Abstract
Background
Ileal involvement in Crohn’s disease (CD) is associated with NOD2 mutations and Granulocyte-Macrophage Colony Stimulating Factor auto-antibodies (GM-CSF Ab), and GM-CSF blockade promotes ileitis in Nod2/Card15 deficient (C15KO) mice. RALDH2 expressing dendritic cells (DC) and IL-4 promote CCR9 imprinting and small bowel homing of T lymphocytes, in conjunction with CCL25 expression by ileal epithelial cells (IEC). We hypothesized that GM-CSF neutralization promotes ileal disease by modulating expression of CCL25 by IEC and CCR9 by T lymphocytes via Nod2 dependent and independent pathways.
Methods
CCL25 and CCR9 expression were determined in pediatric CD patients stratified by GM-CSF Ab. Ileitis was induced in C15KO mice via GM-CSF Ab administration followed by NSAID exposure, and expression of CCL25, CCR9, FOXP3, intracellular cytokines, and RALDH2 was determined in IEC and immune cell populations.
Results
The frequency of CCL25+ IEC and CCR9+ T lymphocytes was increased in CD patients with elevated GM-CSF Ab. In the murine model, GM-CSF blockade alone induced IEC CCL25 expression, and reduced the frequency of mesenteric lymph node (MLN) CD4+FOXP3+ cells, while Card15 deficiency alone enhanced MLN DC RALDH2 expression. Both GM-CSF neutralization and Card15 deficiency were required for down-regulation of MLN DC IL-10 expression; under these conditions NSAID exposure led to an expansion of IL-4+ and IL-17+ CCR9+ lymphocytes in the ileum.
Conclusions
GM-CSF prevents ileal expansion of CCR9+ lymphocytes via Nod2 dependent and independent pathways. CCR9 blockade may be beneficial in CD patients with elevated GM-CSF Ab.
Keywords: RALDH2, CCL25, Crohn’s Disease, dendritic cell
Introduction
The pathogenesis of Crohn’s Disease (CD) involves a complex interplay between host genetic variation and environmental factors, leading to maladaptive immune responses to endogenous and exogenous antigens. The fundamental importance of innate immunity in the development of CD has become evident in recent years. Genetic studies have discovered CARD15/NOD2 polymorphisms in patients with CD that alter their innate immune responses.1 The precise mechanisms of NOD2 mutations in the pathogenesis of CD are unknown, although functional NOD2 modulates toll-like receptor signaling and NFκB activation.2 Although nod2 deficient (C15KO) mice have normal ileal histology, they exhibit abnormal Peyer’s patch homeostasis with increased permeability and increased TLR expression altering their response to bacterial products after injury.3-4 While heterozygous NOD2 polymorphisms confer a modest increased risk of developing ileal CD, the polymorphism is also found in healthy individuals.5-6 Therefore, it would be expected that additional alterations in the innate immune system may interact with NOD2 mutations to regulate CD development within the ileum.
Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) is required for priming of myeloid cell antimicrobial functions. Our group has recently reported that neutralizing auto-antibodies to GM-CSF (GM-CSF Ab) are increased in a subset of CD patients.7-8 In addition to having abnormal neutrophil function, these patients also have an increased risk of ileal location and structuring/penetrating behavior. To test for a pathogenic role for GM-CSF Ab, we developed a novel murine model of transmural ileitis involving GM-CSF neutralization in card15-/- mice (C15KO) and non-steroidal anti-inflammatory drug (NSAID) exposure.7
However, the mechanism for ileal specificity with loss of GM-CSF bioactivity is unknown. Recruitment of lymphocytes from the mesenteric lymph node (MLN) to the intestine is a highly selective process involving adhesion molecules, chemokines and the appropriate cognate receptors. Specifically in regards to the small intestine, it is interactions between mucosal addressin cell adhesion molecule-1 (MadCAM-1) on the intestinal epithelial cell (IEC) and β7 integrins and interactions between the chemokine/chemokine receptor pair CCL25/CCR9 that maintain lymphocyte trafficking and localization to the small intestinal lamina propria.9-10 CCR9 imprinting occurs within the MLN, and requires the presence of IL-4 and RALDH2 expression by MLN dendritic cells (DC) to promote lymphocyte CCR9 expression.11-13 Alterations in CCL25/CCR9 have been described in CD patients with small bowel involvement as well as in the SAMP1/YitFc model of ileitis.14-15 However, the role of GM-CSF Ab and nod2 in this regard has not been described.
In addition to being required for CCR9 lymphocyte expression within the MLN, a role for IL-4 and Th2 cytokines has been described in the early phases of ileal Crohn’s and in the SAMP1/YitFc model of ileitis. Early post-operative recurrent ileal Crohn’s lesions were associated with a significant increase in IL-4 mRNA expression and a decrease of IFN-γ mRNA compared to normal mucosa.16 Further, in the spontaneous model of ileitis in SAMP1/YitFc mice, ileitis can be ameliorated through administration of IL-4 antibodies and transfer of IL-4+ lymphocytes from affected mice with ileitis to a SCID recipient was sufficient to induce intestinal inflammation.17
In our current study, we asked whether CCL25 and CCR9 expression would vary between pediatric CD patients with high and low levels of GM-CSF Ab, and employed our animal model of ileitis involving GM-CSF neutralization in the C15KO host to test for alterations in CCR9+ lymphocyte expansion. We determined that there was increased ileal CCL25 expression and peripheral and lamina propria CCR9 expansion in CD patients with high levels of GM-CSF Ab.
In our corresponding animal model of ileitis, we also found increased ileal CCL25 expression with loss of GM-CSF bioactivity which was nod2 independent. In card15 deficient mice which develop ileitis following GM-CSF neutralization and NSAID exposure, we observed a mixed ileal expansion of Th2 and Th17 CCR9+ lymphocytes. This expansion of CCR9 lymphocytes in the card15 deficient host corresponded to persistent expression of RALDH2 in the MLN DC and an expansion of IL-4+ T lymphocytes within the MLN.
Materials and Methods
Human Subjects
Patient-based studies were approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board. CD patients were diagnosed using established criteria, with phenotype per the Montreal criteria.18 The Pediatric Crohn’s Disease Activity Index (PCDAI) was used to measure disease activity.19 Ileal biopsies were obtained at the time of diagnostic colonoscopy and scored using the Crohn’s Disease Histological Index of Severity (CDHIS).20 Blood samples were obtained either during diagnostic endoscopy or during a routine blood draw after diagnosis and medication exposures were documented. Patients were genotyped for CARD15 mutations and GM-CSF Ab were quantified in serum by enzyme-linked immunosorbent assay.21 Based upon our previous report, CD patients were classified as GM-CSF Ab high if their GM-CSF Ab level was ≥1.6 μg/mL.7
Murine Model of Ileitis
The animal studies were approved by the CCHMC Institutional Animal Care and Use Committee. Mice were housed in conventional conditions and allowed ad lib access to drinking water and chow. We employed our previously described model of acute ileitis involving C15KO mice, GM-CSF neutralization and piroxicam (NSAID) exposure.7
Flow Cytometry Analysis of Patient Samples
Staining of peripheral blood samples were performed in 1% FBS in PBS using anti-human PerCP-CD3, FITC-CD4, and PE-CCR9 or PE-CXCR3 (BD Biosciences, San Jose, CA). Samples were evaluated on a FACSCalibur (BD Biosciences) and analyzed using Cell Quest Pro (BD Biosciences).
