Abstract
Bipolar disorder (BP) in youth is an impairing psychiatric disorder associated with high rates of relapse and recurrence. High rates of psychiatric and medical co-morbidities account for additional illness burden in pediatric BP. The elevated risk of overweight and obesity in this population is of particular concern. One of the likely etiologies for weight gain in youth with BP is use of mood-stabilizing medications. Although these medications can be effective for mood stabilization, excessive weight gain is a common side effect. Obesity is associated with a host of medical problems and is also correlated with worse psychiatric outcomes in BP, rendering the prevention of weight gain in this population particularly clinically relevant. In this article, we describe the rationale and development of a brief motivational intervention for preventing weight gain among youth with BP initiating mood-stabilizing pharmacological treatment and then present a case example illustrating the principles of the intervention.
Introduction
Bipolar disorder (BP) in youth is a serious psychiatric illness associated with high rates of recurrence, profound psychosocial impairment, and suicide (Geller et al. 2008; Birmaher et al. 2009). Recent studies indicate that youth with BP are at substantially elevated risk of overweight and obesity (OW/OB; Correll 2007; Goldstein et al. 2008; Gracious et al. 2010)—a grave public health concern due to risk for morbidity and mortality associated with cardiovascular disease among adults with BP (Osby et al. 2001; Goldstein et al. 2009).
One of the etiologies for weight gain in BP is the use of mood-stabilizing medications (e.g., lithium) and second-generation antipsychotics (e.g., olanzapine) (Correll 2007). Although these medications have demonstrated efficacy for mood stabilization in youth with BP (Chang 2008), substantial weight gain early in treatment is a common side effect that occurs at even greater rates among children and adolescents (Correll 2007; Correll et al. 2010). Potential mechanisms of medication-associated weight gain in BP are multifactorial and include direct effects on appetitive drive and metabolism putatively linked to medication-related changes in various neurotransmitters, chemokines, cytokines, and insulin as well as indirect effects such as reduced energy expenditure caused by sedation and globally reduced psychomotor activity (Virk et al. 2004; Sporn et al. 2005).
OW/OB is not only associated with a host of medical problems, but also correlated with worse psychiatric outcomes among adults with BP, including greater illness severity, higher rates of recurrence, hospitalizations, and suicidality (Fagiolini et al. 2005; Wang et al. 2006; Calkin et al. 2009), rendering the prevention of weight gain in this population particularly clinically relevant. A study of children and adolescents with BP found that those who were OW/OB were more likely to have had a history of physical abuse, psychiatric hospitalization, and co-morbid substance use disorders than those who were not OW/OB (Goldstein et al. 2008).
Researchers have thus called for preventive strategies targeting OW/OB in BP as a means of improving overall course of BP illness and decreasing the substantial associated morbidity and mortality (Jolin et al. 2007). Studies support the efficacy of nonpharmacological interventions for preventing medication-associated weight gain among adults with psychiatric disorders, and these studies have employed cognitive behavior therapy (CBT) strategies and nutritional counseling interventions (Littrell et al. 2003; Evans et al. 2005; Alvarez-Jiménez et al. 2006; Scocco et al. 2006). A recent meta-analysis of nonpharmacological interventions found that preventive interventions appear at least as beneficial for body weight change as treatment interventions designed to reduce weight (Alvarez-Jiménez et al. 2008). However, at present, there are no studies examining weight gain prevention interventions for youth with BP. We thus describe the development of a brief motivational intervention for preventing weight gain among youth with BP who recently initiated mood-stabilizing pharmacological treatment and then present a case example illustrating the principles of the intervention. Through the conduct of a series of pilot cases such as the one we describe in this study, we aim to iteratively develop the intervention while examining the feasibility and acceptability of the approach for this population.
Methods
Brief motivational interventions: a promising approach
A recent meta-analysis elucidated several effective strategies for obesity prevention in the general pediatric population (Stice et al. 2006). One such approach, a brief motivational intervention, has been successfully applied in pediatric healthcare settings (Erickson et al. 2005). Brief motivational interventions are based on motivational interviewing, an evidence-based approach originally developed to enhance behavior change among adults with substance use problems (Miller and Rollnick 1991), with demonstrated sustained effects on behavior change over time (Dunn et al. 2001).
