Abstract
Objective
To determine pharmacokinetic parameters for oseltamivir in all trimesters of pregnancy.
Methods
Thirty pregnant women, 10 per trimester, receiving oseltamivir phosphate 75 mg were recruited to study first-dose pharmacokinetics. Plasma samples were obtained at 0, 0.5, 1, 2, 4, 8, and 12 hours after the first dose. Samples were analyzed for oseltamivir and oseltamivir carboxylate levels. Using a noncompartmental model, area-under-the-curve (AUC), maximum concentration (Cmax), time-to-maximum concentration (Tmax), and half-life (T1/2) were estimated.
Results
There were no significant differences in the pharmacokinetics of oseltamivir by trimester except for an increased T1/2 in the first trimester for oseltamivir phosphate and an increased Cmax in the third trimester for oseltamivir carboxylate. The levels of oseltamivir carboxylate observed were within the range needed to achieve IC50 concentrations for pandemic H1N1.
Conclusion
The pharmacokinetics of oseltamivir do not change significantly according to trimester of pregnancy.
Keywords: Influenza, oseltamivir, pahrmacokinetics, pregnancy
INTRODUCTION
Increased morbidity and mortality from influenza in pregnancy have been reported for seasonal and pandemic influenza, but the emergence of pandemic influenza A H1N1 in early 2009 heightened awareness of the dangers of influenza in pregnancy (1–2). Reports of pandemic influenza A H1N1 in pregnancy demonstrate that pregnant women are at increased risk for hospital admission, intensive care admission, death, and adverse neonatal outcomes (1, 3–10). In the United States, up to 5% of reported influenza-related deaths occurred in pregnant patients (7). Recognizing the increased risk of H1N1 influenza infection in pregnancy, the Centers for Disease Control and Prevention (CDC) recommended that all pregnant women with suspected influenza A H1N1 receive influenza antiviral treatment with oseltamivir and that pregnant women with close exposure to a person with H1N1 receive oseltamivir prophylaxis (10–11).
Oseltamivir is a neuraminidase inhibitor that is effective against both influenza A (including pandemic H1N1) and influenza B (12–13). The neuraminidase inhibitor binds to the active neuraminidase enzyme site, preventing enzymatic cleavage of the sialic acid bonds, and resulting in entrapment of newly replicated viruses on the host cell surface, thereby preventing progeny virion release. It also inhibits viral penetration into the respiratory tract epithelial cells (14, 15, 16). In healthy volunteers, oseltamivir has been shown to reduce the duration of viral replication, the severity and duration of influenza symptoms, the levels of biochemical markers of host inflammatory response, and the incidence of secondary infections (17–19). Early data from the H1N1 pandemic suggest that prompt initiation of antiviral treatment in pregnant patients infected with influenza results in decreased risk of ICU admission and death (7).
There are no studies, however, to guide dosing of oseltamivir during pregnancy. Oseltamivir is absorbed by the gastrointestinal tract, rapidly hydrolyzed by hepatic esterases to the active metabolite oseltamivir carboxylase, and is excreted by the kidney through both glomerular filtration and secretion (14, 18). Given the physiologic changes of pregnancy that include a 50% increase in glomerular filtration rate and a 40–45% increase in blood volume (20), some investigators have advocated higher doses of oseltamivir in acutely ill pregnant patients with severe disease requiring ventilator support (21). In a meeting of experts convened by the CDC anticipating future pandemic influenza, however, Rassmussen and colleagues noted, “No data are available to address whether dosage adjustment is needed; thus, no dosage alteration for pregnant women are recommended at this time”(2). To provide some guidance on this issue, we studied the pharmacokinetics of first-dose oseltamivir in all trimesters of pregnancy in thirty pregnant patients with influenza or influenza exposure.