Immunohistochemistry
Immunohistochemistry was performed on paraffin-imbedded ileal biopsy sections obtained from human samples and mouse sections as described previously using primary antibodies for either CCL-25 (Santa Cruz Biotechnology, Santa Cruz, CA) or CCR9 (Abcam, Cambridge, MA).22
Flow Cytometry Analysis of Murine Samples
Single cell suspensions were prepared from the spleen and MLN. Lamina propria mononuclear cells from mouse ileal specimens were isolated using collagenase digestion, as described previously.23 Surface staining was performed in 1% FBS/PBS using anti-mouse PerCp-CD3, FITC- or PerCp-CD4, PE-CD44, APC-CD62l, PE- or FITC-CCR9, APC-CD11c , (BD Bioscience), APC-CD25, APC-F4/80 and PE-CD11b (eBioscience, San Diego, CA). In certain samples, cells were then fixed and permeabilized according to manufacture recommendations for PE-FoxP3, FITC-IL-4, IL-6, IL-10, IL-17, or IFN-γ intracellular staining (eBioscience). Samples were evaluated on a FACSCalibur (BD Biosciences) and analyzed using CellQuest Pro (BD Biosciences).
Murine Dendritic Cell Gene Expression Quantification with Real Time PCR
Dendritic cells (DC) were harvested from MLN as described previously.24 Briefly, MLN underwent Liberase (Roche, Indianapolis, IN) digestion and cells were stained with FITC-CD11c PE-CD3 (eBioscience) and 7-AAD (BD Pharmingen). CD11chi DC were sorted by the CCHMC Flow Cytometry Core using FACSAria II (BD Biosciences). Total RNA was isolated using the Rneasy Mini Kit (Qiagen, Valencia, CA) and transcribed using oligo primers and Superscript III (Invitrogen, Carlsbad, CA). Real time-PCR (RTPCR) was performed using the Brilliant II SYBR Green Kit (Agilent, Santa Clara, CA) with primer sequences as provided in Supplemental Table 1. Expression data were analyzed using the Stratagene Mx3000P (Agilent).
Statistical Analysis
Statistical analyses were performed using GraphPad PRISM version 5.02 (GraphPad Inc, San Diego, CA). Continuous variables were analyzed using 2-sample t test and 1-way ANOVA with Bonferroni test for multiple comparisons. Discrete variables were analyzed using the Fisher exact test or χ2 analysis. Results were considered statistically significant for p values ≤0.05.
Results
CCR9 and CCL25 are Up-regulated in Pediatric CD Patients with Elevated GM-CSF Ab
Our studies included 30 pediatric-onset CD patients and 15 healthy controls (Supplemental Table 2). Ileal sections were obtained at the time of diagnostic colonoscopy and peripheral blood samples were obtained either at the time of diagnosis or while on treatment. Age, gender, disease location, or clinical disease activity did not vary between GM-CSF Ab Lo (serum GM-CSF Ab < 1.6 mcg/mL) patients and GM-CSF Ab Hi (serum GM-CSF Ab ≥ 1.6 mcg/mL) patients. While the overall frequency of CARD15 mutations did not differ between the GM-CSF Ab Lo and Hi groups, there were insufficient samples to test for an effect of combined elevated GM-CSF Ab with a CARD15 mutation. For peripheral blood samples obtained following diagnosis, there was no difference in duration of disease between the two groups (2.5±1.6 yrs vs. 1.6±2.4 yrs, p=0.5). Exposure to 5-ASA products was higher in the GM-CSF Ab Lo patients compared to the GM-CSF Ab Hi patients (89% vs. 31%, p<0.05). Otherwise, the frequency of exposure to corticosteroids, immune modulators, or biologics did not differ between the groups.
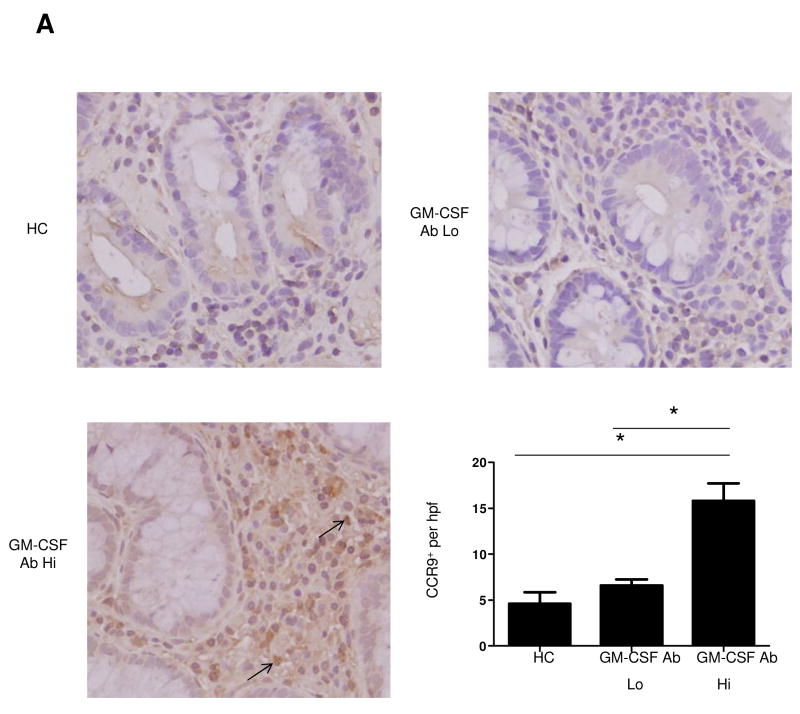
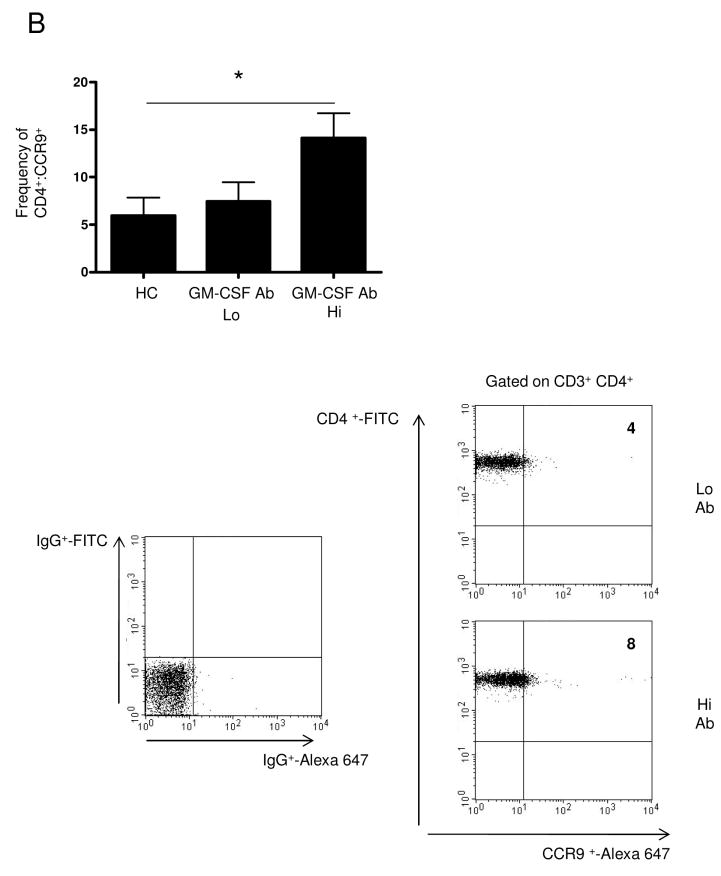
The frequency of ileal CCR9+ lamina propria mononuclear cells (LPMC) was increased in GM-CSF Ab Hi patients compared to GM-CSF Ab Lo patients or controls (Figure 1A). Examining the frequency of CCR9+ CD4+ lymphocytes in the peripheral blood, we observed an expansion of this cell population in GM-CSF Ab Hi patients compared to controls or GM-CSF Ab Lo patients (Figure 1B). This peripheral expansion was specific to the CCR9+ lymphocyte, as there were no differences in granulocyte CCR9 expression or CXCR3 expression between the three groups (data not shown).