A brief motivational intervention is potentially efficacious for preventing weight gain in youth with BP commencing psychotropic medications because (1) evidence supports the use of brief motivational interventions for behavior change among youth (see Suarez and Mullins 2008, as below); (2) this approach can be delivered in few sessions, thus minimizing burden on families and healthcare systems; (3) the focus on eliciting intrinsic motivation for change is especially well suited for adolescents given the emerging developmental need for autonomy; (4) the skills and strategies are relatively easy to teach, learn, and adherently deliver and thus amenable to delivery by community treatment practitioners within extant clinical infrastructures. Brief motivational interventions have been applied toward behavior change among adolescents between 11 and 18 years of age in various domains including diabetes, obesity, dental hygiene, and reproductive health (for a review, see Suarez and Mullins 2008). Similar interventions targeting parents have also been employed to improve health-related outcomes among infants 6–18 months (Weinstein et al. 2004) and preschool and school-aged children between 3 and 7 years (Schwarts et al. 2007).
“The Big 5”: specific behaviors associated with weight gain in youth
We examined the literature to identify specific and measurable weight-related health behaviors amenable to a focus within a brief intervention. “The Big 5” developed by Rao (2006) includes five specific behaviors associated with weight in youth: (1) sweet beverages consumed per day; (2) fast food meals consumed per week; (3) dinners eaten with at least one parent per week; (4) media time per day (e.g., television, video games); and (5) physical activity for at least 30 minutes per week. Each of the Big 5 behaviors is associated with a doctor-recommended “dose” that is associated with the maintenance of healthy weight.
Development of a brief motivational intervention
We describe a five-session brief motivational intervention (three 45-minute in-person sessions and two 15-minute phone sessions) designed to prevent excessive weight gain among youth with BP initiating treatment with mood stabilizers and/or antipsychotics. Given the preventive nature of the intervention, we consider eligible youth to be those who are prescribed a medication associated with weight gain, regardless of the youth's current body mass index (BMI). To maximize feasibility and generalizability, the intervention may be delivered within the infrastructure of a mental health clinic, pediatrician's office, or other healthcare facility.
Structure of the intervention
In light of findings regarding increased risk for excessive weight gain early in the course of pharmacological treatment, the intervention is designed to be delivered in five sessions over a 10–12-week time period. Sessions 1, 3, and 5 last 45 minutes and are delivered in-person at weeks 1, 4–5, and 8–10 of treatment; Sessions 2 and 4 are 15-minute telephone booster sessions (weeks 2 and 6). This design overlaps with pharmacological management visit schedules common in clinical settings where youth with BP are treated.
Parent involvement
Unfortunately, there are no guidelines (e.g., age, pubertal level) to determine the level of parental involvement in weight-focused interventions for youth (Resnicow et al. 2006), and published data on the specific type, amount, and level of parental involvement in prior studies are limited (see Suarez and Mullins 2008). In general, given the strong association between parents' and their children's BMI, food intake, and physical activity (Correll and Carlson 2006), some level of parental involvement appears warranted with youth. We therefore recommend parental involvement be clinically determined based on the adolescent's developmental level, motivation, and familial preferences.