MATERIALS & METHODS
This study was approved by the Internal Review Board at the University of Texas Southwestern Medical Center, Dallas, Texas. Pregnant subjects with a singleton gestation, admitted to the hospital and receiving oseltamivir for treatment or prophylaxis of influenza were eligible for the study. In the 2008–2009 influenza season, only subjects who presented within forty-eight hours of symptom onset and who had a laboratory confirmed diagnosis of influenza were treated with antiviral therapy. During that influenza season, seasonal influenza A showed resistance to oseltamivir, and subjects with influenza A also received treatment with an M2 ion channel inhibitor (22). During the 2009–2010 H1N1 pandemic season, subjects were treated with oseltamivir based on clinically diagnosed and/or laboratory confirmed influenza and were treated regardless of time since symptom onset (11). In both influenza seasons, subjects being treated for influenza received oseltamivir phosphate (Tamiflu®) 75 mg capsule twice daily for five days. Pregnant subjects who were hospitalized for other conditions, exposed to influenza, and elected to receive prophylaxis with oseltamivir were also eligible for the study. They received the approved adult influenza prophylaxis dosing of oseltamivir phosphate 75 mg orally once daily for ten days. Subjects were excluded if they had an allergy or prior adverse reaction to oseltamivir or known kidney or liver disease.
After the decision to treat had been made by the obstetrical team caring for the patient, the researchers were notified. Subjects who consented to participate in the study had blood samples drawn at the time of the first dose of medication and at 0.5, 1, 2, 4, 8, and 12 hours after the first dose of oseltamivir, for a total of seven blood draws. Plasma samples were separated and prepared for storage (−80° Celsius) for batch analysis.
Plasma samples were shipped to BASI Laboratory (United Kingdom) and analyzed for oseltamivir phosphate and oseltamivir carboxylate content using a validated tandem mass spectrometric method (method on file). The method was accurate and sensitive ranging from 1 ng/mL and 10 ng/mL (RSD < 5%) for oseltamivir phosphate and oseltamivir carboxylate, respectively. Oseltamivir and oseltamivir carboxylate concentrations (ng/mL) were plotted versus time (hours) for each of the respective subjects. Data from the 30 subjects were analyzed both as a single group and as trimester subgroups. Using a noncompartmental model, area-under-the-curve (AUC), maximum concentration (Cmax), time-to-maximum concentration (Tmax), and half-life (T1/2) were estimated using Thermo Kinetica 5.0 (Thermo Fischer Scientific, Waltham, MA). The pharmacokinetic estimates were summarized. An Analyses of Variance and Ryan-Einot-Gabriel-Welsh Multiple Range Test were used to determine significance between study variables (SAS 9.2, Cary NC).
RESULTS
Thirty-five subjects receiving oseltamivir were identified and approached for study participation, and thirty were enrolled. All but two patient were Hispanic (reflective of our patient population), and they ranged in age from 16–34 years (Table 1). Twenty-nine subjects were admitted with influenza: 13 with pandemic H1N1, 6 with 2008–2009 influenza B, and 10 with 2008–2009 Influenza A. One subject was admitted for gestational diabetes and elected prophylaxis for an influenza B exposure. All subjects received 75 mg oseltamivir phosphate capsules, and the subjects with 2008–2009 influenza A also received an M2 ion channel inhibitor (6 rimantidine/4 amantadine). All but subject number 11 had singleton gestations; she was enrolled in the study but was later determined to be ineligible due to a twin gestation. All but two study participants completed the seven scheduled plasma collections. Subject 4 elected to withdraw after four draws when her IV stopped working, and subject 21 did not have her 12-hour level analyzed because she received her second dose of medicine before the collection was completed. None of the subjects had severe influenza requiring mechanical ventilation or intensive care unit admission. The average BMI (kg/m2) was in the class I obesity range (1st trimester 30 ± 5, second trimester 33 ± 8, third trimester 30 ± 3), ranging from 21–48 kg/m2. There was no significant difference in the BMI of subjects by trimester (p=0.282).
Table 1.
