Abstract
Stroke induces a biphasic effect on the peripheral immune response that involves early activation of peripheral leukocytes followed by severe immunosuppression and atrophy of the spleen. Peripheral immune cells, including T lymphocytes, migrate to the brain and exacerbate the developing infarct. Recombinant T-cell receptor (TCR) Ligand (RTL)551 is designed as a partial TCR agonist for myelin oligodendrocyte glycoprotein (MOG)-reactive T cells and has demonstrated the capacity to limit infarct volume and inflammation in brain when administered to mice undergoing middle cerebral artery occlusion (MCAO). The goal of this study was to determine if RTL551 could retain protection when given within the therapeutically relevant 4h time window currently in clinical practice for stroke patients. RTL551 was administered subcutaneously 4h after MCAO, with repeated doses every 24h until the time of euthanasia. Cell numbers were assessed in the brain, blood, spleen and lymph nodes and infarct size was measured after 24 and 96h reperfusion. RTL551 reduced infarct size in both cortex and striatum at 24h and in cortex at 96h after MCAO and inhibited the accumulation of inflammatory cells in brain at both time points. At 24h post-MCAO, RTL551 reduced the frequency of the activation marker, CD44, on T-cells in blood and in the ischemic hemisphere. Moreover, RTL551 reduced expression of the chemokine receptors, CCR5 in lymph nodes and spleen, and CCR7 in the blood and lymph nodes. These data demonstrate effective treatment of experimental stroke with RTL551 within a therapeutically relevant 4h time window through immune regulation of myelin-reactive inflammatory T-cells.
BACKGROUND
It is well known that inflammatory cells in the periphery contribute to brain damage induced by ischemic stroke. Experimental stroke in male mice induces a biphasic response on the peripheral immune system characterized by an initial activation phase (6–22 h) followed by an immunosuppressive phase (96h) which is accompanied by a pronounced atrophy of spleen and thymus. Peripheral immune cells including those that reside in the spleen home to the brain. The developing infarct is exacerbated by the influx of inflammatory cells and the time course and degree of accumulation of multiple inflammatory cell types in brain has been extensively studied (Gelderblom et al. 2009;Stevens et al. 2002;Wang et al. 1993).
T-cells may be major offenders in mediating the post-stroke inflammatory response, contributing to increased brain damage. When activated, T-cells produce cytokines that initiates an inflammatory cascade involving recruitment of other inflammatory cells to sites of injury (Jin et al. 2010). T-cells are observed in brain at within hours of the ischemic insult (Gelderblom et al. 2009;Schroeter et al. 1994) and T-cell deficient animals have reduced infarct size after stroke (Yilmaz et al. 2006). One overarching hypothesis is that T-cells promote an autoaggressive response to brain antigens. As the blood brain barrier is breached after an ischemic event, myelin-reactive antigens leak out and are exposed to the peripheral immune system which recognizes them as foreign and propagates an autoaggressive immune response, facilitating the infiltrating of immune cells, into the brain. Evidence to support this contention includes increased influx of myelin oligodendrocyte glycoprotein (MOG)-specific T-cells into the brain (Dirnagl et al. 2007) and reduced infarct size attained by nasal vaccination with a MOG peptide (Frenkel et al. 2003) after experimental stroke.
Despite the severity and increasing incidence of the disease, few therapeutic options are currently available (Jauch et al. 2006). Most studies have focused on brain outcome, but general immunosuppressive therapy is a danger for increasing risk of fatal infection. Recombinant T-cell receptor ligands (RTL) can selectively modulate harmful autoaggressive CD4+ T-cells by delivering partial agonist signals through the T-cell receptor (Burrows et al. 2001; Wang et al. 2003), resulting in reversal of clinical paralysis in an experimental model of multiple sclerosis (Burrows et al. 1999;Burrows et al. 2000). Thus, RTLs that target brain-reactive T-cells have the potential to inhibit T-cell mediated inflammation in the CNS without inducing general immunosuppression. RTL551 is synthesized as a single peptide chain comprising membrane distal α1 and β1 domains of the I-Ad class II molecule expressed by C57BL/6 mice used in MCAO studies covalently linked to the encephalitogenic MOG-35-55 peptide (Sinha et al. 2007). We demonstrated previously that targeting MOG-specific T-cells with RTL551 not only improved stroke outcome, but also partially prevented general immunosuppression that occurs in later stages after MCAO (Subramanian et al. 2009). RTL551, but not RTLs with mismatched class II molecules or correctly matched class II with non-CNS peptides, were effective in reducing infarct volume and the number of infiltrating immune cells in the mouse model of reversible focal ischemia when administered at the time of reperfusion (Subramanian et al. 2009). However, to be further considered as a potential therapy for stroke, treatment must be proven effective when administered hours after the brain attack (Jauch et al. 2006).
The current study extends these findings by demonstrating the ability of RTL551 to similarly reduce infarct size and infiltration of immune cells into the CNS when administered within a therapeutically relevant time window, 4h after induction of MCAO in C57BL/6 mice. In addition, treatment of MCAO mice with RTL551 reduced expression of T-cell activation (CD44) and CNS homing receptor (CCR5 and CCR7) markers known to be important in stroke. These results suggest that RTLs hold promise as a novel therapeutic for treatment of stroke.
METHODS
Animals
All experiments were conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals. Age-matched sexually mature 8–10 weeks of age, male mice C57BL/6J; Charles River Laboratories, body weight 20–28 g were used in all experiments.
RTL Construction and Production
RTL551 was designed to inhibit inflammatory responses of MOG-35-55 specific, I-Ab-restricted T-cells. RTL551 consists of the α1 and β1 domains of the I-Ab MHC class II molecule covalently linked to mouse (m)MOG-35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) and was expressed as a single polypeptide as described previously (Sinha et al. 2007;Subramanian et al. 2009). Protein purification was as previously described (Burrows et al. 1999) with a 30–40mg yield of purified protein per liter of bacterial cell culture with negligible levels of lipopolysaccharide (LPS).
RTL treatment in a therapeutically relevant time window
100µl of Vehicle (20mM Tris pH 8.5 in 4% Dextrose) or 100µg RTL551 in 100µl vehicle was administered by subcutaneous injection 3h after a 60min reperfusion followed by subsequent administration at 24 and 72h after reperfusion. Therefore, mice euthanized at 24h received one injection and mice euthanized at 96h received three injections of RTL551 or Vehicle.