Figure 1. Frequency of Lymphocytes Expressing CCR9 is Up-regulated in Pediatric CD Patients with Elevated GM-CSF Ab.
(A) Immunohistochemistry for CCR9 was performed on ileal biopsies obtained at the time of initial diagnostic endoscopy from HC, GM-CSF Ab Lo CD patients, and GM-CSF Ab Hi CD patients as shown. The number of positive lamina propria mononuclear cells was determined in 10 high powered fields. (B) Peripheral blood samples were obtained from patients at the time of diagnosis or during routine blood draws after diagnosis. Samples from healthy controls (HC), CD patients with low (GM-CSF Ab Lo) or high (GM-CSF Ab Hi) serum GM-CSF antibody were analyzed by flow cytometry. Cells were gated on CD3+CD4+ lymphocytes to determine the frequency of CCR9+ CD3+CD4+ cells as shown with representative scatter plots. n=8-13 per group, data are shown as the mean ± SEM, *p<0.05.
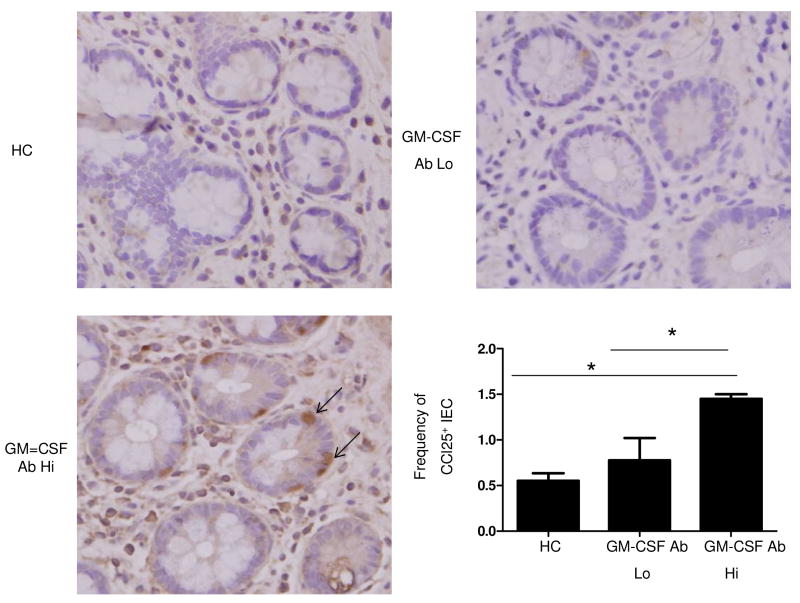
We then examined expression of the cognate chemokine for CCR9, CCL25, within ileal IEC. The frequency of CCL25+ IEC was increased in GM-CSF Ab Hi patients compared to either GM-CSF Ab Lo patients or controls (Figure 2).
Figure 2. Up-regulation of Ileal Epithelial Cell CCL25 Expression in Pediatric CD Patients with Elevated GM-CSF Ab.
Immunohistochemistry for CCL25 was performed on ileal biopsy samples obtained at the time of initial diagnostic endoscopy from HC, GM-CSF Ab Lo CD patients, and GM-CSF Ab Hi CD patients as shown. The number of intestinal epithelial cells (IEC) expressing CCL25 was determined in 10 high powered fields and divided by the total number of IEC to determine the frequency of CCL25+ IEC. n=4 per group, data are shown as the mean ± SEM, *p<0.05.
Expansion of CCR9 and CCL25 in Murine Ileitis due to GM-CSF Ab Administration
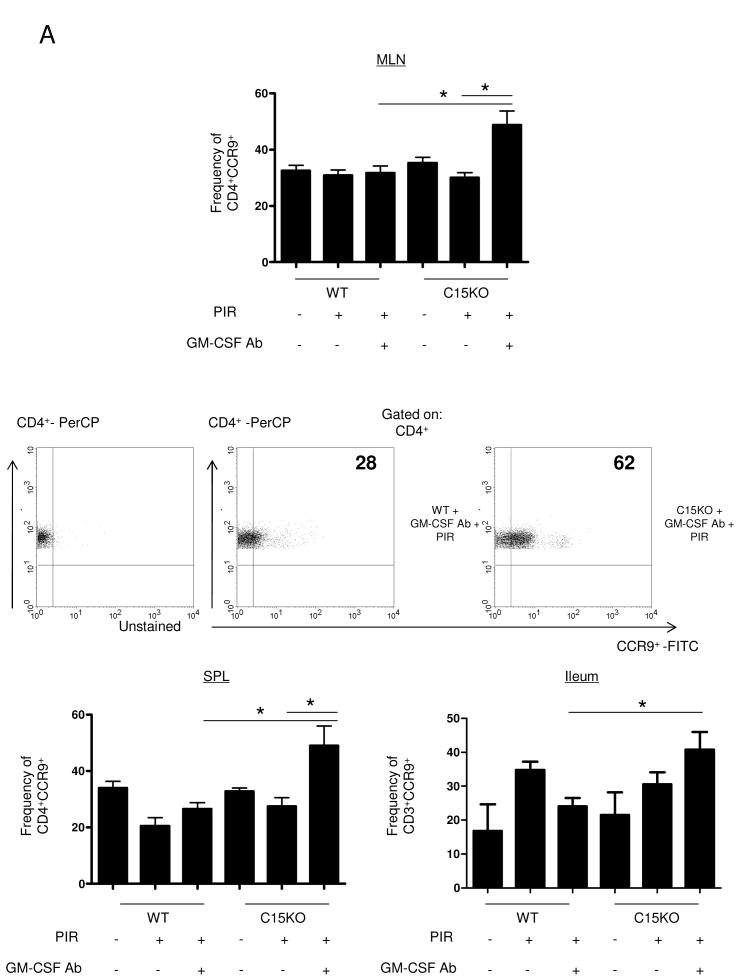
We have previously reported that when C15KO mice have GM-CSF bioactivity blocked with a neutralizing GM-CSF Ab and are on chow containing a NSAID, they develop transmural ulcerating ileal disease.7 To determine if this was due to up-regulation of CCR9 as we observed in pediatric CD patients with elevated GM-CSF Ab, we performed flow cytometric analysis using this murine model of ileitis. The frequency of CCR9+ lymphocytes was increased in the MLN, ileum, and spleen in C15KO mice following neutralization of GM-CSF bioactivity and NSAID exposure (Figure 3A). The frequency of CCR9+ lymphocytes did not vary from baseline values under any other conditions tested including in WT mice with GM-CSF neutralization and NSAID exposure or C15KO mice with isotype control IgG administration and NSAID exposure. Therefore, ileal injury was specifically associated with expansion of CCR9+ lymphocytes.
