunostained with CGRP (red, A), a widely used marker of nociceptors, or GAP43 (red, E) which labels regenerating and sprouting nerve fibers. These sections were co-labeled with an antibody to TrkA (green, B, D), which is the cognate receptor for NGF. Note that the great majority of CGRP+ and GAP43+ nerve fibers co-express TrkA. Each confocal image was acquired with a 40× objective, projected from 40 optical sections at 0.5μm intervals.
Anti-NGF therapy blocks the sprouting of sensory nerve fibers that innervate the periosteum
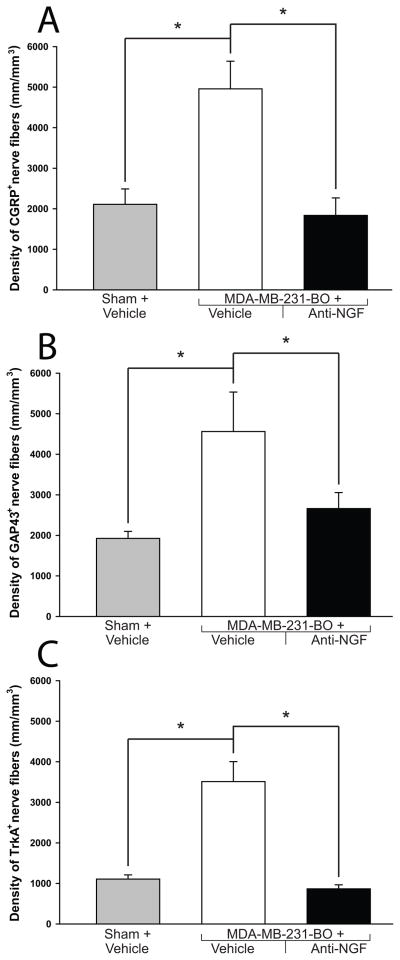
In light of these findings, the present study tested the effect of a highly specific NGF sequestering therapy23 on the pathological nerve sprouting observed in MDA-MB-231-BO-injected mice. Data from these experiments demonstrated that treatment with anti-NGF significantly attenuated the sprouting of CGRP+ (Fig. 4C, 7A), GAP43+ (Fig. 4F, 7B), and TrkA+ (Fig. 7C) nerve fibers at late stages of disease. Whereas vehicle-treated sham animals displayed a CGRP+ nerve fiber density of 2110±377 mm/mm3, MDA-MB-231-BO + vehicle treated animals had a significantly increased density (4956±682 mm/mm3). Anti-NGF therapy reduced the CGRP+ nerve fiber density in tumor-bearing mice back to baseline levels (1835±431 mm/mm3). GAP43+ nerve fiber density in vehicle-treated sham mice was 1923±174 mm/mm3, increased to 4561±974 mm/mm3 in MDA-MB-231-BO + vehicle-treated animals, and approached baseline levels in MDA-MB-231-BO + anti-NGF treated animals (2660±395 mm/mm3). TrkA+ nerve fiber density was found to be at a baseline level in the vehicle-treated sham animals (1105±105 mm/mm3), elevated levels in MDA-MB-231-BO + vehicle-treated animals (3511±493 mm/mm3), and nearly baseline density in the MDA-MB-231-BO + anti-NGF treated animals (867±98 mm/mm3). It is important to note that the majority of CGRP+ cell bodies have been shown to co-localize with TrkA2, 69 and recent work in our lab has demonstrated that sympathetic and most sensory nerve fibers of the periosteum express the TrkA receptor11. Although this would suggest that the TrkA+ nerve fiber density of the periosteum should be higher than what is currently reported, the antigenicity of the TrkA receptor has been notoriously difficult to detect in decalcified tissues and is largely eclipsed by the robust staining of CGRP.
Figure 7. Quantitative analysis of the effects of anti-NGF therapy on tumor-induced nerve sprouting.