Middle cerebral artery occlusion (MCAO) in mice
Reversible focal cerebral ischemia was induced by MCAO via the intraluminal suture technique under isoflurane anesthesia for 60 min as previously described (Offner et al. 2006a;Subramanian et al. 2009). Body and head temperatures were controlled at 36.5±1.0°C, with a warming blanket and heat lamps during surgery and ischemia. A small laser-Doppler probe was affixed to the skull to monitor cortical perfusion and verify vascular occlusion and reperfusion. A silicone-coated 6-0 nylon monofilament was inserted into the right internal carotid artery via the external carotid artery until a drop in laser-Doppler signal was observed. Occlusion was confirmed by a decrease in laser-Doppler signal to less than 25% of baseline. After securing the filament in place, the surgical site was closed, and the animal was awakened. At 60 min of occlusion, the mouse was re-anesthetized, the laser-Doppler probe re-positioned over same site on the skull, and the occluding filament withdrawn to allow for reperfusion. Mice were then allowed to recover for either 24 or 96h after occlusion. For the sham MCAO animal group, the same procedure was conducted except the external carotid was cut and the common and internal carotid arteries were exposed but were otherwise left undisturbed.
Infarct Volume Assessment
Infarction volume was measured by TTC histology as in previous studies (Offner et al. 2006a;Offner et al. 2006b) with the following modification. Infarct size was measured at 24 or 96h after MCAO from a 1-mm thick coronal brain section using 2,3,5-triphenyltetrazolium chloride staining and digital image analysis. The section was incubated in 1.2% 2,3,5-triphenyltetrazolium chloride in saline for 15 min at 37°C, and then fixed in 10% formalin for 24h. Slices were photographed, and analyzed for infarction size with SigmaScan Pro 5.0 software (Systat Software Inc., San Jose, CA, USA). The percent infarct was calculated from integrating the area from the anterior and posterior sides. This method yielded a similar result with RTL vs vehicle treatment by the standard 5 slice method (data not shown). The remaining ipsilateral and contralateral hemispheres were used for isolation of brain mononuclear cells, antibody staining and flow cytometry.
Isolation of leukocytes from spleen, thymus, blood and brain
Spleens and lymph nodes (superficial cervical, mandibular, axillary, lateral axillary, superficial inguinal and mesenteric) from individual sham- and MCAO-treated mice were removed and a single-cell suspension was prepared by passing the tissue through a 100µm nylon mesh (BD Falcon, Bedford, MA). The cells were washed using RPMI 1640 and the red cells lysed using 1x red cell lysis buffer (eBioscience, Inc., San Diego, CA) and incubated for 3 min. The cells were then washed twice with RPMI 1640, counted, and resuspended in stimulation medium (RPMI, containing 10% FBS, 1% sodium pyruvate, 1% L-glutamine, .4% βME. Cardiac blood was collected and placed in heparinized tubes (Fisher Scientific, Pittsburgh, PA). Cells were pelleted and 1x red cell lysis buffer was added to the cell pellet and incubated for 7 min. Cells were washed twice with RPMI, counted and resuspended in RPMI 1640. The brain was divided into the ischemic (right) and nonischemic (left) hemisphere, digested for 60 min with 1 mg/ml Type IV collagenase (Sigma Aldrich, ST. Louis, MO) and DNase I (50 mg/ml, Roche diagnostics, Indianapolis, IN) at 37C with shaking at 200 rpm. Samples were triturated with a 1 ml pipette every 15 mins. The suspension was washed 1x in RPMI, resuspended in 80% Percoll overlayed with 40% Percoll and centrifuged for 30 min at 1600 RPM. The cells were then washed twice with RPMI 1640, counted, and resuspended in stimulation medium.
Analysis of cell populations by fluorescence-activated cell sorting (FACS)
All antibodies were purchased (BD Biosciences, San Jose, CA or eBioscience, Inc., San Diego, CA) as published. Four-color (FITC, PE, APC and PerCP) fluorescence flow cytometry analyses were performed to determine the phenotypes of splenocytes, thymocytes and blood leukocytes, as previously published (Offner et al. 2006a). One million cells were washed with staining medium (PBS containing 0.1% NaN3 and 1% bovine serum albumin (Sigma, Illinois) and incubated with the combinations of the following monoclonal antibodies: CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD11b (MAC-1), CD45 (Ly-5) and CD11c (HL-3), CD19 (1D3), GR-1 (IA8), CD44 (IM7), CCR5 (7A4), CCR7 (EB-1) for 10 min at 4°C. 1 ml of staining buffer was added to wash the cells. Propidium iodide was added to identify dead cells.
Statistical analysis
Differences between infarct volumes were analyzed with Student’s t-test. Spleen, lymph node and blood cell counts and percentages of cellular subtypes for FACS analysis were analyzed by one-way analysis of variance (ANOVA) followed by a Neuman-Kuels post hoc test. Fisher’s exact text was used for 24 and 96h survival analysis. The criterion for statistical significance was p≤0.05. All values are reported as mean ± SEM.