Five essential treatment ingredients
The intervention consists of five thematically related “essential treatment ingredients” (ETIs) that build upon one another. In this way, the clinician has flexibility to spend relatively more or less time on a specific treatment ingredient, as determined by the child and family's needs and clinical judgment. The clinician progresses from one ETI to the next in a way that balances achieving the aims of each ETI, with the family's autonomy and readiness for change. The content of each ETI is as follows:
ETI 1: personalized feedback
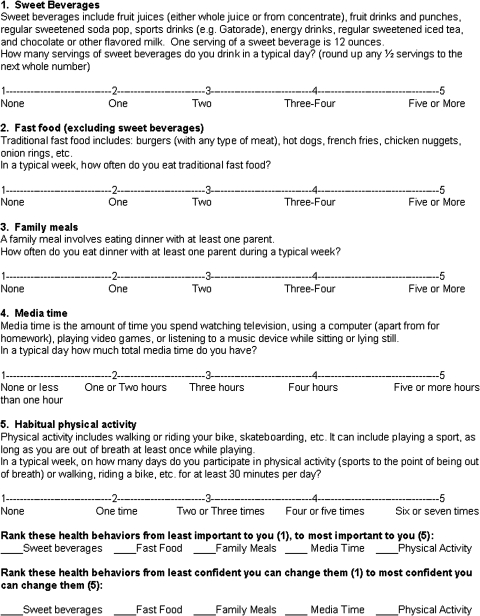
Prior to Session 1, the child rates his/her Big 5 behaviors (Fig. 1), and the clinician obtains the child's height and weight to calculate BMI (see http://apps.nccd.cdc.gov/dnpabmi/). The clinician delivers the first ETI with the child and parent together. Primarily, the clinician elicits each family member's understanding and experience with weight management. What is their understanding of how weight is influenced by symptoms of BP and medications for BP? The clinician then provides information about the balance between energy expenditure and intake in determining weight and shares information about how the child's medications influence weight. Next, all explore concerns about the child's current weight and propensity for weight gain. The clinician concludes by providing individualized feedback about the child's BMI; a standard chart depicting BMI and classification can be utilized.
FIG. 1.
“The Big 5”: A form for rating health behaviors.
ETI 2: the Big 5
The clinician begins the second ETI by reviewing the child's Big 5 behavior ratings and eliciting thoughts and reactions to the items. Then, the clinician invites family members to describe a typical week with respect to weight-related habits. This provides a segue into discussion of the recommended doses of the Big 5 behaviors: (1) sweet beverages: maximum one serving/day; (2) fast food: maximum one per week; (3) family meals: at least 1 parent most days of the week; (4) media time: maximum 2 hours/day; (5) physical activity: minimum 30 minutes/day, 5 days/week. The clinician then provides feedback on how the child's Big 5 behaviors correspond with the recommended doses.
ETI 3: evaluating readiness for change and exploring ambivalence
The goal in this ETI is to help the child identify at least two behaviors to focus on changing, prioritizing those the child deems most important to change and those the child feels most confident he/she can change. The child rank-orders (1–5; least to most) the Big 5 behaviors in terms of both relative importance and confidence (indices of readiness; Fig. 1). If the child ranks a behavior high (i.e., relatively easy to change and confident he/she can change it), the clinician considers proposing a focus on that behavior. If a behavior is ranked relatively low for both importance and confidence, the clinician either targets another behavior or explores reasons for relatively low ratings. Ultimately, the relative indices of readiness and the child's choice guide the decision about which behaviors to focus on. The clinician encourages the child to consider what would happen if he/she decides not to make any changes. Next, the clinician encourages the child to talk about weight-related behaviors, highlighting and exploring any discrepancies between concerns, goals, and current health behaviors. Once the child and clinician agree on specific risk behaviors to target, the clinician and child discuss feelings about trying to change targeted risk behaviors.
ETI 4: develop a change plan
Once the child has committed to behavior change in two areas of the Big 5, the clinician leads the child in the creation of a change plan for each targeted behavior. The change plan should be collaboratively established and determined to be reasonable and attainable by all parties. The clinician can offer a menu of options if the child has difficulty generating a plan.
ETI 5: evaluate the change plan
The final ETI aims to increase motivation for sustained change. The clinician reviews the change plan and reassesses feelings about importance and confidence related to behavior change for the specific behavior selected. Here, we recommend the clinician use the standard 1–10 readiness ruler as it pertains to the selected behavior, as described in standard Motivational Interviewing practice (Miller and Rollnick 1991). Importance refers to the individual's desire for change, whereas confidence refers to the individual's assessment of his/her ability to affect change. Specifically, the clinician asks “How important is this [specific behavior] change to you right now?” and “How confident are you about making this [specific behavior] change?” The clinician highlights successes and works together to problem-solve barriers that interfered with the change plan. By the final session, it is ideal for the clinician to have at least three measurements of height/weight/BMI. A graph of the child's BMI trajectory over time (including additional factors that influenced the trajectory) can guide discussion of the ways in which Big 5 behaviors contributed to the BMI trajectory. If the plan has been mostly successful, the discussion includes encouragement for continued success and troubleshooting future challenges. If the plan has been less successful, then discussion includes providing empathy regarding the difficulty of changing behaviors, soliciting thoughts regarding current weight, and instilling hope that change can occur. Finally, the clinician elicits ongoing short- and long-term goals related to BMI and Big 5 behaviors.