At day 42 post cell injection, the density of CGRP+ (A), GAP43+ (B) and TrkA+ (C) nerve fibers is significantly greater in MDA-MB-231-BO + vehicle-treated mice compared to sham + vehicle-treated mice. This tumor-induced increase in the density of CGRP+, GAP43+ and TrkA+ nerve fibers is attenuated by administration of anti-NGF (10 mg/kg; i.p., given at days 14, 19, 24, 29, 34, and 39 post cell or vehicle injection). The volume of periosteum that was analyzed was an average 400 μm (length), 70 μm (width), 20 μm (depth). The Z-stacked images were analyzed with Image-Pro Plus v. 6.0 (Media Cybernetics) and nerve fibers were manually traced to determine the length of nerve fibers. Nerve sprouting was reported as total length of nerve fibers per volume of periosteum81. Both imaging acquisition and analysis were performed in a blinded fashion. Brackets indicate the groups being compared. *p<0.05. Bars represent the mean ± SEM. The number of animals used for this analysis was n≥5 for every experimental group and marker.
In addition to impeding the sprouting of sensory nerve fibers in the periosteum of tumor-bearing femurs, anti-NGF therapy also caused these nerves to maintain a normal, linear morphology similar to what is seen in the periosteum of sham and naïve mice (Fig 4C, F). Importantly, administration of anti-NGF did not affect the organization or density of CGRP+ or GAP43+ fibers in the contralateral, non-tumor-bearing bones when compared to sham mice (data not shown).
DISCUSSION
Breast cancer metastasis to bone
Breast cancer is the most commonly diagnosed cancer in females and the second leading cause of death from cancer in women29. In the USA, it was estimated that 207,090 new cases and 39,840 deaths from breast cancer occurred in 201029. Bone is a common site for breast cancer metastasis and studies have shown that 75% of patients dying of breast cancer have evidence of metastatic bone disease14. Breast cancer growth in bone frequently results in both ongoing and breakthrough bone cancer pain, hypercalcemia, anemia, increased susceptibility to infection, skeletal fractures, compression of the spinal cord, spinal instability and decreased mobility; all of which compromise the patient’s survival and quality of life14, 15.
Currently, the majority of what is known about the neurobiology of bone cancer pain has been obtained from clinical studies on how to best manage pain in cancer patients54 or from sarcoma models of bone cancer pain in mice49 70 25, 46, 47, 72, 50. Clearly, the mechanisms that generate bone cancer pain due to breast cancer may, in part, be different from sarcoma. For this reason we have developed a model of human breast cancer-induced bone pain in an attempt to more closely model what occurs in patients with breast cancer metastases to bone.
The breast model of bone cancer used here appears to closely resemble many of the aspects of the tumor growth and bone remodeling observed in the majority of human patients as 70% of breast lesions in bone are osteolytic, 15% are osteoblastic, and 15% take on a mixed growth pattern78. Thus, following injection and confinement of breast cancer cells into the mouse femur, there is a highly stereotypic tumor-induced growth and destruction of bone similar to what is observed in humans. This destruction of trabecular and cortical bone is driven by breast cancer-induced proliferation and hypertrophy of osteoclasts13, 78 and is in sharp contrast to the tumor growth and bone formation that is observed in primarily osteoblastic tumors such as prostate67, 78. Whereas prostate cancer cells tend to grow in small separate colonies of tumor cells, form copious amounts of woven bone, and remain highly vascularized for long periods of time34, the human breast cancer cells utilized in this study are significantly more osteolytic, grow more rapidly and show signs of necrosis at much earlier time points than prostate tumors growing in bone. These data suggest that this breast cancer model has the potential to not only provide clinically useful insight into the mechanisms that generate and maintain breast cancer-induced bone pain, but also the factors that drive breast cancer growth and bone remodeling.