RESULTS
RTL551 reduced infarct size at 24 and 96h post-MCAO
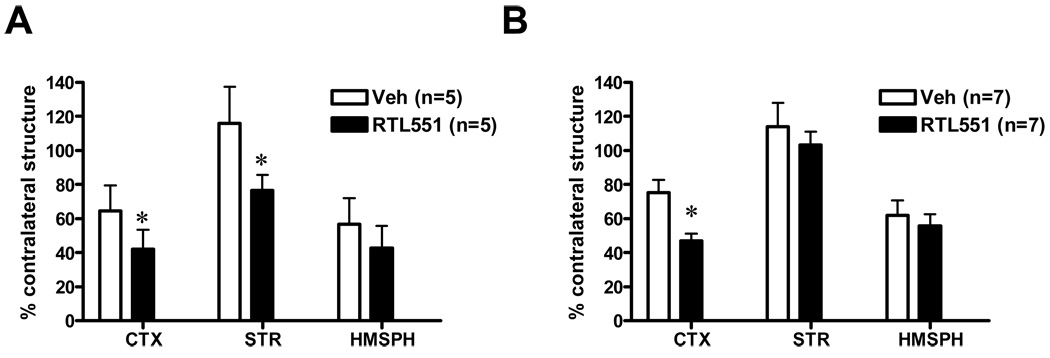
Treatment of MCAO with RTL551, administered 3h after reperfusion, resulted in approximately 35% reduction in infarction volume in the cortex (64.6±6.7, Veh versus 42.0±5.2, RTL551-treated mice) and 34% in the striatum (116±9.6, Veh versus 76.5±4.2, RTL551-treated mice) compared with vehicle treatment 24h after MCAO. Total infarction in RTL551-treated mice was 42.6±5.7 versus 56.8±6.8 in Vehicle-treated animals 24h after MCAO (Figure 1A). RTL551 when administered 3h after reperfusion followed by three daily injections reduced resulted in approximately a 38% reduction in infarction volume in the cortex (75.3±7.4, Veh versus 46.8±4.2, RTL551-treated mice) but not in the striatum 96h after MCAO. Total infarction volume in RTL551-treated mice was 51.5±5.5 versus 61.9±3.5 in Vehicle-treated animals (Figure 1B). There were no differences in mortality between Vehicle- and RTL551-treated groups; 5% in vehicle versus 11% with RTL551 treatment at 24h and 40% in vehicle versus 42% with RTL551 treatment at 96h. There were no differences in LDF between groups during MCAO or upon reperfusion.
FIGURE 1. RTL551 treatment reduced infarct size 24h and 96h after MCAO.
Transient MCAO was treated with RTL551 (100µg/100µl RTL in Tris-HCl, pH 8.5 in 4% dextrose) or Vehicle 3h after a 60 min occlusion and reperfusion and then daily until the time of euthanasia. Infarction volume was assessed by TTC staining as a percentage of the ischemic structure relative to the nonischemic contralateral structure for cortex (CTX), striatum (STR) and total hemisphere (HMSPH) at 24h (A) and 96h (B) following reperfusion. *indicates significant reduction in infarct size.
RTL551 reduced cell numbers and early infiltration of dendritic cells and neutrophils in brain
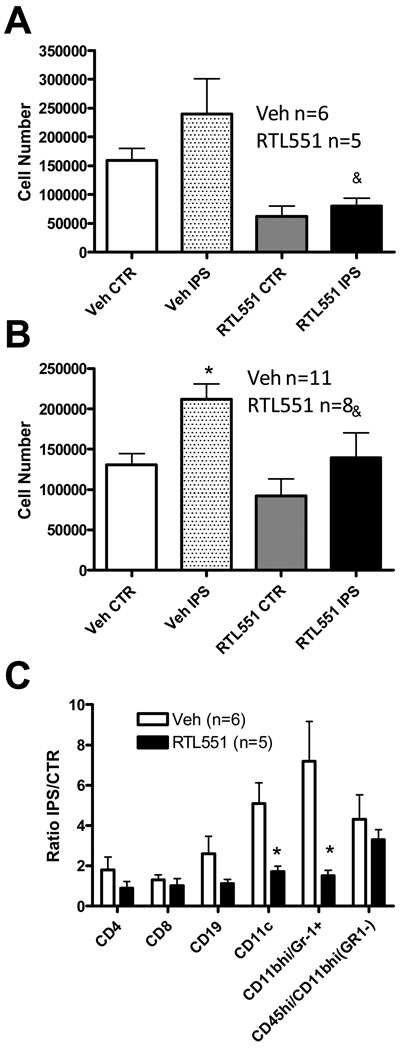
We found previously that RTL551 treatment when administered at the time of reperfusion reduced inflammatory cell infiltration into the brain after stroke. In the current study, we determined if RTL551 could also diminish the influx of inflammatory cells into brain when administered 3h after the time of reperfusion. RTL551 treatment reduced total brain mononuclear cells at both 24 and 96h compared to Vehicle-treated controls (Figure 2a and 2b). RTL significantly reduced absolute numbers of total viable leukocytes at 24h (from 31,299±16,301 in Vehicle-treated mice to 19,536±7,574 in RTL551-treated mice, n=11–12) from the ischemic ipsilateral hemisphere as well as from those recovered from the unaffected contralateral hemisphere (32,716 ±14,063 in Vehicle-treated mice to 15,234±8,503 in RTL551-treated mice, n=11–12). At 96h RTL551 treatment reduced the absolute number of total viable leukocytes (from 43,975±20,141 in Vehicle-treated mice to 28,250±18,500 in RTL551-treated mice) in the ischemic hemispheres (p=0.05) and in the contralateral hemispheres (from 39,135±19,508 in Vehicle-treated mice to 26,182±20,655 in RTL551-treated mice, n=13–14). Using 4-color flow cytometry, a reduction was observed in the percentage of most cell types in the ischemic (ipsilateral) relative to unaffected hemisphere (contralateral) in RTL551-treated compared to Vehicle-treated mice, with a statistical significance in the dendritic cell (CD11b/CD11c+) and granulocyte (CD11b/Gr-1+) populations (Figure 2c). The reduction in the percentage of dendritic cells was not observed after 96h, and although there was a similar decrease in the percentage of granulocytes with RTL551 treatment, the difference was not statistically significant (data not shown).
FIGURE 2. RTL551 treatment reduced influx of inflammatory cells 24 and 96h after MCAO.
Total brain mononuclear cells were isolated from non-ischemic hemispheres or ischemic hemispheres 24h (A) and 96h (B) after MCAO and flow cytometry was used to evaluate the percentage of T-cell (CD4, CD8), B-cell (CD19), granulocyte (CD11b/Gr-1+) and activated macrophages/microglia (CD45hi/CD11bhi/Gr-1−) populations at 24h post-MCAO (C). The percentages were expressed as a ratio of the ipsilateral ischemic hemisphere (IPS) relative to the non ischemic contralateral hemisphere (CTR). *significant difference from vehicle treated animals.
RTL551 did not alter peripheral cell numbers
To determine whether RTL551 would affect stroke-induced atrophy, cell numbers in the spleen, lymph nodes and blood were counted in post-ischemic Vehicle and RTL551-treated mice and compared to respective sham-treated animals. There were no differences between Vehicle vs. RTL551 treatments in the number of leukocytes isolated from blood, lymph nodes or spleen at either 24 or 96h post-MCAO (Tables 1 and 2). MCAO reduced the number of splenocytes by 96h in Vehicle-treated animals as expected but the reduction was observed equally with RTL551 treatment at 96h post-MCAO. Similarly, MCAO reduced the number of lymph node cells in both Vehicle and RTL551-treated groups by 96h post-MCAO, with no differences observed between groups (Table 2).