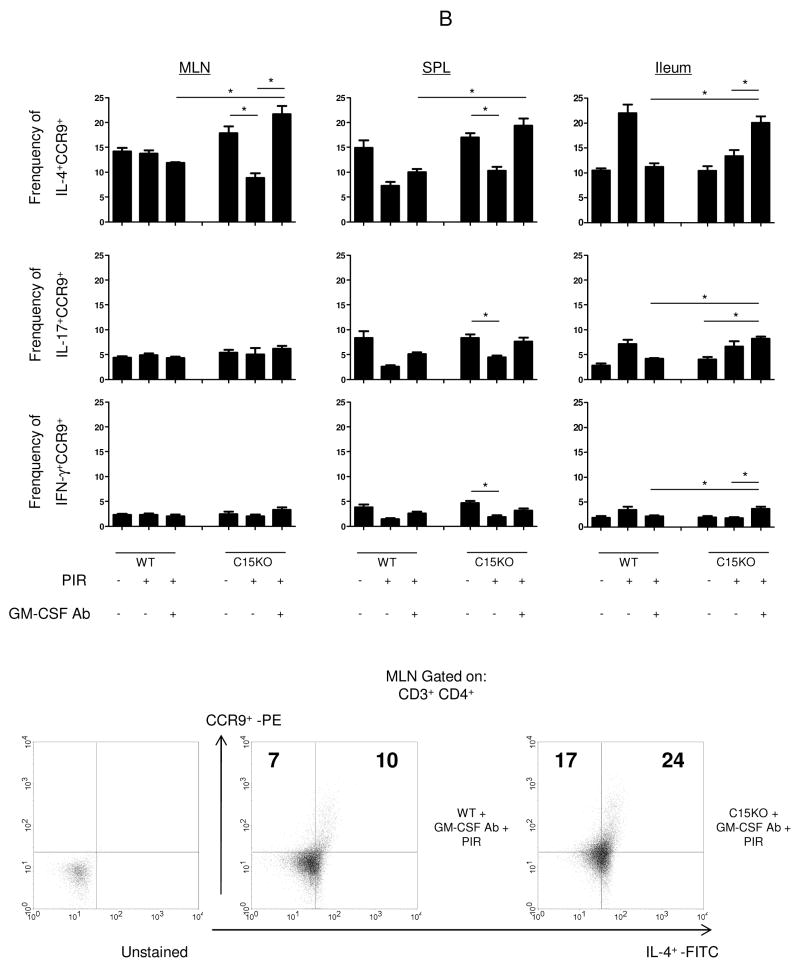
Figure 3. Up-regulation of CCR9 Expression on Lymphocytes in Murine Ileitis.
Mesenteric lymph nodes (MLN), spleen and ileum were isolated at the time of sacrifice for flow cytometric analysis from wild type (WT) or card15 deficient mice (C15KO) on regular chow, animals pre-treated with an IgG isotype control or a neutralizing GM-CSF antibody (GM-CSF Ab, 50 mcg, IP) on chow containing the NSAID piroxicam (PIR, 200 ppm). (A) Cells were gated on CD3+CD4+ lymphocytes to determine the frequency of CCR9+ CD3+CD4+ cells as shown with representative scatter plots from MLN. (B) After surface staining, cells were then fixed and permeabilized before intra-cellular staining for IL-4, IL-17, or IFN-γ was completed. Cells were gated on the CD3+CD4+ population to determine the frequency of CCR9+cytokine+ CD3+CD4+cells as shown with representative scatter plots n=4-7 per group, data are shown as the mean ± SEM, *p<0.05.
To further characterize the CCR9+ lymphocyte, we analyzed intracellular staining for Th1, Th2, and Th17 cytokines. Within the ileum, we found a mixed expansion of IL-4+, IL-17+ and IFN-γ+ CCR9+ lymphocytes in C15KO mice that develop ileal injury compared to WT mice after NSAID exposure and GM-CSF neutralization (Figure 3B). Within the C15KO mice where ileal injury occurred, the frequency of ileal IL-4+CCR9+ lymphocytes were greater than both the frequency of IL-17+ and IFN-γ+CCR9+ lymphocytes (20% vs. 8% vs. 4%, p<0.05).
IL-4 and RALDH2 have been found to be necessary for CCR9 imprinting within the MLN.11, 13 There was a significant increase in IL-4+CCR9+ lymphocytes within the MLN of the C15KO mouse that developed ileitis after NSAID exposure and GM-CSF neutralization compared to either the C15KO mouse with NSAID exposure alone or WT mouse exposed to NSAID and GM-CSF neutralization where injury was not seen (Figure 3B).
RNA from MLN CD11chi DC was isolated to examine for changes in RALDH2 expression. We observed a nod2-dependent reduction of RALDH2 expression when WT mice were exposed to NSAID that was GM-CSF-independent (Figure 4). This resulted in a significant difference in RALDH2 expression between WT mice after NSAID exposure and GM-CSF neutralization and C15KO mice that develop a CCR9+ lymphocyte expansion and ileitis.
Figure 4. RALHD2 Expression within the MLN Dendritic Cell in Murine Ileitis.
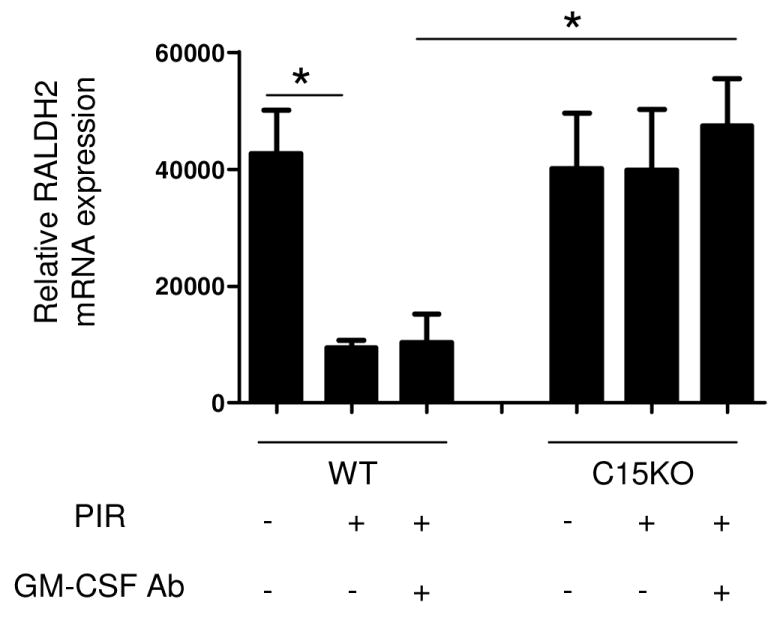
Mesenteric lymph nodes were isolated at the time of sacrifice from wild type (WT) or card15 deficient mice (C15KO) on regular chow, animals pre-treated with an IgG isotype control or a neutralizing GM-CSF antibody (GM-CSF Ab, 50 mcg, IP) on chow containing the NSAID piroxicam (PIR, 200 ppm). RNA was isolated from sorted CD11chi dendritic cells and RALDH2 gene expression was assayed by quantitative PCR and normalized relative to expression of GAPDH. n=4-7 per group, data are shown as the mean ± SEM, *p<0.05.