Breast cancer-induced sprouting of periosteal sensory nerve fibers that express CGRP, TrkA and GAP43
Despite significant advances in our understanding of the mechanisms that drive ongoing cancer pain, the question of whether there is something fundamentally different between the mechanisms that drive ongoing vs. breakthrough cancer pain remains unanswered. One possibility is that with time the tumor environment induces a pathological sprouting and remodeling of sensory nerve fibers and that this newly formed substrate itself drives cancer pain. Clearly there is precedent for this as studies have shown that inappropriate sprouting and ectopic remodeling of sensory nerve fibers can give rise to hyperalgesia, allodynia, and spontaneous ectopic discharges that are perceived as highly painful in humans44, 28, 7, 12.
To examine this possibility we focused on a major class of nociceptive sensory nerve fibers that innervate the bone which are the unmyelinated or thinly myelinated nerve fibers that express CGRP52, 11. In the present study we report for the first time that human breast cancer cells induce a pathological reorganization of CGRP+ nerve fibers in the bone of nude mice. It should be emphasized that we have never observed this ectopic, dense, and highly disorganized appearance of CGRP+ nerve fibers in the periosteum of the hundreds of naïve and sham-injected bones of nude mice that we have previously examined with the same methodologies48, 61, 51, 33.
To further define the phenotype of the nerve fibers that undergo sprouting, we also examined whether these nerve fibers expressed the TrkA receptor and indeed the great majority of sprouting CGRP+ or GAP43+ nerve fibers also expressed TrkA. Interestingly, the immunofluoresence of TrkA in the newly sprouted fibers near the breast cancer cells appeared significantly higher than TrkA+ nerve fibers in naïve periosteum. The most parsimonious explanation for this increase in TrkA staining is that there was a significant up-regulation of the TrkA protein in nerve fibers near tumor cells as a similar up-regulation has been reported in the DRG of sensory nerve fibers in response to inflammation63. These newly sprouted TrkA+ nerve fibers also expressed GAP43 but the immunofluorescence level of GAP43+ nerve fibers in the breast cancer cells did not appear to be significantly different from the GAP43+ nerve fibers in the naïve or sham periosteum. As GAP43 has been shown to be involved in nerve sprouting and regeneration in both the central and peripheral nervous system4, 5, these data suggest that it is the CGRP+ nerve fibers that are actively sprouting as they co-express both TrkA and GAP43.
NGF released from tumors and their associated stromal cells drives bone cancer pain and ectopic sprouting of sensory nerve fibers
To begin to address the factor(s) that might be involved in driving the sprouting and remodeling of sensory nerve fibers observed here we focused on NGF, as nearly all the sprouting CGRP+ nerve fibers expressed TrkA, and TrkA is the cognate receptor for NGF. Additionally, previous reports have demonstrated that even in the adult, NGF can induce marked sprouting of TrkA+ sensory nerve fibers39, 62, 21, 32, 50. Using a mouse monoclonal antibody against NGF (anti-NGF), that is highly specific for NGF and shows virtually no cross-reactivity to other neurotrophins23, we show that early and sustained administration of anti-NGF results in a marked reduction of sprouting by CGRP+ nerve fibers in the tumor-bearing bone and reduces the accompanying cancer pain-related behaviors by nearly 70% while producing no change in the density of CGRP+ sensory nerve fibers in the normal periosteum. It should be noted however, that previous studies in our laboratory have shown that anti-NGF therapy is effective at reducing pain-related behaviors even before the appearance of nerve sprouting and neuroma-like structures34. Thus, we believe that NGF is involved in both sensitizing TrkA+ sensory nerve fibers as well as causing them to ectopically sprout in the diseased tissue. Whether NGF is driving this sprouting through binding to the TrkA or p75 receptor is yet unknown although previous studies suggest that TrkA is more involved in driving sprouting20, while p75 is more involved in apoptosis3.