Table 1.
Cell numbers (× 106) in blood, lymph nodes (LN) and spleen of Vehicle and RTL551-treated Mice 24h after MCAO.
| Vehicle 24h | RTL551 24h | |||
|---|---|---|---|---|
| Sham (4) | MCAO (n) | Sham (4) | MCAO (n) | |
| †Blood | 3±0 | 2±0 (17) | 3±1 | 2±0 (15) |
| LN | 29±4 | 22±3 (20) | 17±2 | 24±2 (18) |
| Spleen | 52±9 | 32±10 (11) | 19±1 | 32±5 (17) |
cell number × 106 per ml
Table 2.
Cell numbers (× 106) in blood, lymph nodes (LN) and spleen of Vehicle and RTL551-treated Mice 96h after MCAO.
| Vehicle 96h | RTL551 96h | |||
|---|---|---|---|---|
| Sham (4) | MCAO (n) | Sham (3–4) | MCAO (n) | |
| †Blood | 4±1 | 3±0 (9) | 3±0 | 2.8±1 (8) |
| LN | 26±7 | 6±2*(8) | 41±13 | 4.9±0*(8) |
| Spleen | 78±12 | 38±8*(7) | 83±1 | 22±7*(7) |
cell number × 106 per ml
indicates significant difference compared to corresponding sham-treated mice
Cellular populations of CD4+ and CD8+ T lymphocytes and CD19+ B lymphocytes were determined by flow cytometry in remaining viable cells from spleen and lymph nodes and no differences were observed between Vehicle vs. RTL551-treatment at either 24 or 96h post-MCAO in either organ (data not shown). In blood, however, the percentage of viable CD4 lymphocytes decreased 24h after MCAO in vehicle, but not in RTL551-treated mice, while the percentage of CD8+ T lymphocytes was higher with RTL551 treatment relative to vehicle.
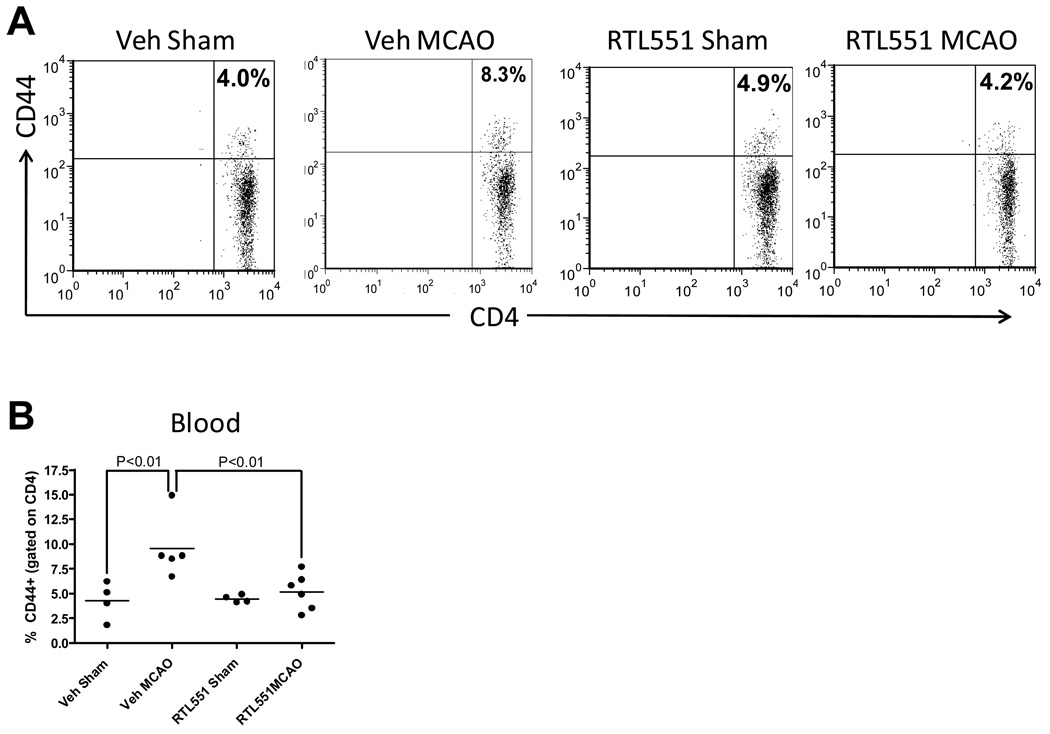
CD44 is a memory T-cell marker that is involved in T-cell activation, binding to selectins on the endothelium during transmigration across the blood-brain barrier and release of cytokines in brain to amplify the inflammatory response. Thus, RTL551 modulation of CD44 might account for the observed reduction in cellular infiltration into brain and reduced infarct volumes. As shown in Fig. 3, MCAO increased the percentage of CD44+, CD4+ T-cells in the blood of Vehicle-treated mice at 24h, the time when MCAO induces activation. RTL551 treatment prevented this increase, resulting in a significant decrease in CD44 expression compared to Vehicle-treated mice (Figure 3). In contrast to blood, no difference in the percentage of CD44+ CD4+ T-cells was observed in the lymph nodes or spleen (data not shown).
FIGURE 3. RTL551 treatment reduced the percentage of CD44+ CD4+ T-cells in blood.
Blood mononuclear cells were isolated from vehicle and RTL551-treated mice 24h after MCAO and evaluated for expression of CD44+ on CD4+ T-cells by flow cytometry. The presence of CD44+ cells was determined by gating on the CD4+ population and the percentage of CD44+CD4+ double positive cells represents events within the CD4+ population. Representative flow cytometry plots (A) and from combined data from two experiments (B).