Case Presentation
We now present a case example to demonstrate the principles of the treatment. We briefly summarize the content of each session and highlight the shift from one ETI to the next. The case presentation is based on an actual course of treatment conducted by one of the authors (M.B.M.) and supervised by another (A.D.). Identifiable patient characteristics are modified to protect confidentiality. “Tom” was a 15-year-old Caucasian boy diagnosed with bipolar I disorder receiving psychiatric care at a specialty clinic for youth with mood disorders. He was referred by his psychiatrist, who had recently prescribed a second-generation antipsychotic for the management of Tom's BP. At baseline, Tom was 5′7" tall and weighed 153 pounds, with a BMI of 24.0 (83rd percentile, high end of healthy weight).
Session 1 (week 1; in-person)
Start ETI 1: Tom attended the first session with his father. The clinician began by engaging them in a discussion about Tom's weight. Tom and his father were well versed in the factors that contribute to weight; although weight had never been a problem for Tom, others in the family had struggled with weight. Next, the clinician discussed the relationship between weight gain, BP, and Tom's medication. Tom noted that symptoms of his current depressive episode (i.e., anhedonia and fatigue) led him to be less active. Tom's father expressed concern about Tom's recent inactivity and its implications for his health and self-esteem. The clinician then provided individualized feedback about Tom's current BMI—on the upper end of the normal range.
Start ETI 2: The clinician then reviewed Tom's ratings of his Big 5 behaviors. He reported fast food <1 time/week, media time <2 hours/day, and majority of meals eaten together in the home. Tom's ratings indicated little to no physical activity, and an average of four sweet beverages/day. Because he had become depressed he reported drinking more soda to get energized from its caffeine, especially during the schooldays. Tom then described a typical week related to his Big 5 behaviors. The clinician discussed the recommended doses of the Big 5 and concluded by asking Tom to keep a daily log of his Big 5 behaviors and their association with his energy level (global daily rating of 1–10) for the next 2 weeks.
Session 2 (week 3; phone)
ETI 2 continued: The phone session occurred 2 weeks after the initial visit. Tom noted that his energy level remained low as his depression persisted. He recognized that the more he lay around, the less energy he had to do things. Additionally, although his energy and motivation increased in the short term after drinking a soda, he then “crashed” and felt even more tired. The clinician reinforced Tom's efforts and offered hope.
Session 3 (week 4; in-person)
The clinician weighed Tom prior to the session; Tom was 5′7′′ tall and weighed 150 pounds, with a BMI of 23.5 (81st percentile, high end of healthy weight, 3 pound weight loss).
Start ETI 3: The clinician reviewed the content of Session 2 with Tom individually. Tom then ranked the relative importance and confidence of each of the Big 5 behaviors (Fig. 1); physical activity was most important to him (5) followed by sweet beverages (4). His confidence ratings reflected most confidence in being able to change sweet beverages (5) followed by physical activity (4). These ratings, taken with prior session content, led the clinician to propose a focus on increasing physical activity and decreasing intake of sweet beverages; Tom agreed.
Next, the clinician invited Tom to consider the pros and cons of making changes in these areas. Tom identified many pros of change, including having more energy and feeling better; he was particularly motivated to play sports with his younger brother—something he had ceased doing recently. Cons included the possibility of failure. They then considered potential components of a change plan. Tom felt he could eliminate soda altogether and drink water instead. With respect to his physical activity, he proposed to shoot baskets after school and take walks with his dad.