In light of this breast cancer-induced nerve sprouting, an important and clinically relevant question remains: What is the source of NGF that is driving the sprouting of sensory nerve fibers? Previous studies have shown that MDA-MB-231 cells express NGF18, 19 in addition to tumor-associated inflammatory, immune, and / or stromal cells including: macrophages9 32, mast cells43, 58, 77, 80, endothelial cells41, 76, lymphocytes68, eosinophils74, 37 and fibroblasts83 45, 56, 62. As these stromal cells comprise 20–80% of the cells in breast tumors36, 59, 6, data from the present study suggest that therapies aimed at blocking the NGF / TrkA induced ectopic nerve sprouting should be useful in most breast cancers where either the tumor or tumor-associated stromal cells are expressing and releasing significant amounts of NGF.
Previous studies have demonstrated that MDA-MB-231 cells express NGF and TrkA, and the growth of MDA-MB-231 tumors is impeded by the sequestration of NGF1. However, in the present study we did not observe significant expression of TrkA immunoreactivity by the MDA-MB-231-BO cancer cells growing in bone although anti-NGF therapy did produce a small but statistically insignificant effect on slowing tumor growth. Whether these differences in TrkA expression in the present and previous studies are due to differences in the techniques employed, the tumor types examined, or the tumor cells growing in vitro vs. in vivo is not clear. However, the present results do clearly demonstrate that anti-NGF therapy significantly reduced MDA-MB-231-BO induced bone cancer pain and the tumor-induced sprouting of CGRP+ / TrkA+ / GAP43+ nerve fibers in nude mice. If these results can be confirmed in breast cancer patients, using therapies that block the NGF / TrkA axis may provide significant analgesia without many of the unwanted side effects of currently available analgesics.
CONCLUSIONS
These results suggest that tumor cells, along with their associated inflammatory and immune cells, release NGF which induces a marked and pathological remodeling of sensory nerve fibers that may contribute to cancer pain. Therapies that target NGF or it’s cognate receptor TrkA, may be efficacious at impeding this ectopic sensory nerve fiber sprouting and attenuating breast cancer induced bone pain (Fig 8).
Figure 8. Schematic depicting how Nerve Growth Factor (NGF) released by breast cancer and associated stromal cells appears to drive sprouting of TrkA+, CGRP+ and GAP43+ primary afferent nerve fibers in the tumor-bearing bone.
Primary afferent neurons have their cell body in the dorsal root ganglia (DRG) and transmit sensory information from the periphery to the spinal cord and brain (A). Cancer and tumor-associated stromal cells produce a variety of pro-nociceptive factors, such as NGF, that may directly activate or sensitize nociceptors. NGF binds to it’s cognate receptor TrkA, and the NGF/TrkA complex is retrogradely transported to the nucleus of the sensory neuron resulting in increased synthesis and anterograde transport of neurotransmitters, receptors, ion channels, and scaffolding molecules from the cell body to the peripheral nerve terminals located in the peripheral tissue and spinal cord. Thus, the NGF/TrkA complex may serve as an upstream regulator of nociceptor function by modulating the sensitivity or increasing the expression of several other receptors and ion channels, contributing to increased excitability of nociceptors in the vicinity of the tumor. The binding of NGF to TrkA may also induce a pathological sprouting and neuroma formation by sensory nerve fibers that may contribute to ongoing and breakthrough cancer pain (B). Anti-NGF therapy resulted in a significant reduction of the tumor-induced pain and nerve sprouting without significantly modifying disease progression (C).
Acknowledgments
The authors wish to thank Drs. Todd W. Vanderah and Setsuko K. Chambers for their outstanding comments on the discussion.