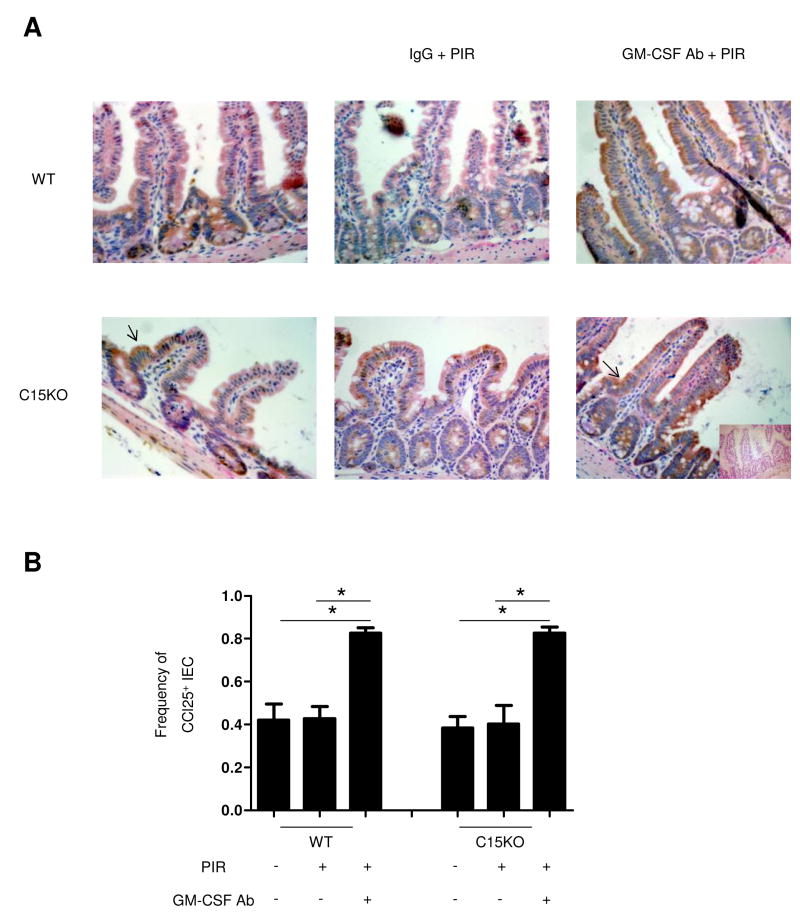
To determine if there were corresponding changes in CCL25 expression, we performed immunohistochemistry. We detected basal expression of ileal CCL25 in both genotypes, localized predominantly at the base of the crypt that did not change after only NSAID-containing chow exposure. However, following GM-CSF neutralization, CCL25 expression increased in ileal IEC in both WT and C15KO mice (Figure 5). Under these conditions, there was continued expression at the base of the crypt, but also expression in the majority of intestinal villi.
Figure 5. Ileal Epithelial Cell CCL25 Expression is Up-regulated after GM-CSF Neutralization and NSAID Exposure.
(A) Immunohistochemistry for CCL25 was performed on ileal samples obtained at the time of sacrifice wild type (WT) or card15 deficient mice (C15KO) on regular chow, animals pre-treated with an IgG isotype control or a neutralizing GM-CSF antibody (GM-CSF Ab, 50 mcg, IP) on chow containing the NSAID piroxicam (PIR, 200 ppm). A negative control employed isotype sera instead of antibody (shown as insert). (B) Intestinal epithelial cells (IEC) expressing CCL25 were determined in 10 high powered fields and divided by the total number of IEC to determine the frequency of CCL25+ IEC. n=6 per group, data are shown as the mean ± SEM.
To understand if CCR9+ lymphocyte expansion was specific or if it was associated with an overall expansion of activated/memory T-cells (CD44+CD62L-,Ta/m) we examined the frequency of Ta/m. We found that in both WT and C15KO mice, there was an increased frequency of Ta/m after GM-CSF neutralization and NSAID exposure compared to the basal frequency within the MLN (Supplemental Figure 1). In the spleen, there was a lower frequency of Ta/m in the C15KO compared to WT mice under basal conditions. In contrast to the MLN, the frequency of these cells increased only in C15KO mice, but not in WT mice, after GM-CSF Ab administration and piroxicam exposure. The frequency of Ta/m was highest within the ileum, however did not change under any conditions in either genotype.
GM-CSF Dependent Regulatory T-cell Induction after NSAID Exposure
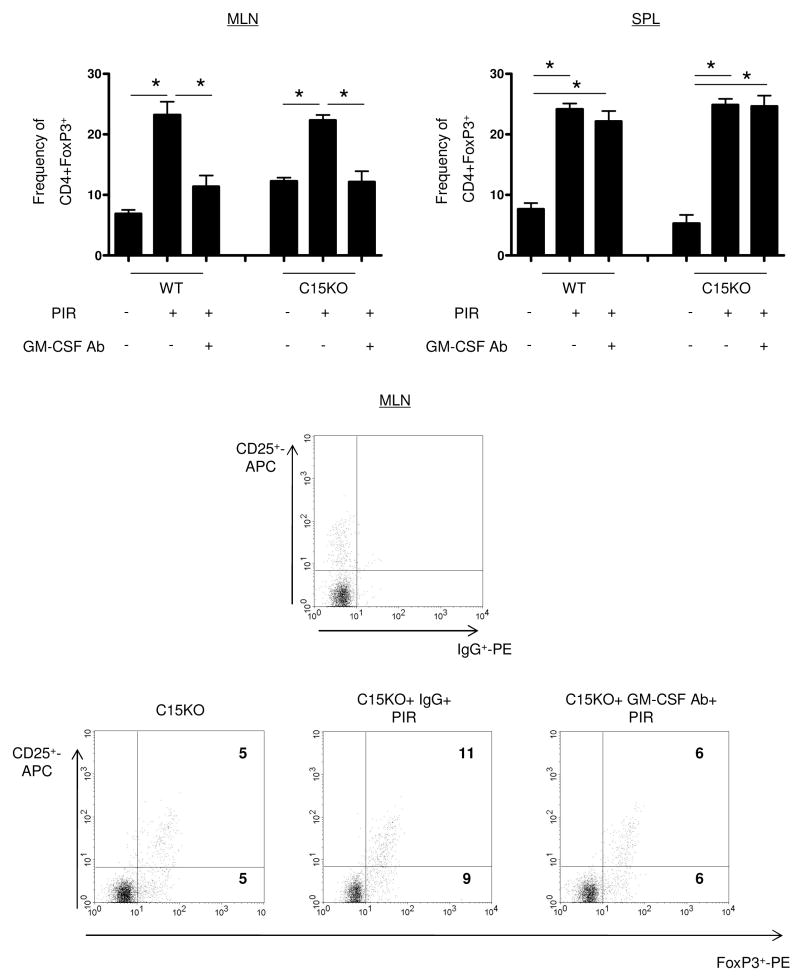
Expansion and function of CCR9+ lymphocytes may be inhibited by regulatory T-cells (Treg), so we next determined if expansion of CCR9+ lymphocytes corresponded to changes within Treg population. Using flow cytometry, a GM-CSF dependent expansion of CD3+CD4+Foxp3+ cells in the draining MLN was observed in both WT and C15KO hosts after NSAID exposure (Figure 6). Expansion in both the population of CD25+FoxP3+ cells and of CD25-FoxP3+ cells was observed. At baseline, WT and C15KO hosts had a similar frequency of Tregs in the MLN. The frequency of Tregs increased after NSAID exposure in both genotypes and this induction of FoxP3+ cells was completely inhibited by GM-CSF neutralization. In the spleen, there was an up-regulation of Tregs after NSAID exposure, but this was GM-CSF independent in both genotypes.
Figure 6. GM-CSF Dependent Regulatory T-cell Up-regulation after NSAID Exposure.
Mesenteric lymph nodes (MLN) and spleen were isolated at the time of sacrifice for flow cytometric analysis from wild type (WT) or card15 deficient mice (C15KO) on regular chow, animals pre-treated with an IgG isotype control or a neutralizing GM-CSF antibody (GM-CSF Ab, 50 mcg, IP) on chow containing the NSAID piroxicam (PIR, 200 ppm). Cells were gated on CD3+CD4+ lymphocytes to determine the frequency of FoxP3+ CD3+CD4+ cells as shown with representative scatter plots from MLN. n=5-16 per group, data are shown as the mean ± SEM, *p<0.05.