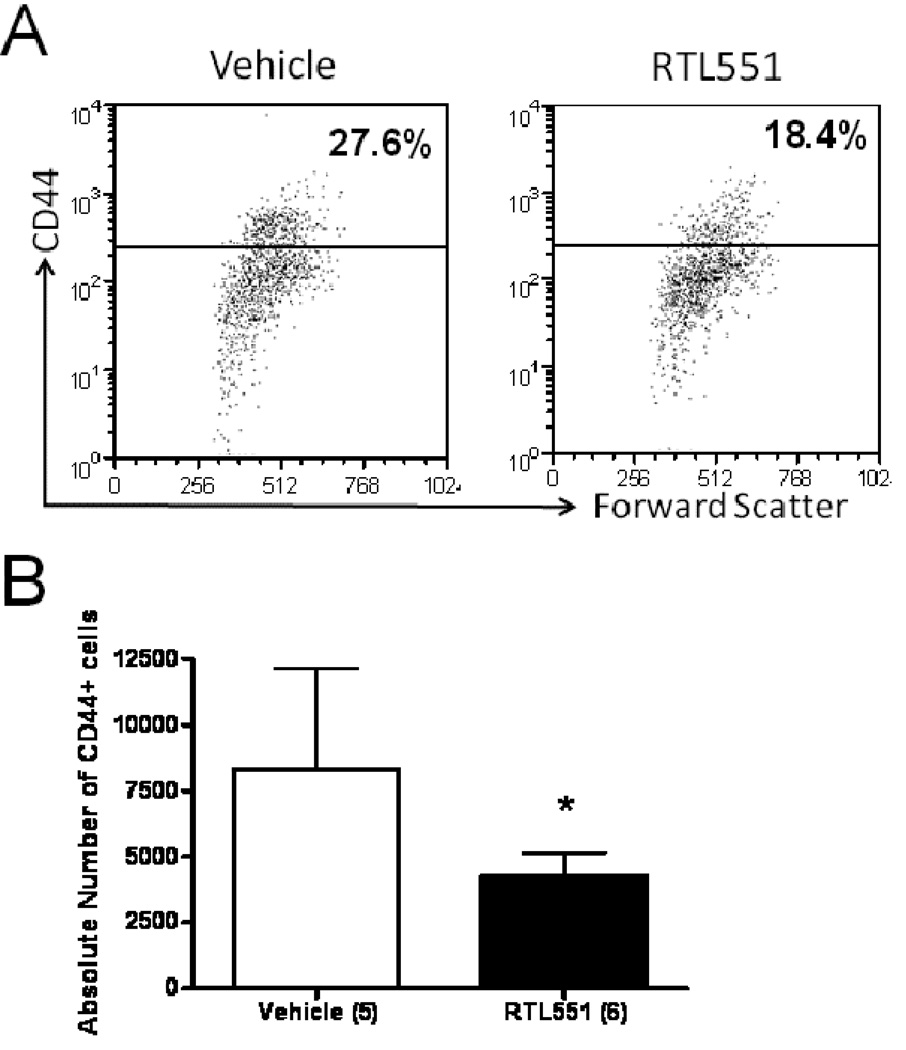
To determine if decreased frequency of CD44 expression in the periphery of RTL551-treated mice resulted in reduced CD44 expression in CNS, brain mononuclear cells were evaluated from ischemic and non-ischemic hemispheres 24h after MCAO. As shown in Fig. 4, the absolute number as well as the percentage of CD44+ cells was significantly reduced in the ischemic hemisphere 24h after MCAO in RTL551- vs. Vehicle-treated mice. To further investigate the possibility that RTL551 might reduce activation of antigen presenting cells, we evaluated expression of the costimulatory molecules, CD80 and CD86, as well as MHC Class II on CD11b+ myeloid cells, CD19+ B-cells and CD11c+ dendritic cells. No differences in these antigen presenting cell (APC) activation markers were observed between Vehicle and RTL551-treated groups 24h after MCAO (data not shown).
FIGURE 4. RTL551-treatment reduced absolute numbers of CD44+ cells in brain 24h after MCAO.
Total brain mononuclear cells were isolated from ischemic hemispheres 24h after RTL1000 or Vehicle treatment and flow cytometry was used to evaluate the percent of CD44+ T-cells (A). Absolute numbers were obtained by multiplying the percent positive T-cell by the total number of cells recovered. (B) represents data analyzed and combined from 5–6 mice per group. * denotes significant difference
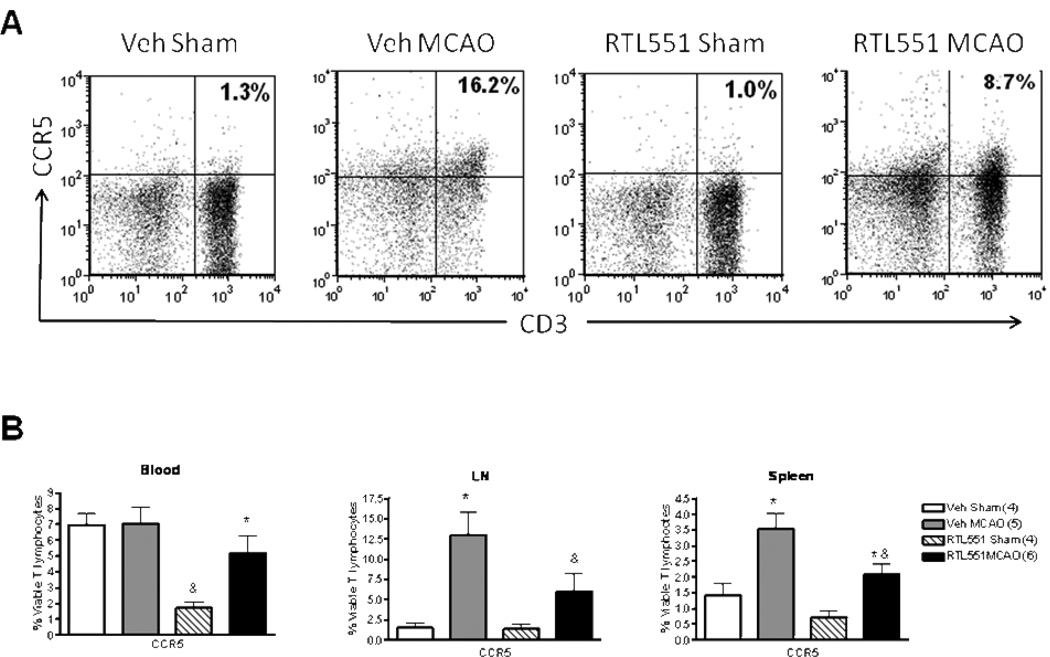
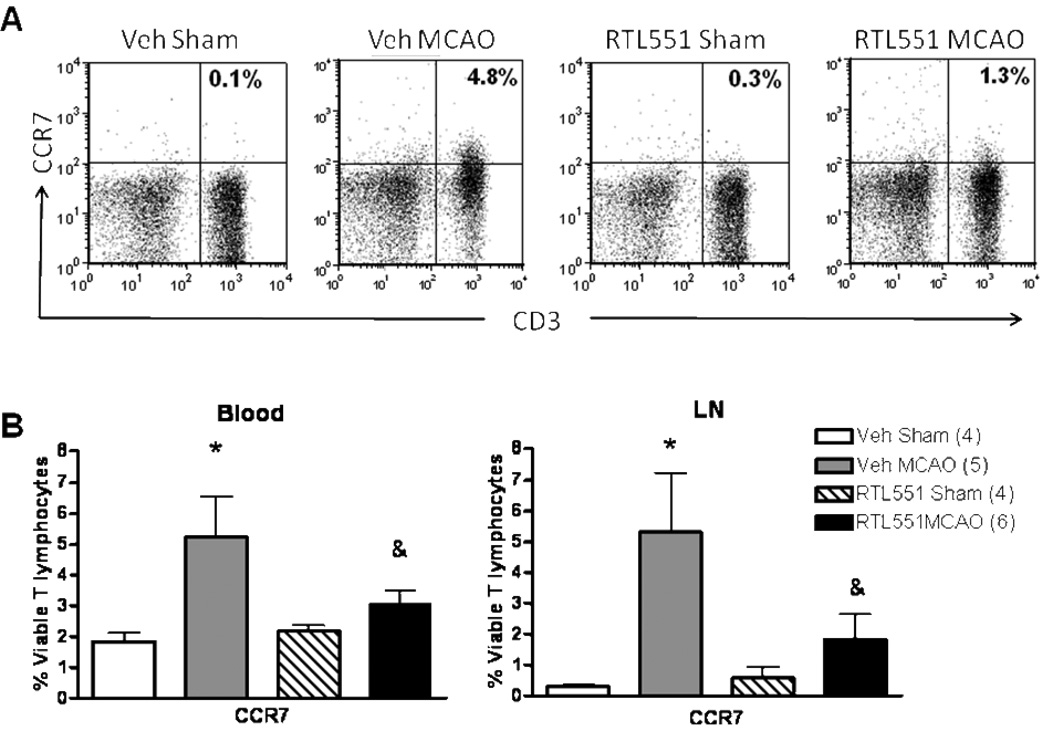
To determine whether RTL551 might inhibit chemokine receptors possibly involved in recruitment of inflammatory cells into the brain after MCAO, CCR5 and CCR7 expression was evaluated on CD3+ T lymphocytes as well as CD11b and CD19 positive APCs 24h after MCAO. As shown in Fig. 5, RTL551 treatment reduced the expression of CCR5 on T lymphocytes in the lymph nodes and spleen after MCAO compared to Vehicle-treated mice. However, RTL551 also reduced the percentage of CCR5+ T-cells in the blood of sham-treated mice, suggesting an RTL551-dependent reduction in CCR5 expression on circulating T-cells independent of ischemia (Figure 5). RTL551 treatment also reduced the percentage of CCR7 on circulating T cells in blood as well as in cells from lymph nodes (Figure 6), but had no effect in the spleen. Neither CCR5 nor CCR7 expression was affected by RTL551 treatment in myeloid or B-cells from blood, LN or spleen (data not shown).
FIGURE 5. RTL551 treatment reduces expression of CCR5 24h after MCAO in blood, lymph nodes and spleen.