Footnotes
DISCLOSURES
This work was supported by the National Institutes of Health grant (NS23970), by the Department of Veteran Affairs, Veteran Health Administration, Rehabilitation Research and Development Service Grants (04380-I and A6707-R) and by the Calhoun Fund for Bone Pain. None of the authors of this study claim a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X, Hondermarck H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68:346–351. doi: 10.1158/0008-5472.CAN-07-1183. [DOI] [PubMed] [Google Scholar]
- 2.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz LI, Perrone-Bizzozero NI, Finklestein SP. Molecular properties of the growth-associated protein GAP-43 (B-50) J Neurochem. 1987;48:1640–1647. doi: 10.1111/j.1471-4159.1987.tb05713.x. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 6.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 7.Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64:644–653. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- 8.Body JJ. Metastatic bone disease: clinical and therapeutic aspects. Bone. 1992;13 (Suppl 1):S57–62. doi: 10.1016/s8756-3282(09)80011-2. [DOI] [PubMed] [Google Scholar]
- 9.Bronzetti E, Artico M, Pompili E, Felici LM, Stringaro A, Bosco S, Magliulo G, Colone M, Arancia G, Vitale M, Fumagalli L. Neurotrophins and neurotransmitters in human palatine tonsils: an immunohistochemical and RT-PCR analysis. Int J Mol Med. 2006;18:49–58. [PubMed] [Google Scholar]
- 10.Brownlow HC, Reed A, Joyner C, Simpson AH. Anatomical effects of periosteal elevation. J Orthop Res. 2000;18:500–502. doi: 10.1002/jor.1100180325. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207. doi: 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Muller MW, Giese T, Buchler MW, Giese NA, Friess H. Pancreatic neuropathy and neuropathic pain-a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186. e171. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Clohisy DR, Palkert D, Ramnaraine ML, Pekurovsky I, Oursler MJ. Human breast cancer induces osteoclast activation and increases the number of osteoclasts at sites of tumor osteolysis. Journal of Orthopaedic Research. 1996;14:396–402. doi: 10.1002/jor.1100140309. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 15.Coleman RE. Risks and benefits of bisphosphonates. Br J Cancer. 2008;98:1736–1740. doi: 10.1038/sj.bjc.6604382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Descamps S, Lebourhis X, Delehedde M, Boilly B, Hondermarck H. Nerve growth factor is mitogenic for cancerous but not normal human breast epithelial cells. J Biol Chem. 1998;273:16659–16662. doi: 10.1074/jbc.273.27.16659. [DOI] [PubMed] [Google Scholar]
- 19.Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22:5592–5601. doi: 10.1038/sj.onc.1206805. [DOI] [PubMed] [Google Scholar]
- 20.Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh GW, Bloom AP, Kuskowski MA, Mantyh PW. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. 2010;6:1–12. doi: 10.1186/1744-8069-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65:9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 23.Hongo JS, Laramee GR, Urfer R, Shelton DL, Restivo T, Sadick M, Galloway A, Chu H, Winslow JW. Antibody binding regions on human nerve growth factor identified by homolog- and alanine-scanning mutagenesis. Hybridoma. 2000;19:215–227. doi: 10.1089/02724570050109611. [DOI] [PubMed] [Google Scholar]
- 24.Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, O'Keefe PF, Ramnaraine ML, Clohisy DR, Mantyh PW. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6:521–528. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 25.Honore P, Mantyh PW. Bone cancer pain: from mechanism to model to therapy. Pain Med. 2000;1:303–309. doi: 10.1046/j.1526-4637.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SS, Chang VT, Kasimis B. Cancer breakthrough pain characteristics and responses to treatment at a VA medical center. Pain. 2003;101:55–64. doi: 10.1016/s0304-3959(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Probability of Breast Cancer in American Women. 2010. [Google Scholar]