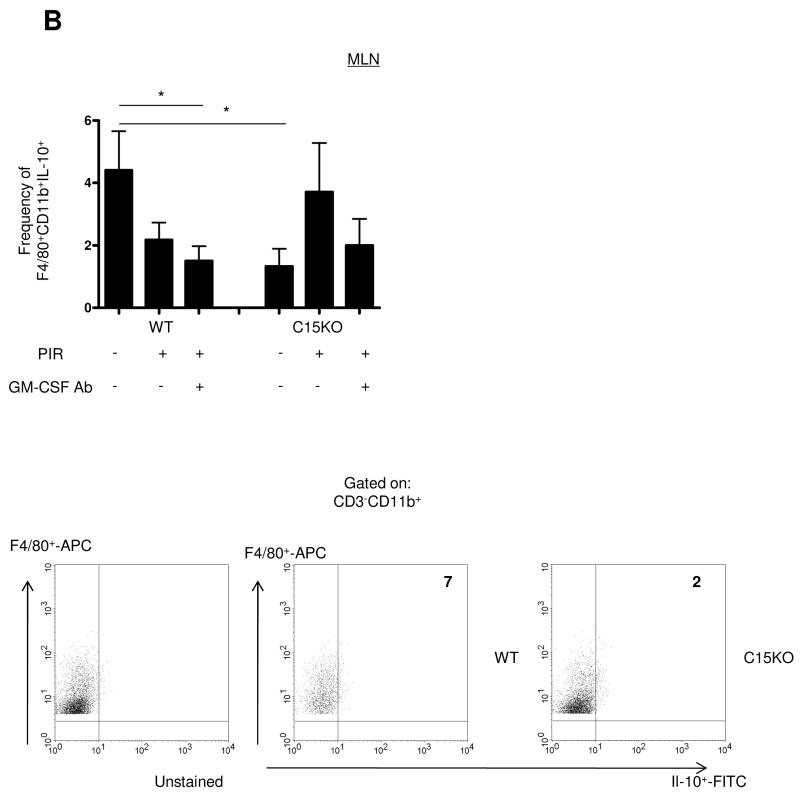
C15KO Mice Exhibit a GM-CSF Dependent Expansion of IL-10 Producing Dendritic Cells
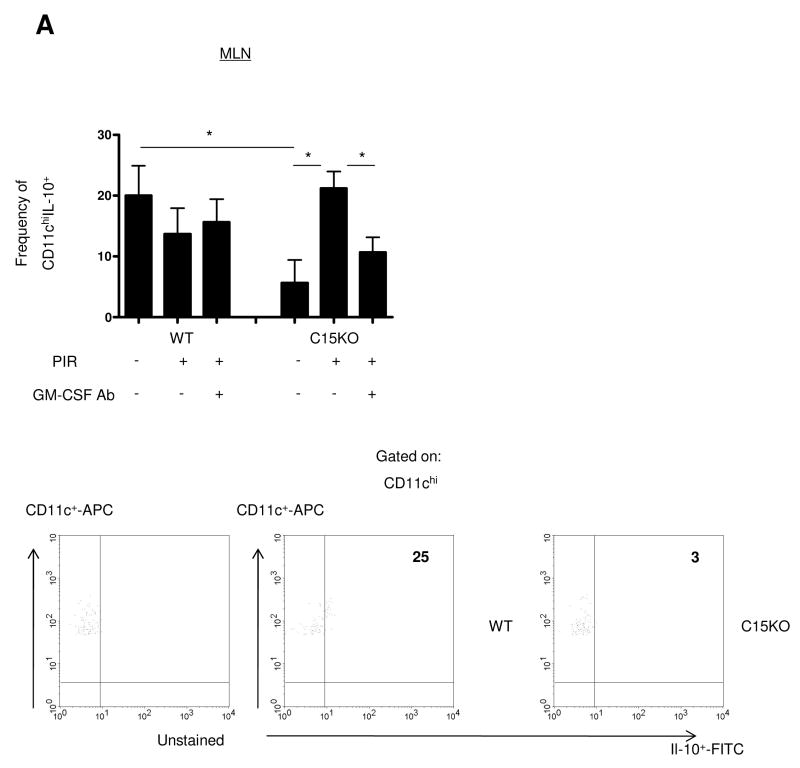
The epithelial and immune response to gut injury may be influenced by IL-10 secreted by antigen presenting cells. We therefore performed intracellular cytokine staining for FACS analysis examining the frequency of IL-10+ DC and macrophages in the different stages of the animal model. Within the MLN, we observed a significantly lower frequency of IL-10+CD11chi DC in the C15KO host compared to the WT (Figure 7A). In the WT, there were no changes in the frequency of these cell populations under any condition in the animal model. In the C15KO host, there was an increase in the frequency of IL-10+CD11chi populations after NSAID exposure which approached the frequency detected in WT animals. This up-regulation was GM-CSF dependent, as GM-CSF neutralization abrogated this response in the C15KO host. Similarly, a decreased basal frequency of MLN IL-10+CD11b+F4/80+ macrophages was observed in the C15KO host compared to the WT (Figure 7B). These differences appeared to be specific to the MLN, as the spleen and ileum exhibited no differences in the frequency of IL-10+ DC under any conditions (Supplemental Figure 2).
Figure 7. IL-10 Expression by Dendritic Cells Requires GM-CSF in the Card15 Deficient Host.
Mesenteric lymph nodes (MLN) were isolated at the time of sacrifice for flow cytometric analysis from wild type (WT) or card15 deficient mice (C15KO) on regular chow, animals pre-treated with an IgG isotype control or a neutralizing GM-CSF antibody (GM-CSF Ab, 50 mcg, IP) on chow containing the NSAID piroxicam (PIR, 200 ppm). (A) Cells were gated on the CD3- CD11chigh population to determine the frequency of IL-10+ CD3-CD11chi cells as shown with representative scatter plots. (B) Cells were gated on the CD3-CD11b+ F4/80+ population to determine the frequency of IL-10+ CD3-CD11b+F4/80+cells as shown with representative scatter plots. n=6-12 per group, data are shown as the mean ± SEM, *p<0.05.
To determine if the changes observed were specific to IL-10, we performed intracellular staining for IL-6. Similar to the frequency of IL-10+ antigen presenting cells, there was a decreased basal frequency of IL-6+ DC and IL-6+ macrophages in C15K0 mice compared to WT (Supplemental Figure 3). The frequency of IL-6+ DC in the C15KO host increased with NSAID exposure to the level observed in WT animals. Collectively, these data demonstrated a substantial, GM-CSF dependent reduction in the frequency of IL-10 expressing DC in the MLN of C15KO mice, relative to WT controls.
Discussion
In this study, we have identified alterations in CCL25 and CCR9 that are associated with loss of GM-CSF bioactivity in pediatric CD and in a model of murine ileitis. Patients with high level of antibodies to GM-CSF exhibited an up-regulation of IEC CCL25 expression, increased ileal CCR9+ LPMC, and increased expression of CCR9 on peripheral blood lymphocytes. We observed similar findings in our mouse model, where animals in the experimental group that develop transmural ileal disease had increased ileal CCL25 expression and expansion of CCR9 in the splenic, MLN and ileal lymphocyte populations. Expansion of CCR9 was associated with an expansion of IL-4+ MLN lymphocytes in C15KO mice after GM-CSF neutralization, and persistent expression of RALDH2 in C15KO MLN DC that was GM-CSF independent. We also identified several novel GM-CSF-dependent intestinal homeostatic responses, including an up-regulation of Tregs and an expansion of IL-10+ MLN DC in the card15 deficient host in response to NSAID exposure.