Demographic data of individual patients including maternal age (years), gestational age (weeks), weight (pounds), BMI (kg/m2), and influenza type.
| Gestational | |||||
|---|---|---|---|---|---|
| Patient | Age | Age | Weight | BMI | Influenza Type |
| 1 | 22 | 10–11 | 115 | 21 | B |
| 2 | 30 | 5–6 | 160 | 29 | B |
| 3 | 23 | 10–11 | 204 | 35 | A |
| 4 | 30 | 9–10 | 177 | 30 | A |
| 5 | 29 | 12–13 | 182 | 33 | A |
| 6 | 25 | 9–10 | 174 | 33 | H1N1 |
| 7 | 16 | 9–10 | 182 | 36 | H1N1 |
| 8 | 26 | 11–12 | 134 | 27 | H1N1 |
| 9 | 21 | 10–11 | 169 | 30 | H1N1 |
| 10 | 22 | 8–9 | 133 | 23 | H1N1 |
| 11 | 33 | 18–19 | 165 | 29 | A |
| 12 | 23 | 16–17 | 153 | 26 | A |
| 13 | 31 | 21–22 | 264 | 48 | B |
| 14 | 25 | 21–22 | 153 | 27 | A |
| 15 | 21 | 18–19 | 169 | 33 | H1N1 |
| 16 | 22 | 17–18 | 202 | 34 | H1N1 |
| 17 | 26 | 25–26 | 162 | 34 | H1N1 |
| 18 | 25 | 25–26 | 246 | 45 | H1N1 |
| 19 | 30 | 22–23 | 120 | 26 | H1N1 |
| 20 | 24 | 21–22 | 165 | 32 | H1N1 |
| 21 | 20 | 32–33 | 193 | 37 | B |
| 22 | 20 | 34–35 | 176 | 34 | B |
| 23 | 27 | 28–29 | 150 | 28 | A |
| 24 | 25 | 37–38 | 218 | 36 | Exposure |
| 25 | 25 | 34–35 | 184 | 30 | A |
| 26 | 18 | 38–39 | 180 | 32 | A |
| 27 | 24 | 38–39 | 164 | 30 | A |
| 28 | 22 | 36–37 | 210 | 36 | B |
| 29 | 34 | 34–35 | 194 | 34 | H1N1 |