Blood mononuclear cells, lymph node cells and splenocytes isolated from sham-treated and MCAO-treated mice that were administered either vehicle or RTL551 3h after reperfusion were evaluated for expression of CCR5 on CD3+ T lymphocytes by flow cytometry. Representative flow cytometry plot from lymph nodes (A) and combined data (B). LN=lymph nodes. *significant difference in MCAO- vs. sham-treated mice. &significant difference in RTL551 treated vs. vehicle-treated mice after MCAO.
FIGURE 6. RTL551 treatment reduces expression of CCR7 24h after MCAO in blood and lymph nodes.
Blood mononuclear cells and lymph node cells from sham-treated and MCAO-treated mice that were administered either vehicle or RTL551 3h after reperfusion were evaluated for expression of CCR7 on CD3+ T lymphocytes by flow cytometry. Representative flow cytometry plot from lymph nodes (A) and combined data (B). LN=lymph nodes; *significant difference in stroke mice vs. sham-treated mice. &significant difference in RTL551 treated vs. vehicle-treated mice after MCAO.
DISCUSSION
Previously, we demonstrated that RTL551 reduced infarct size and infiltration of inflammatory cells after MCAO when administered at the time of reperfusion (Subramanian et al. 2009). In the current study, our results are expanded to demonstrate that RTL551 is similarly effective in reduction of infarct size and infiltration of inflammatory cells when administered in a therapeutically relevant time window, 4h after MCAO. RTL551 also reduced CD44 expression in brain and on circulating T cells, suggesting diminished T-cell activation and trafficking. Furthermore, chemokine receptors CCR5 and CCR7 were reduced on T cells in the periphery.
RTLs were originally designed as therapy for multiple sclerosis, a devastating inflammatory demyelinating disease mediated by CD4+ T cells which become dysregulated and recognize myelin protein antigens as foreign (Burrows et al. 2001). Similar to stroke, the blood brain barrier is disrupted in EAE and although the disease is mediated via CD4+ T cells, secondary infiltration of primarily macrophages is observed in the CNS. Sinha et al. showed that RTL treatment strongly reduced the secondary infiltration of immune cells including macrophages as well as the expression of chemokines and chemokine receptors required for entry into the CNS (Sinha et al. 2007). RTLs reverse clinical and histological symptoms of experimental autoimmune encephalomyelitis when administered i.v. or s.c. after onset of the disease, suggesting that RTLs can reverse both inflammatory and neurodegenerative processes in vivo (Offner et al. 2008).