Start ETI 4: They collaboratively developed the following change plan: Reduce sweet beverage intake to one cup/day and increase physical activity to 30 minutes/day by walking with his father and playing basketball. Tom invited his father into the session and presented the change plan; Tom's father agreed to support him in his plan.
Session 4 (week 6; phone)
Start ETI 5: Session 4 started with review of Tom's progress toward implementing his change plan. He was successful in implementing the sweet beverage change plan. He described feeling more sluggish the first 2 days he implemented the plan (he attributed this to the decreased caffeine intake). Tom stated he was mostly unable to execute his physical activity change plan (walking with his dad) because of inclement weather. They explored other ways Tom could increase his activity level by identifying alternative options (e.g., push-ups, sit-ups, stationary bicycle). Tom reported his continued depressive symptoms (i.e., insomnia and hopelessness) were also negatively impacting his motivation to implement the physical activity change plan. Together, they brainstormed solutions to improve Tom's motivation. Tom agreed to write down the change plans after the telephone call, review them with his father, and post them on the refrigerator.
Session 5 (week 11; in-person)
The clinician weighed Tom prior to the session; Tom was 5′7′′ tall and weighed 148 pounds, with a BMI of 23.0 (78th percentile, healthy weight, total 5 pound weight loss).
ETI 5 continued: With Tom and his father, the clinician reviewed experience implementing the change plan. Tom continued with the sweet beverage change plan. In addition, he walked for 30 minutes four times/week over the past 2 weeks and started playing basketball with his younger brother after school. Tom described feeling more energetic and less depressed.
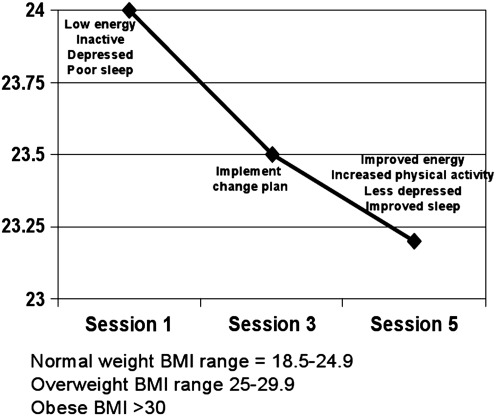
The clinician and family reviewed a chart of Tom's change in BMI during the intervention (Fig. 2). Tom was encouraged to see the graph and expressed interest in continuing to chart his BMI trajectory at home to maintain gains. They then revisited the Big 5 behaviors and Tom's associated goals and change plan. Tom's ratings of importance of sustained change in these areas and confidence in his ability to maintain the positive changes he had made remained high (importance 9 and confidence 8 of 10). He identified improvements in mood and sleep associated with increased physical activity; his father agreed, noting less irritability since Tom had been more active. The clinician concluded by providing reinforcement for Tom's hard work and encouragement for continued success.
FIG. 2.
Tom's body mass index (BMI) trajectory over treatment.
Discussion
OW/OB is highly prevalent among youth with BP and is associated with greater illness severity. Although multiple factors are associated with OW/OB in this population, studies demonstrate a clear link between mood-stabilizing medications and weight gain in youth. It is therefore vital that the treatment of pediatric BP incorporates an emphasis on the prevention of OW/OB. Given competing demands from medication management and psychosocial treatment visits targeting the stabilization of BP, it is important that adjunctive weight-related intervention in this population be time efficient. Preventive brief interventions such as the one we describe in this study have the potential to mitigate medication-associated weight gain in BP without placing undue burden on patients, families, or healthcare systems.
The content and structure of the brief motivational intervention we describe are designed to be flexible to allow for delivery to youth of various age and developmental levels, cognitive capacities, and social and environmental influences. Family members can be incorporated to the extent that their involvement facilitates positive change. The provision of ETIs rather than prescribed session content allows the clinician to individualize the pace of the intervention based on the child's and family's level of knowledge and readiness for change. The case example demonstrates how an adolescent with BP was engaged in the process of understanding the link between medication, mood, and weight, identifying and enacting changes in his health behaviors, and ultimately experiencing positive health and clinical outcomes.