- 28.Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687–697. doi: 10.1016/s1474-4422(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 30.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport. 2008;19:183–186. doi: 10.1097/WNR.0b013e3282f3c781. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: Therapeutic opportunity for treating skeletal pain. Bone. 2010;46:306–313. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimenez Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. Journal of Neuroscience. 2010;30:14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerba M, Wu JS, Duan Q, Hagen NA, Bennett MI. Neuropathic Pain Features in Patients With Bone Metastases Referred for Palliative Radiotherapy. J Clin Oncol. 2010;28:4892–4897. doi: 10.1200/JCO.2010.28.6559. [DOI] [PubMed] [Google Scholar]
- 36.Key ME. Macrophages in cancer metastases and their relevance to metastatic growth. Cancer Metastasis Rev. 1983;2:75–88. doi: 10.1007/BF00046906. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214–2220. doi: 10.1182/blood.v99.6.2214. [DOI] [PubMed] [Google Scholar]
- 38.Kruger L, Silverman JD, Mantyh PW, Sternini C, Brecha NC. Peripheral patterns of calcitonin-gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol. 1989;280:291–302. doi: 10.1002/cne.902800210. [DOI] [PubMed] [Google Scholar]
- 39.Kryger GS, Kryger Z, Zhang F, Shelton DL, Lineaweaver WC, Buncke HJ. Nerve growth factor inhibition prevents traumatic neuroma formation in the rat. J Hand Surg Am. 2001;26:635–644. doi: 10.1053/jhsu.2001.26035. [DOI] [PubMed] [Google Scholar]
- 40.Laird BJ, Walley J, Murray GD, Clausen E, Colvin LA, Fallon MT. Support Care Cancer. Characterization of cancer-induced bone pain: an exploratory study. [DOI] [PubMed] [Google Scholar]
- 41.Lecht S, Arien-Zakay H, Marcinkiewicz C, Lelkes PI, Lazarovici P. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of Erk phosphorylation. J Mol Neurosci. 2010;41:183–192. doi: 10.1007/s12031-009-9318-0. [DOI] [PubMed] [Google Scholar]
- 42.Lentz SI, Edwards JL, Backus C, McLean LL, Haines KM, Feldman EL. Mitochondrial DNA (mtDNA) Biogenesis. Visualization and Duel Incorporation of BrdU and EdU Into Newly Synthesized mtDNA In Vitro. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.954701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindqvist A, Rivero-Melian C, Turan I, Fried K. Neuropeptide- and tyrosine hydroxylase-immunoreactive nerve fibers in painful Morton's neuromas. Muscle Nerve. 2000;23:1214–1218. doi: 10.1002/1097-4598(200008)23:8<1214::aid-mus9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 45.Lindsay TH, Jonas BM, Sevcik MA, Kubota K, Halvorson KG, Ghilardi JR, Kuskowski MA, Stelow EB, Mukherjee P, Gendler SJ, Wong GY, Mantyh PW. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119:233–246. doi: 10.1016/j.pain.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Luger NM, Honore P, Sabino MA, Schwei MJ, Rogers SD, Mach DB, Clohisy DR, Mantyh PW. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001;61:4038–4047. [PubMed] [Google Scholar]
- 47.Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, Rathbun M, Clohisy DR, Honore P, Yaksh TL, Mantyh PW. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99:397–406. doi: 10.1016/S0304-3959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 48.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 49.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 50.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–598. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin CD, Jimenez-Andrade JM, Ghilardi JR, Mantyh PW. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett. 2007;427:148–152. doi: 10.1016/j.neulet.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34:623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- 53.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 54.Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev. 1998;24:425–432. doi: 10.1016/s0305-7372(98)90005-6. [DOI] [PubMed] [Google Scholar]
- 55.Mercadante S, Villari P, Ferrera P, Casuccio A. Optimization of opioid therapy for preventing incident pain associated with bone metastases. J Pain Symptom Manage. 2004;28:505–510. doi: 10.1016/j.jpainsymman.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Nassenstein C, Schulte-Herbruggen O, Renz H, Braun A. Nerve growth factor: the central hub in the development of allergic asthma? Eur J Pharmacol. 2006;533:195–206. doi: 10.1016/j.ejphar.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 57.NCI. Service Epidemiology and End Results. 2010. [Google Scholar]