We found RTL551 reduced infarct volume in the cortex and striatum at 24h post MCAO and in the cortex at 96h after MCAO. This is in agreement with our previous finding demonstrating a reduction in cortical infarct at 96h after MCAO and is expected, since areas that receive some degree of microcirculatory reperfusion such as the cortex would receive more benefit than areas with poor microcirculatory function such as the striatum because T-cells as well as other immunocytes require vascular access to infiltrate the brain. The populations most effected by RTL551 treatment were dendritic cells (CD11c) and granulocytes (CD45hi/CD11bhi Gr-1+) at 24h post-MCAO. This is also consistent with our previous observations of decreased absolute number of dendritic cells as well as CD45hi/CD11bhi cells which includes the Gr-1+ population when RTL551 treatment was given at the time of reperfusion and showed a concurrent reduction of infarct size and all infiltrating cell types at 96h in the ischemic hemisphere (Subramanian et al. 2009). In the current study a non-significant reduction in CD45hi/CD11bhi cells was also observed at 96h. One difference between the current study and our previous study was that we performed analyses on individual, rather than pooled hemispheres, thus increasing the variability and is a limitation of the study.
Previous work has defined characteristic changes in peripheral immune cell populations after brain injury in mice. Focal cerebral ischemia induces spleen and thymic degeneration, in part through apoptotic death mechanisms (Brait et al. 2010;Liesz et al. 2009;Offner et al. 2006b;Prass et al. 2003) and in part due to cellular emigration from spleen to brain with deleterious consequences (Ajmo, Jr. et al. 2008;Yilmaz et al. 2006). Surprisingly the decrease in emigrating cells from the periphery into brain did not partially preserve spleen cell numbers at 96h after MCAO as previously observed with RTL551 treatment given at the time of reperfusion. We found MCAO induced a similar decrease in the number of cells in the lymph nodes, suggesting overall damage to lymph nodes in response to ischemic injury. It is possible that cells may be homing to peripheral organs such as lymph nodes and or spleen, where they reside but eventually undergo cell death. RTLs have completed Phase I safety trial with no harmful effects to humans (Offner et al. 2010). At minimum, RTL551 treatment did not further reduce overall cell numbers in spleen, lymph nodes or blood, suggesting that RTL551 does not augment post-stroke immunosupression. However further studies investigating immune response will be required.
When triggered by an injurious response, T cells express a characteristic set of activation markers, chemokine receptors and adhesion molecules required for homing of these cells to secondary lymphoid organs as well as the site of injury (Sallusto et al. 2004). CD44 is involved in multiple functions of inflammatory response including activation and endothelial cell recognition and lymphocyte trafficking (Pure and Cuff 2001). The post-ischemic microvasculature expresses high levels of P-selectin, which recruits leukocytes expressing CD44. Yilmaz et al. demonstrated that CD44 is not expressed in baseline bone marrow derived stromal cells but after exposure to brain extract from ischemic reperfusion injury CD44 is rapidly induced (Yilmaz et al. 2011) and an attenuated recruitment response into the ischemic hemisphere is observed with CD44 deficient BMSCs suggesting CD44 is a ligand on cells that engage selectins required for the recruitment of peripheral immune cells to the vasculature and subsequent infiltration into brain (Yilmaz et al. 2011). Likewise we are the first to our knowledge to report increased frequency of circulating CD44 on CD4+ T cells 24h after MCAO. RTL551 reduced expression of CD44+ CD4+ T-cells which likely decreased infiltration of inflammatory cells into brain. In EAE, An antibody against CD44 prevents clinical symptoms by partially targeting the primary influx of encephalitogenic T-cells and by preventing the secondary influx of leukocytes (Brocke et al. 1999). Interestingly, RTL551 mediated-reduction of CD44 expression on circulating T cells was accompanied by an overall decrease in absolute number of CD44+ cells in the brain after MCAO at 24h but with no differences in CD44 expression on T cells between vehicle and RTL551-treatment groups. RTLs have been recently shown to bind to antigen presenting cells, such as dendritic cells, macrophages and B-cells (Sinha et al. 2010). It may be possible that RTL551, in addition to its effects on reducing T cell activation in the periphery, may reduce secondary infiltration of cells via decreased CD44 or may have direct effects in the brain (Wang et al. 2002). Expression of CD44 on resident immune cells such as microglia as well as other APCs in the brain will be a subject for future study.
Chemokines and chemokine receptors are crucial for the recruitment of inflammatory cells to sites of injury. In the current study, decreased expression of CCR5 and CCR7 in the periphery was associated with decreased infarct size in RTL551 treated animals 24h post-MCAO. CCR5 is known to be induced by neuronal insults including ischemia (Cowell et al. 2002;Kremlev et al. 2007;Spleiss et al. 1998). We previously found MCAO induces the chemokine CCL5/RANTES (regulated upon activation normal T expressed and secreted) at 6h after MCAO. The message for the cognate chemokine receptor for RANTES, CCR5 was most dramatically increased after MCAO, associating CCR5 with injury (Offner et al. 2006a). We hypothesized that reduction of CCR5 expression would thus be beneficial for stroke outcome, yet some reports argue a protective role for CCR5 after ischemia. The absence of CCR5 is detrimental for responses to certain types of infection (Barr et al. 2005;Hardison et al. 2006;Khan et al. 2006) and increased infection contributes to exacerbating brain damage after ischemia (Denes et al. 2010) Likewise, CCR5-deficient animals show enlarged infarct area which is associated with behavioral deficits and enhanced neuronal death present in the infarct core (Sorce et al. 2010). We identified RTL reduction of CCR5 specific to T-cells, but not B-cells or CD11b+ myeloid cells, suggesting that the protective effects of CCR5 observed in the brain (Sorce et al. 2010) are not T-cell mediated.
CCR7 is also an indicator of activated T lymphocytes as well as naïve and central memory T-cells (Sallusto et al. 2004), and is involved homing of these cells to lymph nodes (Ebert et al. 2005). These cells home to secondary lymphoid organs, and readily proliferate and differentiate to effectors cells in response to antigenic stimulation. CCR7 ligands are required for the generation of EAE suggesting the pathogenic involvement of CCR7 in this disease (Kuwabara et al. 2009) CCR7 expression is upregulated in murine spleen (Offner et al. 2006b) and in human blood (Yan et al. 2009) after stroke. Reduced CCR7 expression in the periphery induced by RTL551 treatment of MCAO may limit naïve cells from undergoing differentiation into central memory T-cells in response to antigenic stimulation and these cells may not be able to respond to neural antigens, especially in the lymph nodes where brain antigens would be likely present after stroke.