Further treatment development and testing are needed to establish the efficacy of the BMI. Next steps include completion of a pilot case series focused on further treatment development and preliminary examination of targeted outcomes with treatment. Specifically, in addition to measuring change in BMI, behavior change associated with targeted “Big 5” behaviors should be assessed throughout treatment and over follow-up using established subjective (e.g., food diaries) and objective (e.g., pedometers) methods. Further, the association between mood symptoms and change in targeted health outcomes will be an important area of future study. If conduct of the pilot series supports the feasibility and acceptability of the approach for this population, an adequately powered, randomized, controlled trial would be warranted to examine treatment-related outcomes. In the interim, the present article provides a framework for clinicians to work toward the important goal of preventing OW/OB among youth with BP as means of decreasing associated risk for negative psychiatric and medical outcomes.
Clinical Significance
Overweight and obesity are highly prevalent among youth with BP and are associated with greater illness severity. Although multiple factors are associated with overweight and obesity in this population, studies demonstrate a clear link between mood-stabilizing medications and weight gain in youth. Preventive brief interventions such as the one we described in this study have the potential to mitigate medication-associated weight gain in youth with BP without placing undue burden on patients, families, or healthcare systems.
Disclosures
Dr. T.R. Goldstein receives royalties from Guilford Press. Dr. B.I. Goldstein has received an honorarium from Purdue Pharma and has been a consultant for Bristol Drs. Myers Squibb. M.B. Mantz, B. Bailey, and A. Douaihy have no disclosures to report.
Acknowledgments
This work was supported by NIMH grant MH074581 (PI: T.R. Goldstein). The authors thank the faculty and staff of the Child and Adolescent Bipolar Services Clinic at the University of Pittsburgh.
References
- Alvarez-Jiménez M. Gonzalez-Blanch C. Vasquez-Barquero J. Perez-Iglesias R. Martinez-Garcia O. Perez-Pardal T. Ramirez-Bonilla M. Crespo-Facorro B. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naïve first episode psychosis patients: A randomized controlled trial. J Clin Psychiatry. 2006;67:1253–1260. doi: 10.4088/jcp.v67n0812. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jiménez M. Hetrick S. Gonzalez-Blanch C. Gleeson J. McGorry P. Non-pharmacological management of antipsychotic-induced weight gain: Systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2008;193:101–107. doi: 10.1192/bjp.bp.107.042853. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Axelson DA. Goldstein BI. Strober M. Gill MK. Hunt J. Houck M. Ha W. Iyengar S. Kim E. Yen S. Hower H. Smythers C. Goldstein TR. Ryan ND. Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin C. van de Velde C. Ruzickova M. Slaney C. Garnham J. Hajek T. O'Donovan C. Alda M. Can body mass index help predict outcome in patients with bipolar disorder? Bipolar Disord. 2009;11:650–656. doi: 10.1111/j.1399-5618.2009.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KD. The use of atypical antipsychotics in pediatric bipolar disorder. J Clin Psychiatry. 2008;69:4–8. [PubMed] [Google Scholar]
- Correll C. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: A systematic review and pooled analysis of short-term trials. J Am Acad Child Psychiatry. 2007;46:687–700. doi: 10.1097/chi.0b013e318040b25f. [DOI] [PubMed] [Google Scholar]
- Correll C. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Correll C. Sheridan A. DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: A comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12:116–141. doi: 10.1111/j.1399-5618.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Dunn C. DeRoo L. Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: A systematic review. Addiction. 2001;96:1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- Erickson SJ. Gerstle M. Feldstein SW. Brief interventions and motivational interviewing with children, adolescents, and their parents in pediatric health care settings. Arch Pediatr Adolesc Med. 2005;159:1173–1180. doi: 10.1001/archpedi.159.12.1173. [DOI] [PubMed] [Google Scholar]