| 30 | 26 | 34–35 | 166 | 32 | H1N1 |
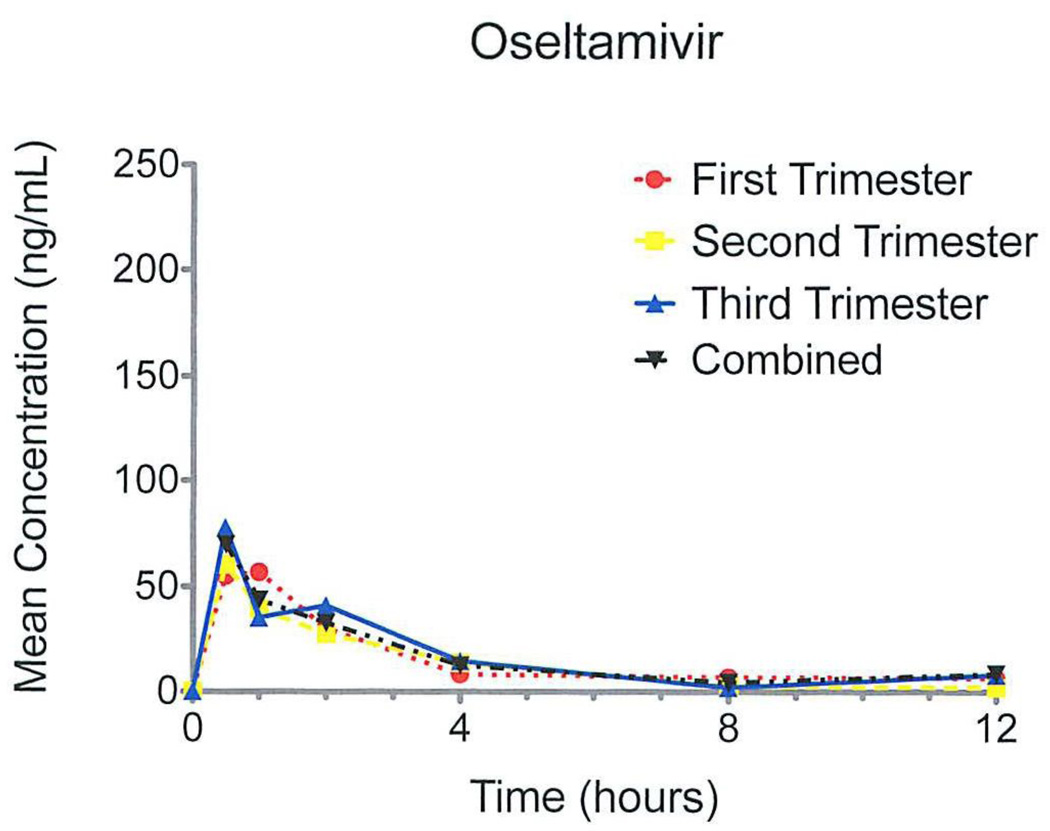
The pharmacokinetic pattern for oseltamivir was similar for each trimester (Figure 1). For oseltamivir, Cmax (ng/mL) and Tmax (hours) were not significantly different between trimesters, ranging from 80–101 ng/mL and 0.9–2.3 hours, respectively (Table 2). The half-life of oseltamivir (T1/2 ) was significantly longer in the first trimester subjects (4.0 hours) compared with the second and third trimester subjects (2.1 and 1.5 hours, respectively). The AUC0-12 (ng·hr/mL) was not significantly different between trimesters, ranging from 151 to 215 ng·hr/mL (Table 2).
Figure 1.
Mean concentrations of oseltamivir after first dose graphed by entire cohort and by trimester.
Table 2.
Pharmacokinetic parameters for oseltamivir phosphate prodrug.
| Trimester 1 | Trimester 2 | Trimester 3 | P-value | |
|---|---|---|---|---|
| Cmax (ng/mL) | 80 ± 18 | 75 ± 56 | 101 ± 59 | 0.43 |
| Tmax (hour) | 0.9 ± 0.5 | 1.1 ± 0.7 | 2.3 ± 3.6 | 0.30 |
| T1/2 (hour) | 4.0 ± 3.1* | 2.1 ± 0.8 | 1.5 ± 0.5 | 0.02 |
| AUC0–12 h (ng·h/mL) | 215 ± 124 | 151 ± 57 | 166 ± 46 | 0.23 |
Data expressed as mean value with standard deviations.
Trimester 1 is significantly different from Trimester 2 and 3.