CCL25 has been recognized as a chemokine central to immune cell homing to the small intestine and may play a role in the pathogenesis of IBD. It is widely recognized that expression of CCL25 is limited to the small intestine in both humans and mice.25-26 Other investigators have reported that CCL25 expression is up-regulated in inflamed small bowel specimens from CD patients.14 However, our study is the first to correlate increased CCL25 expression to loss of GM-CSF bioactivity in CD and occurred both in WT and C15KO mice after GM-CSF neutralization. The factors involved in promoting the expression of CCL25 are largely unknown. It has been reported to be up-regulated in the murine ileum after TNF-α administration.27 However, a separate study found expression to be unchanged after LPS injection and was not different between germ free or conventionally-housed swine.28-29 CCL25 has been implicated in animal models of ileitis. It was shown that SAMP1/YitFc mice that develop spontaneous ileitis have increased ileal expression of CCL25 and that disease could be prevented through administration of a neutralizing antibody to CCL25.15 Yet in the TnfΔARE ileitis model, CCL25 appears to be dispensable as despite the loss of CCL25 expression, animals still develop disease.30 In our animal model, WT mice with loss of GM-CSF bioactivity and NSAID exposure also exhibit increased expression of CCL25, but yet did not develop significant ileitis. Therefore, increased CCL25 expression is not sufficient to cause ileal injury.
CCR9 is the cognate receptor for the chemokine CCL25 and homes activated T-cells from the MLN to the small intestine. MLN stromal cells and antigen-presenting DC from the Peyer’s patches induce activation, proliferation and increased expression of CCR9 on MLN lymphocytes in an IL-4 and retinoic acid dependent manner.11, 13, 31-33 In our animal model, we exhibited an expansion of MLN IL-4+CCR9+ lymphocytes as well as sustained RALDH2 expression, both of which support CCR9 imprinting, after GM-CSF neutralization and NSAID exposure in C15KO mice that develop ileitis. Factors that control RALDH2 expression are largely unknown, and our study is the first to show a nod2-dependent reduction after ileal insult.
Characterizations of CCR9+ lymphocytes have revealed a phenotype of highly activated cells, with increased expression of CD40L and pro-inflammatory cytokine production.34-35 It has been shown that therapeutic blockade of CCR9 can prevent disease in other murine models of ileitis.15, 36-37 In our model, we found a mixed expansion of IL-4+, IL-17+ and IFN-γ+ ileal CCR9+ lymphocytes which only occurred in the experimental group that developed ileitis. With a higher frequency of IL-4+CCR9+ lymphocytes compared to IL-17 and IFN-γ, our acute ileitis model shows a relative Th2 bias which is consistent with increases in IL-4 compared to IFN-γ mRNA within ileal samples of early, post-operative recurrent CD lesions and consistent with the finding that spontaneous ileitis in the SAMP1/YitFc mice can be ameliorated through inhibition of IL-4. 16-17
We found that pediatric CD patients with high levels of GM-CSF Ab had increased circulating CCR9+ lymphocytes. This is consistent with adult CD patients undergoing surgical resection having increased CCR9+ cells within the peripheral blood.14 However, in this study, inflamed portions of small bowel exhibited a decreased frequency of CCR9+ lymphocytes compared to normal small bowel. This is in contrast to our finding that GM-CSF Ab hi CD patients had increased CCR9+ LPMC compared to GM-CSF Ab lo patients or controls. One explanation for this discrepancy may be related to the stage of disease when the samples were obtained. All of our patient samples were obtained at time of diagnosis, while samples in the previous study were taken at time of surgical resection. It has been shown in the SAMP1/YitFc ileitis model that CCR9 expression increases early in the disease course, but decreases at later stages and neutralization of CCR9 is only beneficial to animals early in disease.15 A phase II study of a CCR9 antagonist has demonstrated efficacy in patients with moderate-severe CD.38 Our data suggests that patients with high level of GM-CSF Ab may particularly benefit from therapeutic blockade of this pathway.
Expansion of pro-inflammatory T-cells may result from alterations in the proportion of the Treg population or function of these anti-inflammatory cells. Peripheral contractions of Tregs and intestinal expansion have been identified in IBD patients with active disease.39-41 We identified a novel GM-CSF dependent expansion of FoxP3+ cells within the MLN in response to NSAID exposure. NSAIDs may lead to increased reactive oxygen species within the IEC and increased apoptosis with bacterial adhesion and invasion.42-44 Compromise of the intestinal barrier which leads to increased permeability may lead to expansion of Treg responses.45 GM-CSF may maintain Treg homeostasis through modulation of antigen presenting cell pathways that are important in sustaining Treg expansion and inhibition of auto-immune diseases.46-49 GM-CSF may also act to modulate TLR4 expression which has been shown to be necessary for Treg responses to intestinal injury.50-52 Although retinoic acid is involved in Treg homeostasis, other cytokines such as TGF-β are required and the inflammatory milieu may influence FoxP3 expression and regulatory T-cell function. It has been shown that despite retinoic acid treatment, transferred Treg lose suppressive function under chronic inflammatory conditions and that retinoic acid is also important in the generation of IL-17 producing T lymphocytes which promote intestinal inflammation in a T-cell transfer model of ileitis.53-54 These reports are consistent with our finding that despite persistent RALDH2 expression in the affected C15KO host with ileitis, expansion of Treg was not observed with NSAID exposure following GM-CSF neutralization.
The final novel homeostatic mechanism identified through our animal model was a nod2-dependent basal population of IL-10+ MLN DC and a GM-CSF-dependent expansion of these cells after NSAID exposure in the card15 deficient host. Multiple studies involving peripheral blood monocytes have shown that patients with mutant NOD2 have defective IL-10 responses to MDP, adherent-invasive E. Coli, and Samonella.55-58 In addition, a recent study found that there were basal differences in IL-10 expression between NOD2 mutant and wild type primary human monocytes.59 However, similar differences in basal IL-10 expression have not been found in mouse bone marrow-derived macrophages or splenocytes.59-60 This could be consistent with our findings as the basal difference was specific to the DC and macrophage within the MLN and was not observed in splenic cell populations. The biochemical mechanism for the GM-CSF dependent expansion of IL-10+ DC within the card15 deficient host is not known, although GM-CSF has been shown to be important in expression of TLR4 receptor mRNA and LPS responses.50-51
In conclusion, we have identified alterations in CCL25 and CCR9 expression related to neutralization of GM-CSF bioactivity in pediatric CD and similar changes in our novel model of murine ileitis. We also report several novel, GM-CSF-dependent and nod2-dependent homeostatic mechanisms that may play a role in prevention of disease in genetically susceptible individuals. Our data would suggest that either blockade of the CCL25/CCR9 pathway or restoration of GM-CSF bioactivity may be of therapeutic benefit to this particular group of patients.
Supplementary Material
Acknowledgments
Ramona Bezold and Kathleen Lake provided outstanding support with subject recruitment. This work was supported by the Crohn’s and Colitis Foundation of America (L.A.D.), the Broad Medical Research Program (L.A.D.), AGA Foundation for Digestive Health and Nutrition (C.M.S.), the Integrative Morphology and Flow Cytometry cores of the National Institutes of Health (NIH)-supported Cincinnati Children’s Hospital Research Foundation Digestive Health Center (1P30DK078392-01), and NIH grants T32 DK07727 (C.M.S), R01 DK078683 (L.A.D.) and R01 DK068164 (L.A.D.)
Abbreviations
- Ab
antibody
- C15KO
card15 deficient
- CD
Crohn’s disease
- CDHIS
Crohn’s disease histological index of severity
- DC
dendritic cells
- FACS
flow cytometric analysis
- GM-CSF
granulocyte-macrophage colony stimulating factor
- IEC
intestinal epithelial cell
- LPMC
lamina propria mononuclear cells
- MLN
mesenteric lymph node
- NSAID
non-steroidal anti-inflammatory drug
- PCDAI
pediatric Crohn’s disease activity index
- Ta/m
activated/memory T-cells
- Treg
regulatory T-cells
- WT
wild type
Footnotes
Financial disclosures: The authors have no financial arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
This work was previously presented in abstract form at the 2009 Digestive Diseases Week and CCFA Advances in IBD Meetings.