- Evans S. Newton R. Higgins S. Nutritional intervention to prevent weight gain in patients commenced on olanzapine: A randomized controlled trial. Aust New Zeal J Psychiatry. 2005;39:479–486. doi: 10.1080/j.1440-1614.2005.01607.x. [DOI] [PubMed] [Google Scholar]
- Fagiolini A. Frank E. Scott J. Turkin S. Kupfer DJ. Metabolic syndrome in bipolar disorder: Findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- Geller B. Tillman R. Bolhofner K. Zimerman B. Child bipolar I disorder: Prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI. Birmaher B. Axelson DA. Goldstein TR. Esposito-Smythers C. Strober MA. Hunt J. Leonard H. Gill MK. Iyengar S. Grimm C. Yang M. Ryan ND. Keller MB. Preliminary findings regarding overweight and obesity in pediatric bipolar disorder. J Clin Psychiatry. 2008;69:1953–1959. doi: 10.4088/jcp.v69n1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI. Fagiolini A. Houck P. Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracious BL. Cook SR. Meyer A. Chirieac M. Malhi N. Fischetti A. Finucane T. Ma Y. Prevalence of overweight and obesity in adolescents with severe metal illness: A cross-sectional chart review. J Clin Psychiatry. 2010;71:949–954. doi: 10.4088/JCP.09m05033gre. [DOI] [PubMed] [Google Scholar]
- Jolin EM. Weller EB. Weller RA. The public health aspects of bipolar disorder in children and adolescents. Curr Psychiatry Rep. 2007;9:106–113. doi: 10.1007/s11920-007-0079-6. [DOI] [PubMed] [Google Scholar]
- Littrell K. Hilligoss N. Kirshner C. Petty R. Johnson C. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Sch. 2003;35:237–241. doi: 10.1111/j.1547-5069.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- Miller W. Rollnick W. Motivational Interviewing. Guilford Press; New York: 1991. [Google Scholar]
- Osby U. Brandt L. Correia N. Ekbom A. Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- Rao G. Child Obesity. Amherst: Prometheus Books; 2006. [Google Scholar]
- Resnicow K. Davis R. Rollnick S. Motivational interviewing for pediatric obesity: Conceptual issues and evidence review. J Am Diet Assoc. 2006;106:2024–2033. doi: 10.1016/j.jada.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Schwarts R. Hamre R. Dietz W. Office based motivational interviewing to prevent childhood obesity: A feasibility study. Arch Pediatr Adolesc Med. 2007;161:495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- Scocco P. Longo R. Caon F. Weight change in treatment with olanzapine and a psychoeducational approach. Eat Behav. 2006;7:115–124. doi: 10.1016/j.eatbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Sporn A. Bobb A. Gogtay N. Stevens H. Greenstein D. Clasen L. Tosesell J. Nugent T. Gochman P. Sharp W. Mattai A. Lenane M. Yanovski J. Rapoport J. Hormonal correlates of clozapine-induced weight gain in psychotic children: An exploratory study. J Am Acad Child Adolesc Psychiatry. 2005;44:925–933. doi: 10.1097/01.chi.0000170552.15798.dd. [DOI] [PubMed] [Google Scholar]
- Stice E. Shaw H. Marti C. A meta-analytic review of obesity prevention programs for children, adolescents: The skinny on interventions that work. Psychol Bull. 2006;132:691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez M. Mullins S. Motivational interviewing and pediatric health behavior interventions. J Dev Behav Pediatr. 2008;29:417–428. doi: 10.1097/DBP.0b013e31818888b4. [DOI] [PubMed] [Google Scholar]
- Virk S. Schwartz S. Jindal N. Jones N. Psychiatric medication induced obesity: An aetiologic review. Obes Rev. 2004;5:167–170. doi: 10.1111/j.1467-789X.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- Wang PW. Sachs GS. Zarate C. Marangell LB. Calabrese JR. Goldberg J. Overweight and obesity in bipolar disorders. J Psychiatry Res. 2006;40:762–764. doi: 10.1016/j.jpsychires.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Weinstein P. Harrison R. Benton T. Motivating parents to prevent caries in their young children: One year findings. J Am Dent Assoc. 2004;135:731–738. doi: 10.14219/jada.archive.2004.0299. [DOI] [PubMed] [Google Scholar]