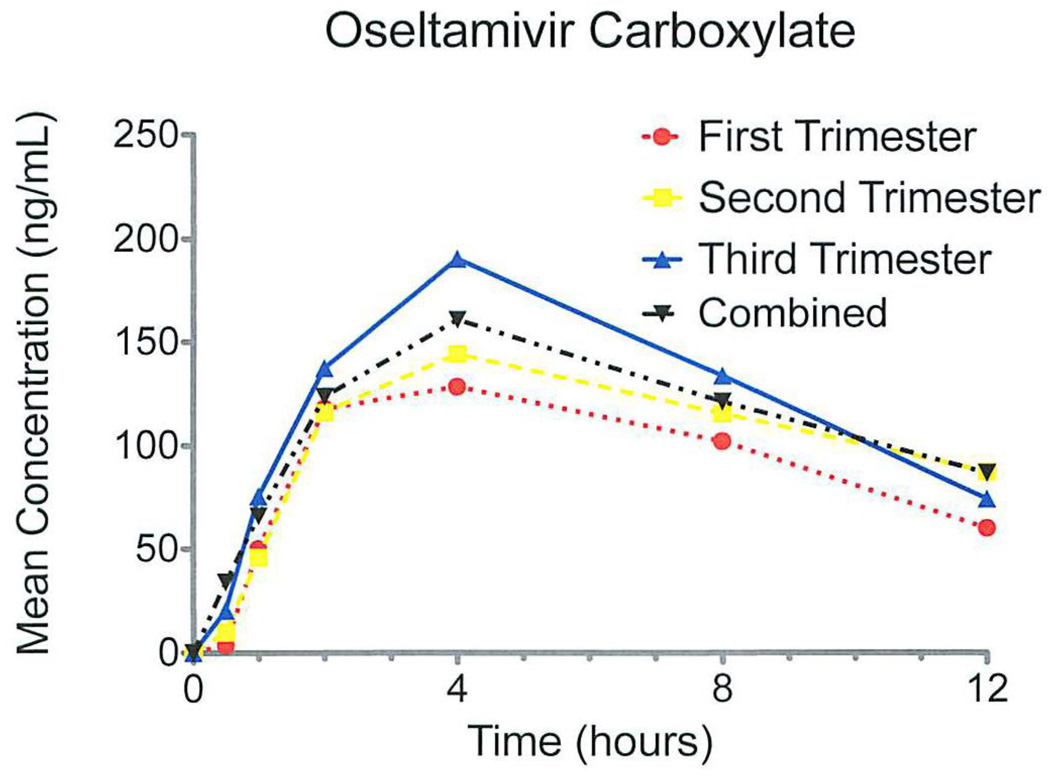
For the active metabolite oseltamivir carboxylate, we also observed similar pharmacokinetics by trimester (Figure 2). The Cmax (ng/mL) was significantly higher in the third trimester, 198 ng/mL, compared with those in the first and second trimester, 150 ng/mL and 153 ng/mL, respectively (Table 3). The AUC0-12 (ng·hr/mL) was not significantly different between trimesters, ranging from 1828 ng·hr/mL in the first trimester to 2367 ng·hr/mL in the third trimester. The Tmax and T1/2 were not significantly different between trimesters and ranged from 3.4–4.6 hours and 6.4–9.4 hours, respectively.
Figure 2.
Mean concentrations of oseltamivir carboxylate after first dose graphed by entire cohort and by trimester.
Table 3.
Pharmacokinetic parameters for active metabolite oseltamivir carboxylate.
| Trimester 1 | Trimester 2 | Trimester 3 | P-value | |
|---|---|---|---|---|
| Cmax (ng/mL) | 150 ± 23 | 153 ± 53 | 198 ± 43* | 0.02 |
| Tmax (hour) | 3.4 ± 1.0 | 4.6 ± 3.1 | 3.8 ± 1.8 | 0.46 |
| T1/2 (hour) | 6.9 ± 2.3 | 9.4 ± 6.1 | 6.4 ± 1.5 | 0.23 |
| AUC0–12 h (ng·h/mL) | 1828 ± 406 | 2325 ± 1008 | 2367 ± 650 | 0.269 |
Data expressed as mean value with standard deviations.
Trimester 3 is significantly different from Trimester 1 and 2
COMMENT
Our study revealed several important observations: 1) the first-dose Cmax for oseltamivir carboxylate in pregnancy were lower than the steady state Cmax reported for non-pregnant subjects; 2) the levels of oseltamivir carboxylate observed were within the range needed to achieve IC50 concentrations for pandemic H1N1; 3) the AUC0-12 for oseltamivir and oseltamivir carboxylate were within previously reported ranges for first-dose oseltamivir phosphate and did not differ among trimester.