In summary, we demonstrated that RTL551 is effective in reducing infarct size and infiltration of harmful immune cells when administered in a therapeutically relevant time window. Interactions with the peripheral immune system and brain are complex and inflammatory processes arise from multiple cell types, with T-cells being an influential component. The mechanisms of RTL551 action remain to be determined but are likely mediated via diminished harmful activation of T-cells, which limits downstream activation and recruitment of other immune cells. These findings demonstrate that RTL551 is a promising therapeutic intervention after stroke by targeting infarct associated brain regions rather than a global inflammatory response.
ACKNOWLEGEMENTS
The authors wish to thank Dr. Sushmita Sinha and Ms. Sandhya Subramanian for helpful discussions and Ms. Eva Niehaus for assistance in preparing the manuscript. This work was supported by NIH Grant NR003521 (PDH), NIH Grant NS047661 (AAV), the Collins Medical Trust (SD), AHA grant 09POST2190040 (SD), and the Biomedical Laboratory R&D Service, Department of Veterans’ Affairs.
Footnotes
CONFLICT OF INTEREST
Drs. Offner, Burrows, Vandenbark, and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
REFERENCES
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr EL, Ouburg S, Igietseme JU, Morre SA, Okwandu E, Eko FO, Ifere G, Belay T, He Q, Lyn D, Nwankwo G, Lillard JW, Jr, Black CM, Ananaba GA. Host inflammatory response and development of complications of Chlamydia trachomatis genital infection in CCR5-deficient mice and subfertile women with the CCR5delta32 gene deletion. J Microbiol Immunol Infect. 2005;38:244–254. [PubMed] [Google Scholar]
- Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci U S A. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows GG, Adlard KL, Bebo BF, Jr, Chang JW, Tenditnyy K, Vandenbark AA, Offner H. Regulation of encephalitogenic T cells with recombinant TCR ligands. J Immunol. 2000;164:6366–6371. doi: 10.4049/jimmunol.164.12.6366. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167:4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Xu H, Galasso JM, Silverstein FS. Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke. 2002;33:795–801. doi: 10.1161/hs0302.103740. [DOI] [PubMed] [Google Scholar]
- Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Hardison JL, Wrightsman RA, Carpenter PM, Kuziel WA, Lane TE, Manning JE. The CC chemokine receptor 5 is important in control of parasite replication and acute cardiac inflammation following infection with Trypanosoma cruzi. Infect Immun. 2006;74:135–143. doi: 10.1128/IAI.74.1.135-143.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Thomas SY, Moretto MM, Lee FS, Islam SA, Combe C, Schwartzman JD, Luster AD. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2:e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremlev SG, Roberts RL, Palmer C. Minocycline modulates chemokine receptors but not interleukin-10 mRNA expression in hypoxic-ischemic neonatal rat brain. J Neurosci Res. 2007;85:2450–2459. doi: 10.1002/jnr.21380. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Ishikawa F, Yasuda T, Aritomi K, Nakano H, Tanaka Y, Okada Y, Lipp M, Kakiuchi T. CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells. J Immunol. 2009;183:2513–2521. doi: 10.4049/jimmunol.0800729. [DOI] [PubMed] [Google Scholar]
- Liesz A, Hagmann S, Zschoche C, Adamek J, Zhou W, Sun L, Hug A, Zorn M, Dalpke A, Nawroth P, Veltkamp R. The Spectrum of Systemic Immune Alterations After Murine Focal Ischemia. Immunodepression Versus Immunomodulation. Stroke. 2009 doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: A Phase I clinical study. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Sinha S, Wang C, Burrows GG, Vandenbark AA. Recombinant T cell receptor ligands: immunomodulatory, neuroprotective and neuroregenerative effects suggest application as therapy for multiple sclerosis. Rev Neurosci. 2008;19:327–339. doi: 10.1515/revneuro.2008.19.4-5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Sinha S, Miller L, Subramanian S, McCarty OJ, Proctor T, Meza-Romero R, Huan J, Burrows GG, Vandenbark AA, Offner H. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;225:52–61. doi: 10.1016/j.jneuroim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, Huan J, Vandenbark AA, Burrows GG, Offner H. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci. 2007;27:12531–12539. doi: 10.1523/JNEUROSCI.3599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Bonnefont J, Julien S, Marq-Lin N, Rodriguez I, Dubois-Dauphin M, Krause KH. Increased brain damage after ischaemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol. 2010;160:311–321. doi: 10.1111/j.1476-5381.2010.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spleiss O, Gourmala N, Boddeke HW, Sauter A, Fiebich BL, Berger M, Gebicke-Haerter PJ. Cloning of rat HIV-1-chemokine coreceptor CKR5 from microglia and upregulation of its mRNA in ischemic and endotoxinemic rat brain. J Neurosci Res. 1998;53:16–28. doi: 10.1002/(SICI)1097-4547(19980701)53:1<16::AID-JNR3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T Cell Receptor Ligand Treats Experimental Stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- Wang PY, Kao CH, Mui MY, Wang SJ. Leukocyte infiltration in acute hemispheric ischemic stroke. Stroke. 1993;24:236–240. doi: 10.1161/01.str.24.2.236. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu L, Wang H, Zhan Y, Pure E, Feuerstein GZ. CD44 deficiency in mice protects brain from cerebral ischemia injury. J Neurochem. 2002;83:1172–1179. doi: 10.1046/j.1471-4159.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Greer JM, Etherington K, Cadigan GP, Cavanagh H, Henderson RD, O'Sullivan JD, Pandian JD, Read SJ, McCombe PA. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009;206:112–117. doi: 10.1016/j.jneuroim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Vital S, Yilmaz CE, Stokes KY, Alexander JS, Granger DN. Selectin-mediated recruitment of bone marrow stromal cells in the postischemic cerebral microvasculature. Stroke. 2011;42:806–811. doi: 10.1161/STROKEAHA.110.597088. [DOI] [PMC free article] [PubMed] [Google Scholar]