- 58.Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295–2301. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 59.Normann SJ. Macrophage infiltration and tumor progression. Cancer Metastasis Rev. 1985;4:277–291. doi: 10.1007/BF00048093. [DOI] [PubMed] [Google Scholar]
- 60.Peleshok JC, Ribeiro-da-Silva A. Delayed reinnervation by nonpeptidergic nociceptive afferents of the glabrous skin of the rat hindpaw in a neuropathic pain model. J Comp Neurol. 2011;519:49–63. doi: 10.1002/cne.22500. [DOI] [PubMed] [Google Scholar]
- 61.Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, Mach DB, Schwei MJ, Sevcik MA, Mantyh PW. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005;193:85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 62.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 63.Pezet S, Onteniente B, Jullien J, Junier MP, Grannec G, Rudkin BB, Calvino B. Differential regulation of NGF receptors in primary sensory neurons by adjuvant-induced arthritis in the rat. Pain. 2001;90:113–125. doi: 10.1016/s0304-3959(00)00393-6. [DOI] [PubMed] [Google Scholar]
- 64.Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 65.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 66.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. doi: 10.1016/s0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 67.Rajarubendra N, Bolton D, Lawrentschuk N. Diagnosis of Bone Metastases in Urological Malignancies-An Update. Urology. 2010;76:782–90. doi: 10.1016/j.urology.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 68.Santambrogio L, Benedetti M, Chao MV, Muzaffar R, Kulig K, Gabellini N, Hochwald G. Nerve growth factor production by lymphocytes. J Immunol. 1994;153:4488–4495. [PubMed] [Google Scholar]
- 69.Schmidt RE, Dorsey DA, Selznick LA, DiStefano PS, Carroll SL, Beaudet LN, Roth KA. Neurotrophin sensitivity of prevertebral and paravertebral rat sympathetic autonomic ganglia. J Neuropathol Exp Neurol. 1998;57:158–167. doi: 10.1097/00005072-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, Kubota K, Kuskowski MA, Boustany L, Shelton DL, Mantyh PW. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115:128–141. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 72.Sevcik MA, Luger NM, Mach DB, Sabino MA, Peters CM, Ghilardi JR, Schwei MJ, Rohrich H, De Felipe C, Kuskowski MA, Mantyh PW. Bone cancer pain: the effects of the bisphosphonate alendronate on pain, skeletal remodeling, tumor growth and tumor necrosis. Pain. 2004;111:169–180. doi: 10.1016/j.pain.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 73.Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116:8–16. doi: 10.1016/j.pain.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 74.Solomon A, Aloe L, Pe'er J, Frucht-Pery J, Bonini S, Levi-Schaffer F. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol. 1998;102:454–460. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 75.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 76.Sornelli F, Lambiase A, Mantelli F, Aloe L. NGF and NGF-receptor expression of cultured immortalized human corneal endothelial cells. Mol Vis. 2010;16:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 77.Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90:1807–1820. [PubMed] [Google Scholar]
- 78.Tang P, Hicks DG. The histopathology of skeletal metastases. In: Heymann D, editor. Bone Cancer: Progression and therapeutic approaches. Elsevier; New York: 2010. pp. 243–250. [Google Scholar]
- 79.Turk DC, Monarch ES, Williams AD. Cancer patients in pain: considerations for assessing the whole person. Hematol Oncol Clin North Am. 2002;16:511–525. doi: 10.1016/s0889-8588(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 80.Xiang Z, Nilsson G. IgE receptor-mediated release of nerve growth factor by mast cells. Clin Exp Allergy. 2000;30:1379–1386. doi: 10.1046/j.1365-2222.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 81.Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495:679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- 82.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 83.Young M, Oger J, Blanchard MH, Asdourian H, Amos H, Arnason BG. Secretion of a nerve growth factor by primary chick fibroblast cultures. Science. 1975;187:361–362. doi: 10.1126/science.1167427. [DOI] [PubMed] [Google Scholar]
- 84.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R162–171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]