Pregnancy does not appear to significantly affect the time to achieve maximum oseltamivir concentration. We observed values in pregnancy consistent with non-pregnant ranges (0.9–2.3 hours for oseltamivir and 3.4–4.6 for oseltamivir carboxylate). Similarly, the reported T1/2 for oseltamivir is 1–3 hours (12, 15, 18) and for oseltamivir carboxylate is 6–10 hours (12, 14, 18), similar to our data. The T1/2 of oseltamivir carboxylate was not different between trimesters and fell within the reported T1/2 in non-pregnant subjects.
Pregnant subjects have similar first dose Cmax for oseltamivir compared to non-pregnant subjects but may have lower Cmax for oseltamivir carboxylate than reported values for non-pregnant subjects. The mean Cmax (ng/ml) reported by the manufacturer for non-pregnant subjects receiving a 75 mg dose of oseltamivir phosphate is 65.2 ng/mL for oseltamivir and 348 ng/mL for oseltamivir carboxylate. (18) Brewster et al reported similar values of 75.1 ng/mL for oseltamivir phosphate and 276 ng/mL for oseltamivir carboxylate. (12) While the Cmax we observed in pregnancy for oseltamivir is well within the prior reported ranges, the average Cmax for the active metabolite in each trimester (150–198 ng/mL) was lower than previously reported values (18, 23).
Another important parameter regarding the concentration of oseltamivir carboxylate is the inhibitory concentration (IC). The concentration required in vitro to achieve a 50% or 90% inhibition of the neuraminidase enzyme is designated the IC50 or IC90. The ICs vary by influenza type, subtype, strain, and clade. The manufacturer reports IC50 of 0.0008 µM to > 35 µM and IC90 of 0.004µM to > 100 µM for oseltamivir carboxylate (18). Other investigators have reported ICs that fall within this range, with influenza B generally having higher ICs (15, 24). The reported IC50 for pandemic H1N1 ranges from 0.28 to 1.41 nM (25). Based on our first dose Cmax values, our subjects had average levels of 0.528 nM in the first trimester, 0.538 nM second trimester, and 0.697 nM third trimester. These values fall within the lower half of the range reported for H1N1. Specifically, the levels recorded in our study would meet the IC50 for approximately half of the strains reported (25). Considering H5N1 avian influenza, however, mean IC50 concentrations range from 0.09 to 11.45 depending on the H5N1 strain (26), which are above the levels we observed.
The AUC0-12h data are more complex. Although the AUC0-12 was not different by trimester, the AUC0-12 (and the Cmax) for the active metabolite oseltamivir carboxylate was highest in the third trimester. This finding is surprising given the increasing blood volume, increasing GFR, and increasing placental unit in the third trimester, which led us to hypothesize that values would be lower during the third trimester compared to the first. The absence of a decrease in levels in the third trimester may be because little oseltamivir or oseltamivir carboxylate reaches the amniotic cavity. Worley et al using an ex vivo placental model detected neither oseltamivir phosphate nor oseltamivir carboxylate in the transplacental perfusate when therapeutic dosing levels were used (27).
When comparing our AUC0-12h data in a pregnant population with influenza to previously published data in non-pregnant subjects, it is important to recognize that the levels in our study reflect first-day, first-dose pharmacokinetics. We found AUC0-12h for oseltamivir ranged from 151–215 ng·h/mL and for oseltamivir carboxylate ranged from 1828–2367 ng·h/mL. These values are within the means reported by Schentag et al for Caucasian (75.9 ng·h/mL and 1092 ng·h/mL, respectively) and for Japanese subjects (100 ng·h/mL and 1367ng·h/mL) following the first dose of oseltamivir phosphate (13). Similarly, He and colleagues calculated first dose AUC0-12h for 50 mg and 100 mg dosing and reported oseltamivir carboxylate levels of 1206 ng·h/mL and 2450 ng·h/mL, respectively (14). By comparison, the manufacturer reports the AUC0-12h for steady state after multiple 75 mg doses to be 112 ng·h/mL for oseltamivir and 2719 ng·h/mL for oseltamivir carboxylate. Schentag and He both reported first dose and steady state AUC0-12h, and the steady state levels were approximately 1.5 times higher than the first dose AUC0-12h (12, 14). If this association is similar in pregnancy, our AUC0-12h at steady state would range from 2742 ng·h/mL first trimester to 3551 ng·h/mL third trimester, comparable with the AUC0-12h reported by the manufacturer.