References
- 1.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–66. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 3.Barreau F, Meinzer U, Chareyre F, et al. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS One. 2007;2:e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreau F, Madre C, Meinzer U, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut. 2010;59:207–17. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 5.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 6.Hugot JP, Zaccaria I, Cavanaugh J, et al. Prevalence of CARD15/NOD2 mutations in Caucasian healthy people. Am J Gastroenterol. 2007;102:1259–67. doi: 10.1111/j.1572-0241.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 7.Han X, Uchida K, Jurickova I, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology. 2009;136:1261–71. e1–3. doi: 10.1053/j.gastro.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–79. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 9.Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9:836–50. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–23. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 11.Molenaar R, Greuter M, van der Marel AP, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 12.Svensson M, Johansson-Lindbom B, Zapata F, et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgueta R, Sepulveda FE, Vilches F, et al. Imprinting of CCR9 on CD4 T cells requires IL-4 signaling on mesenteric lymph node dendritic cells. J Immunol. 2008;180:6501–7. doi: 10.4049/jimmunol.180.10.6501. [DOI] [PubMed] [Google Scholar]
- 14.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121:246–54. doi: 10.1053/gast.2001.27154. [DOI] [PubMed] [Google Scholar]
- 15.Rivera-Nieves J, Ho J, Bamias G, et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518–29. doi: 10.1053/j.gastro.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn’s disease. Gastroenterology. 1997;113:118–26. doi: 10.1016/s0016-5085(97)70116-1. [DOI] [PubMed] [Google Scholar]
- 17.Bamias G, Martin C, Mishina M, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–66. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–47. [PubMed] [Google Scholar]
- 20.D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–7. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 21.Uchida K, Nakata K, Trapnell BC, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–98. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 22.Carey R, Jurickova I, Ballard E, et al. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–57. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 24.Lykens JE, Terrell CE, Zoller EE, et al. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol. 2010;184:877–85. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–8. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadakis KA, Prehn J, Nelson V, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–76. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 27.Hosoe N, Miura S, Watanabe C, et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2004;286:G458–66. doi: 10.1152/ajpgi.00167.2003. [DOI] [PubMed] [Google Scholar]
- 28.Ericsson A, Kotarsky K, Svensson M, et al. Functional characterization of the CCL25 promoter in small intestinal epithelial cells suggests a regulatory role for caudal-related homeobox (Cdx) transcription factors. J Immunol. 2006;176:3642–51. doi: 10.4049/jimmunol.176.6.3642. [DOI] [PubMed] [Google Scholar]
- 29.Meurens F, Berri M, Siggers RH, et al. Commensal bacteria and expression of two major intestinal chemokines, TECK/CCL25 and MEC/CCL28, and their receptors. PLoS One. 2007;2:e677. doi: 10.1371/journal.pone.0000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apostolaki M, Manoloukos M, Roulis M, et al. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn’s disease. Gastroenterology. 2008;134:2025–35. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 31.Hammerschmidt SI, Ahrendt M, Bode U, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 33.Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–21. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadakis KA, Landers C, Prehn J, et al. CC chemokine receptor 9 expression defines a subset of peripheral blood lymphocytes with mucosal T cell phenotype and Th1 or T-regulatory 1 cytokine profile. J Immunol. 2003;171:159–65. doi: 10.4049/jimmunol.171.1.159. [DOI] [PubMed] [Google Scholar]
- 35.Saruta M, Yu QT, Avanesyan A, et al. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. 2007;178:3293–300. doi: 10.4049/jimmunol.178.5.3293. [DOI] [PubMed] [Google Scholar]
- 36.Wei Z, Ertl L, Baumgart T, et al. Cc Chemokine Receptor 9 (ccr9) Antagonist Ameliorates Experimental Ileitis and Colitis. Gastroenterology. 2005;128:A-204–A-205. [Google Scholar]
- 37.Walters MJ, Wang Y, Lai N, et al. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2010;335:61–9. doi: 10.1124/jpet.110.169714. [DOI] [PubMed] [Google Scholar]
- 38.Keshav S, Johnson D, Bekker P, et al. PROTECT-1 Study Demonstrated Efficacy of the Intestine-Specific Chemokine Receptor Antagonist CCX282-B (Traficet-EN) in Treatment of Patients with Moderate to Severe Crohn’s Disease. Gastroenterology. 2009;136:A-65. [Google Scholar]
- 39.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Saruta M, Yu QT, Fleshner PR, et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281–90. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Yu QT, Saruta M, Avanesyan A, et al. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–9. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]
- 42.Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis. 200511:1060–9. doi: 10.1097/01.mib.0000187582.90423.bc. [DOI] [PubMed] [Google Scholar]
- 43.Omatsu T, Naito Y, Handa O, et al. Involvement of reactive oxygen species in indomethacin-induced apoptosis of small intestinal epithelial cells. J Gastroenterol. 2009;44(Suppl 19):30–4. doi: 10.1007/s00535-008-2293-3. [DOI] [PubMed] [Google Scholar]
- 44.Palmerini E, Fan K, Yang K, et al. Piroxicam increases colon tumorigenesis and promotes apoptosis in Mlh1 +/- /Apc1638(N/+) mice. Anticancer Res. 2007;27:3807–12. [PubMed] [Google Scholar]
- 45.Boirivant M, Amendola A, Butera A, et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology. 2008;135:1612–1623. e5. doi: 10.1053/j.gastro.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Cheatem D, Ganesh BB, Gangi E, et al. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol. 2009;131:260–70. doi: 10.1016/j.clim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganesh BB, Cheatem DM, Sheng JR, et al. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–82. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaudreau S, Guindi C, Menard M, et al. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–47. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 49.Yokota A, Takeuchi H, Maeda N, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–77. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bozinovski S, Jones J, Beavitt SJ, et al. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L877–85. doi: 10.1152/ajplung.00275.2003. [DOI] [PubMed] [Google Scholar]
- 51.Shibata Y, Berclaz PY, Chroneos ZC, et al. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–67. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 52.Pasternak BA, D’Mello S, Jurickova II, et al. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn’s disease and murine colitis. Inflamm Bowel Dis. 2010;16:856–69. doi: 10.1002/ibd.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menning A, Loddenkemper C, Westendorf AM, et al. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol. 2010;40:2539–48. doi: 10.1002/eji.200939938. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Kang SG, HogenEsch H, et al. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol. 2010;184:5519–26. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beynon V, Cotofana S, Brand S, et al. NOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytes. Inflamm Bowel Dis. 2008;14:1033–40. doi: 10.1002/ibd.20441. [DOI] [PubMed] [Google Scholar]
- 56.Canto E, Moga E, Ricart E, et al. MDP-Induced selective tolerance to TLR4 ligands: impairment in NOD2 mutant Crohn’s disease patients. Inflamm Bowel Dis. 2009;15:1686–96. doi: 10.1002/ibd.21013. [DOI] [PubMed] [Google Scholar]
- 57.Peeters H, Bogaert S, Laukens D, et al. CARD15 variants determine a disturbed early response of monocytes to adherent-invasive Escherichia coli strain LF82 in Crohn’s disease. Int J Immunogenet. 2007;34:181–91. doi: 10.1111/j.1744-313X.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 58.Salucci V, Rimoldi M, Penati C, et al. Monocyte-derived dendritic cells from Crohn patients show differential NOD2/CARD15-dependent immune responses to bacteria. Inflamm Bowel Dis. 2008;14:812–8. doi: 10.1002/ibd.20390. [DOI] [PubMed] [Google Scholar]
- 59.Noguchi E, Homma Y, Kang X, et al. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10:471–9. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe T, Kitani A, Murray PJ, et al. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–8. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.