Our study has several limitations. First, the study was designed to assess the pharmacokinetics only after the first dose of medication. We do not have antepartum steady state data, and comparisons of our observations with steady state IC50 data may not be valid. Second, we had a racially homogenous group. Limited data from prior studies, however, have not demonstrated racial differences in the pharmacokinetics of oseltamivir (13, 16). Finally, as other influenza strains emerge or oseltamivir resistance develops, IC concentrations for new strains should be considered in determining dosing and timing recommendations in pregnant and nonpregnant adults.
In conclusion, we found few significant differences in the pharmacokinetics of oseltamivir or oseltamivir carboxylate by pregnancy trimester. Furthermore, pregnancy did not appear to change significantly the timing of absorption or of conversion of oseltamivir phosphate to oseltamivir carboxylate. Concentrations of the active metabolite after the first dose were lower than non-pregnant first dose concentrations and lower than levels reported for steady-state concentrations in non-pregnant adults. These first-dose values may underestimate levels expected at steady state. Overall, we found that approved adult dosing of oseltamivir provides similar antepartum plasma concentrations to those achieved in non-pregnant subjects. This critical clinical evidence supports the current treatment recommendations for pregnant women with influenza.
Acknowledgments
Sources of funding: University of Texas Southwestern Medical Center Department of Obstetrics and Gynecology and by Grant # 5-U10-HD046000-05 from National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jamison DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 Influenza virus infection during pregnancy in the USA. The Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, MacFarlane K, Cragan JD, Williams J, Henderson Z. Pandemic Influenza and Pregnant Women: Summary of a Meeting of Experts. Am J Public Health. 2009;99 Supp 2:248–253. doi: 10.2105/AJPH.2008.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 Influenza A and Pregnancy Outcomes in Victoria, Australia. Clin Infect Dis. 2010;50:686–690. doi: 10.1086/650460. [DOI] [PubMed] [Google Scholar]
- 4.Louie JK, Acosta M, Jamieson DJ, Honein M. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. New Eng J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 5.Archer BN, Cohen C, Naidoo D, et al. Interim Report on Pandemic H1N1 influenza virus infections in South Africa, April to October 2009: Epidemiology and Factors Associated with Fatal Cases. Euro Surveill. 2009;14(42) doi: 10.2807/ese.14.42.19369-en. pii=19369. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19369. [DOI] [PubMed]
- 6.Louie JK, Acosta M, Winter K, et al. Factors Associated with Death or Hospitalization Due to Pandemic 2009 Influenza A (H1N1) Infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 7.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 Influenza A (H1N1) Virus Illness Amount Pregnant Women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG. Severity of 2009 Pandemic Influenza A (H1N1) Virus Infection in Pregnant Women. Obstet Gynecol. 2010;115(4):717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 9.Miller AC, Safi F, Hussain S, Subramanian RA, Elamin EM, Sinert R. Novel Influenza A (H1N1) Virus Among Gravid Admissions. Arch Intern Med. 2010;170:10868–10873. doi: 10.1001/archinternmed.2010.126. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2009 Pandemic Influenza A (H1N1) in Pregnant Women Requiring Intensive Care-New York City, 2009. MMWR. 2010;59(11):321–325. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Updated Interim Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. [Accessed February 1, 2011]; Available at: http://www.cdc.gov/h1n1flu/pregnancy/antiviral_messages.htm.
- 12.Brewster M, Smith JR, Dutkowski R, Robson R. Active metabolite from Tamiflu® solution is bioequivalent to that from capsule delivery in healthy volunteers: A cross-over, randomized, open-label study. Vaccine. 2006;24:6660–6663. doi: 10.1016/j.vaccine.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 13.Shentag JJ, Hill G, Chu T, Rayner CR. Similarity in pharmacokietnics of oseltamivir and oseltamivir carboxylate in Japanese and Caucasian subjects. J Clin Pharmacol. 2007;47(6):689–696. doi: 10.1177/0091270007299761. [DOI] [PubMed] [Google Scholar]
- 14.He G, Massarell J, Ward P. Clinical Pharmacokinetics of the Prodrug Oseltamivir and its Active Metabolie Ro 64-0802. Clin Pharmacokinet. 1999;37(6):471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Massarella JW, He GZ, Dorr A, Neiforth K, Ward P, Brown A. The Pharmacokinetics and Tolerability of the Oral Neuraminidase Inhibitor Oseltamivir (Ro 64-0796/GS4104) in Healthy Adult and Elderly Volunteers. J Clin Pharmacol. 2000;40:836–843. doi: 10.1177/00912700022009567. [DOI] [PubMed] [Google Scholar]
- 16.Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65 supp 2:ii5–ii10. doi: 10.1093/jac/dkq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden FG, Treanor JJ, Fritz RS, et al. Use of the Oral Neuraminidase Inhibitor Oseltamivir in Experimental Human Influenza: Randomized Controlled Trials for Prevention and Treatment. JAMA. 1999;282(13):1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 18.Roche FH-L. Tamiflu oseltamivir phosphate: product information. [Accessed February 1, 2011];2004 Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM183850.pdf.
- 19.Ng S, Cowling BJ, Fang VJ, et al. Effects of Oseltamivir Treatment on Duration of Clinical Illness and Viral Shedding and Household Transmission of Influenza Virus. Clin Inf Dis. 2010;50(1):707–714. doi: 10.1086/650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham FG, Leveno KJ, Bloom SC, Hauth JC, Rouse DJ, Spong CY. Williams Obstetrics. 23rd ed. McGraw-Hill Professional; 2009. pp. 114–126. [Google Scholar]
- 21.Saleeby E, Chapman J, Morse J, Bryant A. H1N1 Influenza in Pregnancy: Cause for Concern. Obstet Gynecol. 2009;114(4):885–891. doi: 10.1097/AOG.0b013e3181bb44bb. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Antiviral Drug-Resistant Strains of Seasonal Influenza Virus. [Accessed February 1, 2011]; Available at: http://www.cdc.gov/flu/professionals/antivirals/resistance.htm.
- 23.Widner N, Meylan P, Ivanyuk A, Aouri M, Decosterd LA, Buclin T. Oseltamivir in seasonal, avian H5N1 and pandemic 2009 A/H1N1 influenza. Clin Pharmacokinet. 2010;49(11):741–765. doi: 10.2165/11534730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Bantia S, Parker CD, Ananth SL, et al. Comparison of the Anti-Influenza virus Activity of RWJ-270201 with Those of Oseltamivir and Zanamivir. Antimicrob Agents Chemother. 2001;45(4):1162–1167. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Update: Drug Susceptibility of Swine-Origin Influenza A (H1N1) Viruses, April 2009. MMWR. 2009;58(16):433–435. [PubMed] [Google Scholar]
- 26.Taylor WRJ, Thinh BN, Anh GT, et al. Oseltamivir is Adequately Absorbed Following Nasogastric Administration to Adult Subjects with Severe H5N1 Influenza. PLos One. 2008;3(10):e3410. doi: 10.1371/journal.pone.0003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worley KC, Roberts SW, Bawdon RE. The Metabolism and Transplacental Transfer of Oseltamivir in the Ex Vivo Human Model. Infect Dis Obstet Gynecol. 2008;2008:927574. doi: 10.1155/2008/927574. [DOI] [PMC free article] [PubMed] [Google Scholar]